Analysis of Risk Factors for Cerebral Microbleeds and the Relationship between Cerebral Microbleeds and Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Plasma Analyses
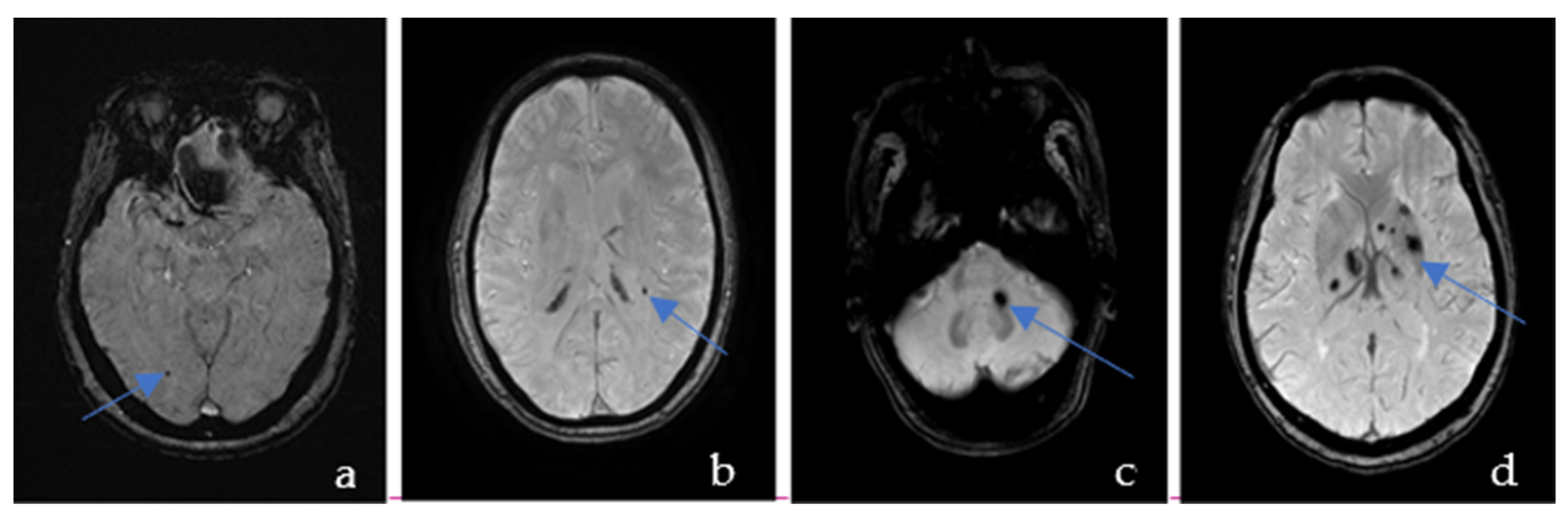
2.3. Brain MRI and SVD Markers
2.4. Assessment of Cognitive Function
2.5. Vascular Risk Factors
2.6. Statistical Analysis
3. Results
3.1. Analysis of Risk Factors of CMBs
3.1.1. The Univariate Analysis of the Risk Factors for CMBs
3.1.2. The Multivariate Analysis of the Risk Factors for CMBs
3.1.3. The Risk Factors for CMBs in Different Regions
3.1.4. The Multivariate Analysis of the Risk Factors for CMBs in Different Regions
3.2. Analysis of the Relationship between CMBs and Cognitive Impairment
3.2.1. The Univariate Analysis of the Characteristics of Cognitive Impairment
3.2.2. The Univariate Analysis of the Imaging Characteristics of Cognitive Impairment
3.2.3. The Multivariate Analysis of the Risk Factors for Cognitive Impairment
3.2.4. The Relationship between the Number of CMBs and MoCA Scores
3.2.5. The Relationship between CMBs in Different Regions and MoCA Scores
3.2.6. The Relationship between CMBs in Different Regions and Specific Cognitive Domains
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorelick, P.B.; Counts, S.E.; Nyenhuis, D. Vascular cognitive impairment and dementia. Biochim. Biophys. Acta 2016, 1862, 860–868. [Google Scholar] [CrossRef]
- Rost, N.S.; Etherton, M. Cerebral Small Vessel Disease. Continuum 2020, 26, 332–352. [Google Scholar] [CrossRef]
- Akoudad, S.; Wolters, F.J.; Viswanathan, A.; De Bruijn, R.F.; Van Der Lugt, A.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A.; Vernooij, M.W. Association of Cerebral Microbleeds with Cognitive Decline and Dementia. JAMA Neurol. 2016, 73, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, D.-H.; Li, H.-Q.; Tan, L.; Xu, W.; Dong, Q.; Tan, L.; Yu, J.-T. Association of Cerebral Microbleeds with Cognitive Decline: A Longitudinal Study. J. Alzheimer’s Dis. 2020, 75, 571–579. [Google Scholar] [CrossRef]
- Oboudiyat, C.; Gefen, T.; Varelas, E.; Weintraub, S.; Rogalski, E.; Bigio, E.H.; Mesulam, M. Cerebrospinal fluid markers detect Alzheimer’s disease in nonamnestic dementia. Alzheimer’s Dement. 2017, 13, 598–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Park, S.; Cho, H.; Jang, Y.K.; Lee, J.S.; Jang, H.; Kim, Y.; Kim, K.W.; Ryu, Y.H.; Choi, J.Y.; et al. Assessment of Extent and Role of Tau in Subcortical Vascular Cognitive Impairment Using 18F-AV1451 Positron Emission Tomography Imaging. JAMA Neurol. 2018, 75, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Leijsen, E.M.; Kuiperij, H.B.; Kersten, I.; Bergkamp, M.I.; van Uden, I.W.; Vanderstichele, H.; Stoops, E.; Claassen, J.A.H.R.; van Dijk, E.J.; de Leeuw, F.-E.; et al. Plasma Aβ (Amyloid-β) Levels and Severity and Progression of Small Vessel Disease. Stroke 2018, 49, 884–890. [Google Scholar] [CrossRef]
