Differential Predictors of Response to Early Start Denver Model vs. Early Intensive Behavioral Intervention in Young Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Design and Data Sources
2.2. Study Inclusion and Exclusion Criteria
2.3. Assessment and Measures
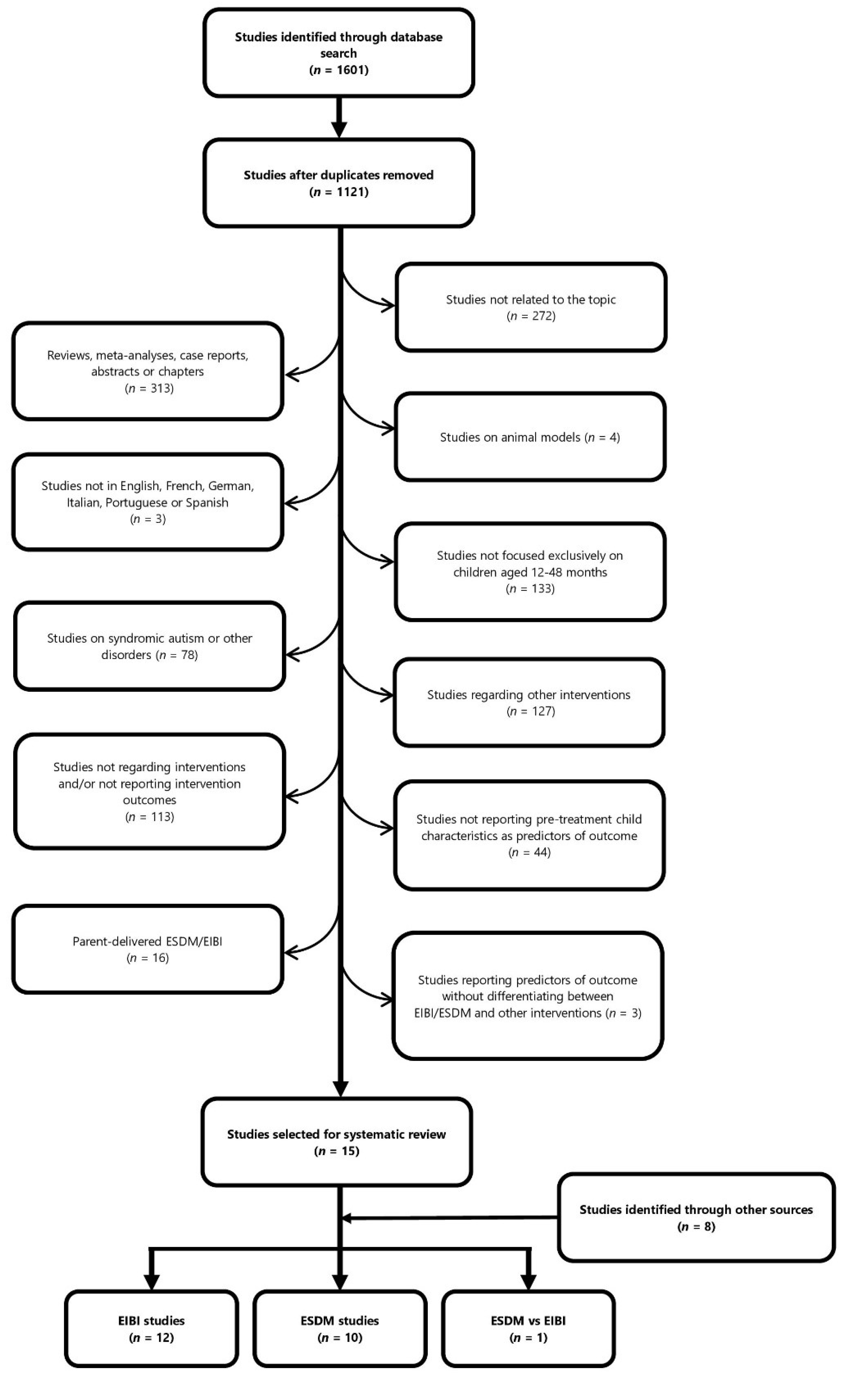
2.4. Study Selection Process
2.5. Meta-Analytical Strategy for Combining p-Values
3. Results
3.1. Early Intensive Behavioral Interventions
3.1.1. Sample Characteristics and Patient Selection Criteria
3.1.2. Treatment
3.1.3. Measures
3.1.4. Predictors of EIBI Treatment Outcome
3.2. Early Start Denver Model
3.2.1. Sample Characteristics and Patient Selection Criteria
3.2.2. Treatment
3.2.3. Measures
3.2.4. Predictors of ESDM Treatment Outcome
4. Discussion
5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Trial Registration
References
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Huguet, G.; Benabou, M.; Bourgeron, T. The Genetics of Autism Spectrum Disorders. In A Time for Metabolism and Hormones; Springer: Berlin, Germany, 2016; pp. 101–129. [Google Scholar]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors With Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, G.B.; Mendelsohn, N.J. Clinical Genetics Evaluation in Identifying the Etiology of Autism Spectrum Disorders: 2013 Guideline Revisions. Genet. Med. 2013, 15, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Butler, M.G. Clinical Assessment, Genetics, and Treatment Approaches in Autism Spectrum Disorder (ASD). Int. J. Mol. Sci. 2020, 21, 4726. [Google Scholar] [CrossRef]
- Fernandez, B.A.; Scherer, S.W. Syndromic Autism Spectrum Disorders: Moving from a Clinically Defined to a Molecularly Defined Approach. Clin. Res. 2017, 19, 20. [Google Scholar] [CrossRef]
- Cucinotta, F.; Ricciardello, A.; Turriziani, L.; Calabrese, G.; Briguglio, M.; Boncoddo, M.; Bellomo, F.; Tomaiuolo, P.; Martines, S.; Bruschetta, M.; et al. FARP-1 Deletion Is Associated with Lack of Response to Autism Treatment by Early Start Denver Model in a Multiplex Family. Mol. Genet. Genom. Med. 2020, 8, e1373. [Google Scholar] [CrossRef] [PubMed]
- Vorstman, J.A.S.; Spooren, W.; Persico, A.M.; Collier, D.A.; Aigner, S.; Jagasia, R.; Glennon, J.C.; Buitelaar, J.K. Using Genetic Findings in Autism for the Development of New Pharmaceutical Compounds. Psychopharmacology 2014, 231, 1063–1078. [Google Scholar] [CrossRef]