- Vernooij, M.W.; van der Lugt, A.; Ikram, M.A.; Wielopolski, P.A.; Niessen, W.J.; Hofman, A.; Krestin, G.P.; Breteler, M. Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study. Neurology 2008, 70, 1208–1214. [Google Scholar] [CrossRef]
- Joseph-Mathurin, N.; Dorieux, O.; Trouche, S.G.; Boutajangout, A.; Kraska, A.; Fontès, P.; Verdier, J.-M.; Sigurdsson, E.M.; Mestre-Francés, N.; Dhenain, M. Amyloid beta immunization worsens iron deposits in the choroid plexus and cerebral microbleeds. Neurobiol. Aging 2013, 34, 2613–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.-P.; Chou, K.-H.; Chen, W.-T.; Liu, L.-K.; Lee, W.-J.; Chen, L.-K.; Lin, C.-P.; Wang, P.-N. Strictly Lobar Cerebral Microbleeds Are Associated With Cognitive Impairment. Stroke 2016, 47, 2497–2502. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Salman, R.A.-S.; Warach, S.; Launer, L.J.; Van Buchem, M.A.; Breteler, M.M. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, S.M.; Chaudhary, U.J.; Brown, M.M.; Yousry, T.A.; Kallis, C.; Jager, H.R.; Werring, D.J. The Microbleed Anatomical Rating Scale (MARS): Reliability of a tool to map brain microbleeds. Neurology 2009, 73, 1759–1766. [Google Scholar] [CrossRef]
- Shaaban, C.E.; Jorgensen, D.R.; Gianaros, P.J.; Mettenburg, J.; Rosano, C. Cerebrovascular disease: Neuroimaging of cerebral small vessel disease. Prog. Mol. Biol. Transl. Sci. 2019, 165, 225–255. [Google Scholar] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Neuroradiol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazekas, F.; Kleinert, R.; Offenbacher, H.; Payer, F.; Schmidt, R.; Kleinert, G.; Radner, H.; Lechner, H. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am. J. Neuroradiol. 1991, 12, 915–921. [Google Scholar]
- Wahlund, L.O.; Barkhof, F.; Fazekas, F.; Bronge, L.; Augustin, M.; Sjogren, M.; Wallin, A.; Ader, H.; Leys, D.; Pantoni, L.; et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001, 32, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Jia, J.; Yang, Z. Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J. Alzheimer’s Dis. 2016, 53, 487–496. [Google Scholar] [CrossRef]
- O’Driscoll, C.; Shaikh, M. Cross-Cultural Applicability of the Montreal Cognitive Assessment (MoCA): A Systematic Review. J. Alzheimer’s Dis. 2017, 58, 789–801. [Google Scholar] [CrossRef]