- Landa, R.J. Efficacy of Early Interventions for Infants and Young Children with, and at Risk for, Autism Spectrum Disorders. Int. Rev. Psychiatry 2018, 30, 25–39. [Google Scholar] [CrossRef]
- Lord, C.; Wagner, A.; Rogers, S.; Szatmari, P.; Aman, M.; Charman, T.; Dawson, G.; Durand, V.M.; Grossman, L.; Guthrie, D.; et al. Challenges in Evaluating Psychosocial Interventions for Autistic Spectrum Disorders. J. Autism Dev. Disord. 2005, 35, 695–708. [Google Scholar] [CrossRef]
- Lovaas, I. Behavioral Treatment and Normal Educational and Intellectual Functioning in Young Autistic Children. J. Consult. Clin. Psychol. 1987, 55, 3–9. [Google Scholar] [CrossRef]
- Lovaas, O.I. Teaching Developmentally Disabled Children: The ME Book; Pro-Ed: Austin, TX, USA, 1981. [Google Scholar]
- Cohen, H.; Amerine-Dickens, M.; Smith, T. Early Intensive Behavioral Treatment: Replication of the UCLA Model in a Community Setting. Dev. Behav. Pediatr. 2006, 27, S145–S155. [Google Scholar] [CrossRef]
- Eikeseth, S.; Klintwall, L.; Jahr, E.; Karlsson, P. Outcome for Children with Autism Receiving Early and Intensive Behavioral Intervention in Mainstream Preschool and Kindergarten Settings. Res. Autism Spectr. Disord. 2012, 6, 829–835. [Google Scholar] [CrossRef]
- Hayward, D.; Eikeseth, S.; Gale, C.; Morgan, S. Assessing Progress during Treatment for Young Children with Autism Receiving Intensive Behavioural Interventions. Autism 2009, 13, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Groen, A.D.; Wynn, J.W. Randomized Trial of Intensive Early Intervention for Children with Pervasive Developmental Disorder. Am. J. Ment. Retard. 2000, 105, 269–285. [Google Scholar] [CrossRef]
- Sheinkopf, S.J.; Siegel, B. Home-Based Behavioral Treatment of Young Children with Autism. J. Autism Dev. Disord. 1998, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Schreibman, L.; Dawson, G.; Stahmer, A.C.; Landa, R.; Rogers, S.J.; McGee, G.G.; Kasari, C.; Ingersoll, B.; Kaiser, A.P.; Bruinsma, Y.; et al. Naturalistic Developmental Behavioral Interventions: Empirically Validated Treatments for Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 2411–2428. [Google Scholar] [CrossRef]
- Rogers, S.J.; Dawson, G. Early Start Denver Model for Young Children with Autism; Guilford: New York, NY, USA, 2009. [Google Scholar]