- Romero, J.R.; Preis, S.R.; Beiser, A.; DeCarli, C.; Viswanathan, A.; Martinez-Ramirez, S.; Kase, C.S.; Wolf, P.A.; Seshadri, S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke 2014, 45, 1492–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackam, D.G.; Woodward, M.; Newby, L.K.; Bhatt, D.L.; Shao, M.; Smith, E.E.; Donner, A.; Mamdani, M.; Douketis, J.D.; Arima, H.; et al. Statins and intracerebral hemorrhage: Collaborative systematic review and meta-analysis. Circulation 2011, 124, 2233–2242. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Ye, H.; Wang, J.; Yan, J.; Wang, J.; Wang, Y. Antiplatelet Therapy, Cerebral Microbleeds, and Intracerebral Hemorrhage: A Meta-Analysis. Stroke 2018, 49, 1751–1754. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Jia, J.-J.; Zhao, H.-L.; Zhou, B.; Wang, W.; Lu, X.-H.; Wang, H.; Wang, Z.-F.; Wu, W.-P. Early MRI imaging and follow-up study in cerebral amyloid angiopathy. Open Med. 2021, 16, 257–263. [Google Scholar] [CrossRef]
- Lee, M.; McGeer, E.; McGeer, P.L. Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: Implications for Alzheimer’s disease pathogenesis. Neurobiol. Aging 2015, 36, 42–52. [Google Scholar] [CrossRef]
- Jung, Y.H.; Jang, H.; Park, S.B.; Choe, Y.S.; Park, Y.; Kang, S.H.; Lee, J.M.; Kim, J.S.; Kim, J.; Kim, J.P.; et al. Strictly Lobar Microbleeds Reflect Amyloid Angiopathy Regardless of Cerebral and Cerebellar Compartments. Stroke 2020, 51, 3600–3607. [Google Scholar] [CrossRef]
- Wang, Z.; Wong, A.; Liu, W.; Yang, J.; Chu, W.C.; Au, L.; Lau, A.; Chan, A.; Xiong, Y.; Soo, Y.; et al. Cerebral Microbleeds and Cognitive Function in Ischemic Stroke or Transient Ischemic Attack Patients. Dement. Geriatr. Cogn. Disord. 2015, 40, 130–136. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Sun, H.; Li, M.; Li, Y.; Zhao, J.; Li, J.; Liu, X.; Cong, Y.; Li, F.; et al. Cerebral Microbleeds Are Associated With Mild Cognitive Impairment in Patients With Hypertension. J. Am. Heart Assoc. 2018, 7, e008453. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Ramirez, S.; Greenberg, S.M.; Viswanathan, A. Cerebral microbleeds: Overview and implications in cognitive impairment. Alzheimer’s Res. Ther. 2014, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sigurðsson, S.; Jónsson, P.V.; Eiriksdottir, G.; Meirelles, O.; Kjartansson, O.; Lopez, O.L.; van Buchem, M.A.; Gudnason, V.; Launer, L.J. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology 2017, 88, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Poels, M.M.F.; Ikram, M.A.; van der Lugt, A.; Hofman, A.; Niessen, W.J.; Krestin, G.P.; Breteler, M.M.B.; Vernooij, M.W. Cerebral microbleeds are associated with worse cognitive function: The Rotterdam Scan Study. Neurology 2012, 78, 326–333. [Google Scholar] [CrossRef]
- Luo, Q.; Tang, H.; Xu, X.; Huang, J.; Wang, P.; He, G.; Song, X.; Huang, Y.; Chen, S.; Yan, F.; et al. The Prevalence and Risk Factors of Cerebral Microbleeds: A Community-Based Study in China. Ther. Clin. Risk Manag. 2021, 17, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.K.; Pan, S.Y. Risk factors for cerebral microbleeds. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 1425–1427. [Google Scholar] [PubMed]
- Poliakova, T.; Levin, O.; Arablinskiy, A.; Vasenina, E.; Zerr, I. Cerebral microbleeds in early Alzheimer’s disease. J. Neurol. 2016, 263, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Calvillo, M.; Fan, D.; Irimia, A. Multimodal Imaging of Cerebral Microhemorrhages and White Matter Degradation in Geriatric Patients with Mild Traumatic Brain Injury. Methods Mol. Biol. 2020, 2144, 223–236. [Google Scholar]
- Gold, B.T.; Shao, X.; Sudduth, T.L.; Jicha, G.A.; Wilcock, D.M.; Seago, E.R.; Wang, D.J. Water exchange rate across the blood-brain barrier is associated with CSF amyloid-β 42 in healthy older adults. Alzheimer’s Dement. 2021, 17, 2020–2029. [Google Scholar] [CrossRef]


| Clinical Characteristics | Non-CMBs (n = 210) | CMBs (n = 196) | t/χ2 Value | p-Value |
|---|---|---|---|---|
| Men/Women | 151/59 | 140/56 | 0.011 | 0.915 |
| Age (mean ± SD), years | 68.10 ± 6.54 | 69.93 ± 6.87 | −2.751 | 0.006 |
| Education (mean ± SD), years | 7.01 ± 2.62 | 6.89 ± 3.44 | 1.801 | 0.075 |
| Smoker (n (%)) | 29(13.80) | 55(28.06) | 12.549 | <0.001 |
| Drinker (n (%)) | 28(13.33%) | 25(12.76%) | 0.030 | 0.863 |
| Hypertension (n (%)) | 87(41.43%) | 148(75.51%) | 23.433 | <0.001 |
| Diabetes (n (%)) | 42(20.00%) | 37(18.88%) | 0.082 | 0.775 |
| CHD (n (%)) | 33(15.71%) | 45(22.96%) | 3.428 | 0.064 |
| Antithrombotic medication | 52(24.76%) | 74(37.76%) | 7.997 | 0.005 |
| Lipid-lowering medication | 36(17.14%) | 22(11.22%) | 0.816 | 0.366 |
| TC (mean ± SD) | 4.08 ± 1.02 | 3.66 ± 0.81 | 4.618 | <0.001 |
| TG (mean ± SD) | 1.48 ± 0.49 | 1.68 ± 1.38 | −1.811 | 0.061 |
| HDL-C (mean ± SD) | 1.13 ± 0.31 | 1.08 ± 0.29 | 1.819 | 0.070 |
| LDL-C (mean ± SD) | 2.39 ± 0.94 | 2.37 ± 0.77 | 0.203 | 0.404 |
| BUA (mean ± SD), μmol/L | 301.30 ± 79.56 | 312.37 ± 79.73 | −1.399 | 0.400 |
| HCY (mean ± SD), μmol/L | 16.93 ± 8.35 | 20.20 ± 10.17 | −3.552 | <0.001 |
| Aβ1-42(mean ± SD), μmol/L | 44.75 ± 24.80 | 70.71 ± 37.49 | −8.171 | <0.001 |
| pTau-81(mean ± SD), μmol/L | 21.29 ± 12.67 | 25.17 ± 10.10 | −3.396 | 0.001 |