- Dawson, G.; Rogers, S.; Munson, J.; Smith, M.; Winter, J.; Greenson, J.; Donaldson, A.; Varley, J. Randomized, Controlled Trial of an Intervention for Toddlers With Autism: The Early Start Denver Model. Pediatrics 2010, 125, e17–e23. [Google Scholar] [CrossRef]
- Fuller, E.A.; Kaiser, A.P. The Effects of Early Intervention on Social Communication Outcomes for Children with Autism Spectrum Disorder: A Meta-Analysis. J. Autism Dev. Disord. 2020, 50, 1683–1700. [Google Scholar] [CrossRef]
- Rogers, S.J.; Estes, A.; Lord, C.; Munson, J.; Rocha, M.; Winter, J.; Greenson, J.; Colombi, C.; Dawson, G.; Vismara, L.A.; et al. A Multisite Randomized Controlled Two-Phase Trial of the Early Start Denver Model Compared to Treatment as Usual. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 853–865. [Google Scholar] [CrossRef]
- Rogers, S.J.; Yoder, P.; Estes, A.; Warren, Z.; McEachin, J.; Munson, J.; Rocha, M.; Greenson, J.; Wallace, L.; Gardner, E.; et al. A Multisite Randomized Controlled Trial Comparing the Effects of Intervention Intensity and Intervention Style on Outcomes for Young Children With Autism. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 710–722. [Google Scholar] [CrossRef]
- Waddington, H.; van der Meer, L.; Sigafoos, J. Effectiveness of the Early Start Denver Model: A Systematic Review. Rev. J. Autism Dev. Disord. 2016, 3, 93–106. [Google Scholar] [CrossRef]
- Eldevik, S.; Eikeseth, S.; Jahr, E.; Smith, T. Effects of Low-Intensity Behavioral Treatment for Children with Autism and Mental Retardation. J. Autism Dev. Disord. 2006, 36, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Remington, B.; Hastings, R.P.; Kovshoff, H.; degli Espinosa, F.; Jahr, E.; Brown, T.; Alsford, P.; Lemaic, M.; Ward, N. Early Intensive Behavioral Intervention: Outcomes for Children With Autism and Their Parents After Two Years. Am. J. Ment. Retard. 2007, 112, 418–438. [Google Scholar] [CrossRef]
- Sinai-Gavrilov, Y.; Gev, T.; Mor-Snir, I.; Vivanti, G.; Golan, O. Integrating the Early Start Denver Model into Israeli Community Autism Spectrum Disorder Preschools: Effectiveness and Treatment Response Predictors. Autism 2020, 24, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, G.; Dissanayake, C.; Zierhut, C.; Rogers, S.J.; Victorian ASELCC Team. Brief Report: Predictors of Outcomes in the Early Start Denver Model Delivered in a Group Setting. J. Autism