| Variable | B | SE | Ward | p | OR (95%CI) |
|---|---|---|---|---|---|
| Age | 0.044 | 0.018 | 6.030 | 0.014 | 1.045 (1.009, 1.082) |
| Hypertension | 1.283 | 0.251 | 26.070 | <0.001 | 3.607 (2.204, 5.901) |
| Smoking | 1.282 | 0.302 | 18.059 | <0.001 | 3.604 (1.995, 6.509) |
| Antithrombotic medication | 0.068 | 0.272 | 0.062 | 0.803 | 1.070 (0.628, 1.822) |
| TC | −0.493 | 0.137 | 12.966 | <0.001 | 0.611 (0.467, 0.799) |
| HCY | 0.010 | 0.014 | 0.498 | 0.481 | 1.010 (0.982, 1.039) |
| Aβ1-42 | 0.027 | 0.005 | 34.876 | <0.001 | 1.028 (1.018, 1.037) |
| pTau-181 | −0.010 | 0.011 | 0.788 | 0.375 | 0.990 (0.969, 1.012) |
| Lobar CMBs (n = 67) OR (95%CI) | Deep CMBs (n = 47) OR (95%CI) | Infratentorial CMBs (n = 27) OR (95%CI) | Mixed CMBs (n = 55) OR (95%CI) | |
|---|---|---|---|---|
| Men | 1.10 (0.44–2.73) | 0.93 (0.33–2.64) | 0.93 (0.23–3.84) | 0.97 (0.37–2.57) |
| Age | 1.12 (1.00–2.12) | 1.08 (1.01–1.16) | 1.32 (1.10–1.44) | 1.11 (1.00–1.20) |
| Smoking | 2.55 (0.95–6.81) | 4.67 (1.62–13.41) | 1.75 (0.34–9.09) | 2.33 (0.79–6.85) |
| Drinking | 1.50 (0.53–4.24) | 1.06 (0.28–4.03) | 2.57 (0.60–10.99) | 1.34 (0.60–3.29) |
| Hypertension | 0.89 (0.39–2.02) | 12.00 (2.66–54.21) | 5.33 (1.08–26.26) | 2.67 (1.06–6.74) |
| Diabetes | 1.02 (0.37–2.81) | 0.72 (0.19–2.68) | 0.46 (0.06–3.78) | 1.36 (0.48–3.84) |
| CHD | 2.24 (0.88–5.67) | 1.74 (0.56–5.39) | 2.24 (0.53–9.48) | 1.37 (0.45–4.16) |
| Antithrombotic medication | 0.91 (0.38–2.17) | 0.37 (0.10–1.35) | 0.24 (0.03–1.92) | 1.27 (0.51–3.17) |
| Lipid-lowering medication | 2.44 (1.04–5.77) | 0.65 (0.18–2.39) | 0.92 (0.18–4.60) | 1.51 (0.56–4.06) |
| TC | 0.43 (0.24–0.75) | 0.49 (0.26–0.92) | 0.61 (0.27–1.35) | 0.82 (0.51–1.32) |
| TG | 1.78 (1.07–2.97) | 1.98 (1.18–3.34) | 1.09 (0.37–3.23) | 1.79 (1.06–3.04) |
| HDL-C | 0.29 (0.07–1.24) | 0.16 (0.03–0.99) | 0.21 (0.02–2.34) | 0.34 (0.07–1.61) |
| LDL-C | 0.91 (0.56–1.47) | 0.89 (0.50–1.58) | 1.04 (0.49–2.19) | 1.14 (0.69–1.87) |
| BUA | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) |
| HCY | 1.07 (1.03–1.12) | 1.05 (0.99–1.09) | 1.05 (0.98–1.11) | 0.98 (0.91–1.05) |
| Aβ1-42 | 1.02 (1.01–1.03) | 1.00 (0.98–1.02) | 1.02 (1.00–1.03) | 1.01 (1.00–1.03) |
| pTau-181 | 1.04 (1.01–1.07) | 1.00 (0.95–1.05) | 1.06 (1.02–1.11) | 1.03 (0.99–1.07) |
| Lobar CMBs (n = 67) OR (95%CI) | Deep CMBs (n = 47) OR (95%CI) | Infratentorial CMBs (n = 27) OR (95%CI) | Mixed CMBs (n = 55) OR (95%CI) | |
|---|---|---|---|---|
| Age | 1.02 (0.94–1.10) | 1.13 (1.04–1.24) | 0.95 (0.83–1.08) | 1.04 (0.97–1.12) |
| Smoking | 2.35 (0.62–8.89) | 6.02 (1.53–23.71) | 0.40 (0.03–5.65) | 2.37 (0.70–7.95) |
| Hypertension | 0.64 (0.24–1.71) | 12.54 (2.21–71.28) | 4.40 (0.78–24.81) | 2.11 (0.78–5.70) |
| Antithrombotic | 0.01 (0.00–0.29) | 0.15 (0.02–1.40) | 1.48 (1.04–2.39) | 0.43 (0.07–5.70) |
| medication | ||||
| Lipid-lowering | 9.46 (4.20–20.65) | 2.55 (0.22–28.94) | 3.48 (1.44–8.39) | 3.27 (0.52–20.79) |
| medication | ||||
| TC | 0.26 (0.13–0.54) | 0.26 (0.12–0.55) | 0.52 (0.19–1.40) | 0.59 (0.32–1.06) |
| TG | 2.57 (1.14–5.83) | 4.48 (1.94–10.39) | 1.19 (0.31–4.59) | 2.91 (1.29–6.59) |
| HDL-C | 0.64 (0.10–4.17) | 1.28 (0.12–13.64) | 0.19 (0.01–5.86) | 0.99 (0.15–6.69) |
| Aβ1-42 | 1.02 (1.00–1.03) | 1.01 (0.99–1.03) | 1.02 (0.99–1.04) | 1.01 (0.99–1.03) |
| pTau-181 | 0.99 (0.95–1.04) | 0.95 (0.89–1.02) | 1.07 (0.99–1.15) | 0.99 (0.95–1.05) |
| Cognitive Impairment (n = 160) | Non-Cognitive Impairment (n = 246) | t/χ2 | p-Value | |
|---|---|---|---|---|
| Men/Women | 115 (71.9%) | 176 (71.5%) | 0.005 | 0.942 |
| Age, years (mean ± SD) | 70.07 ± 6.71 | 68.45 ± 6.45 | 2.429 | 0.016 |
| Education, years (mean ± SD) | 5.84 ± 2.81 | 6.75 ± 2.96 | −3.085 | 0.002 |
| Smoker (n (%)) | 32 (20.0%) | 52 (21.1%) | 0.077 | 0.782 |
| Drinker (n (%)) | 18 (20.0%) | 35 (14.2%) | 0.518 | 0.384 |
| Hypertension (n (%)) | 103 (64.4%) | 132 (53.7%) | 4.567 | 0.033 |
| Diabetes (n (%)) | 34 (21.3%) | 44 (17.9%) | 0.709 | 0.400 |
| CHD (n (%)) | 33 (20.6%) | 45 (18.3%) | 0.707 | 0.401 |
| Antithrombotic medication (n (%)) | 48 (30.0%) | 78 (31.7%) | 0.132 | 0.716 |
| Lipid-lowering medication (n (%)) | 22 (13.8%) | 36 (14.6%) | 0.062 | 0.804 |
| TC, mmol/L (mean ± SD) | 3.74 ± 0.91 | 3.96 ± 0.96 | −2.340 | 0.020 |
| TG, mmol/L (mean ± SD) | 1.67 ± 1.30 | 1.52 ± 0.79 | 1.350 | 0.178 |
| HDL-C, mmol/L (mean ± SD) | 1.01 ± 0.31 | 1.12 ± 0.30 | −0.704 | 0.482 |
| LDL-C, mmol/L (mean ± SD) | 2.35 ± 0.82 | 2.40 ± 0.89 | −0.554 | 0.580 |
| BUA μmol/L (mean ± SD) | 310.01 ± 81.50 | 304.50 ± 78.65 | 0.685 | 0.494 |
| HCY, μmol/L (mean ± SD) | 18.86 ± 8.92 | 17.04 ± 7.91 | 2.093 | 0.037 |
| Aβ1-42, pg/mL (mean ± SD) | 65.25 ± 36.87 | 52.10 ± 31.17 | 3.726 | <0.001 |
| pTau-81, pg/mL (mean ± SD) | 26.07 ± 10.99 | 22.24 ± 11.99 | 3.244 | 0.001 |
| Cognitive Impairment (n = 160) | Non-Cognitive Impairment (n = 246) | χ2/Z | p-Value | |
|---|---|---|---|---|
| Lacunes | 4.308 | 0.516 | ||
| 0 | 16 | 30 | OR | 95%CI |
| 1–5 | 90 | 116 | 1.455 | 0.747–2.832 |
| 6–10 | 27 | 59 | 0.858 | 0.402–1.832 |
| >10 | 27 | 41 | 1.235 | 0.568–2.686 |
| CMBs | 58.623 | <0.001 | ||
| None | 48 | 162 | OR | 95%CI |
| Lobar | 37 | 30 | 4.162 | 2.332–7.429 |
| Deep | 22 | 25 | 2.970 | 1.539–5.731 |
| Infratentorial | 13 | 14 | 3.134 | 1.379–7.121 |
| Mixed | 40 | 15 | 9.001 | 4.582–17.680 |
| WML | 9.829 | <0.001 | ||
| 0 | 69 | 165 | OR | 95%CI |
| 1 | 43 | 46 | 2.235 | 1.353–3.692 |
| 2 | 25 | 20 | 2.989 | 1.558–5.735 |
| 3 | 23 | 15 | 3.667 | 1.805–7.447 |
| Brain atrophy | 0.869 | 0.351 | ||
| inexistence | 124 | 200 | OR | 95%CI |
| existence | 36 | 46 | 1.262 | 0.773–2.061 |
| B | SE | WALD | p | OR (95%CI) | |
|---|---|---|---|---|---|
| Age | 0.032 | 0.019 | 2.729 | 0.099 | 1.032 (0.994, 1.071) |
| Education years | −0.040 | 0.014 | 7.910 | 0.005 | 0.959(0.930, 0.988) |
| Hypertension | 0.212 | 0.257 | 0.681 | 0.409 | 1.237(0.747, 2.048) |
| TC | −0.168 | 0.130 | 1.669 | 0.196 | 0.846 (0.656, 1.091) |
| HCY | 0.086 | 0.050 | 3.000 | 0.083 | 0.918 (0.833, 1.011) |
| AΒ1-42 | 0.004 | 0.004 | 0.778 | 0.378 | 1.004 (0.996, 1.012) |
| pTau-181 | −0.015 | 0.012 | 1.686 | 0.194 | 0.985 (0.962, 1.008) |
| WML | 0.988 | 0.209 | 22.25 | <0.001 | 2.687 (1.782, 4.051) |
| CMBs | |||||
| Lobar | 3.071 | 0.677 | 20.608 | <0.001 | 21.246 (5.728, 21.576) |
| Deep | 3.288 | 0.812 | 16.418 | <0.001 | 26.798 (5.462, 131.488) |
| Infratentorial | 3.297 | 0.842 | 15.342 | <0.001 | 27.028 (5.193, 140.690) |
| Mixed | 4.811 | 0.806 | 35.632 | <0.001 | 122.884 (25.317, 596.446) |
| Model 1 | ||||
| Regions | Standardized | β | p-Value | 95%CI |
| Lobar | −0.487 | <0.001 | (−5.278, −2.605) | |
| Deep | −0.231 | 0.005 | (−3.861, −0.715) | |
| Infratentorial | −0.301 | <0.001 | (−5.666, −1.846) | |
| Mixed | −0.306 | <0.001 | (−3.716, −1.100) | |
| Model 2 | ||||
| Regions | Standardized | β | p-Value | 95%CI |
| Lobar | −0.375 | <0.001 | (−4.188, −1.884) | |
| Deep | −0.201 | 0.081 | (−0.772, 5.613) | |
| Infratentorial | −0.216 | 0.072 | (−0.211, 4.814) | |
| Mixed | −0.292 | <0.001 | (−4.814, −0.211) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Yuan, Y.; Zhang, Z.; Zhang, J. Analysis of Risk Factors for Cerebral Microbleeds and the Relationship between Cerebral Microbleeds and Cognitive Impairment. Brain Sci. 2022, 12, 1445. https://doi.org/10.3390/brainsci12111445
Zheng H, Yuan Y, Zhang Z, Zhang J. Analysis of Risk Factors for Cerebral Microbleeds and the Relationship between Cerebral Microbleeds and Cognitive Impairment. Brain Sciences. 2022; 12(11):1445. https://doi.org/10.3390/brainsci12111445
Chicago/Turabian StyleZheng, Huiwen, Yong Yuan, Zuohui Zhang, and Jing Zhang. 2022. "Analysis of Risk Factors for Cerebral Microbleeds and the Relationship between Cerebral Microbleeds and Cognitive Impairment" Brain Sciences 12, no. 11: 1445. https://doi.org/10.3390/brainsci12111445





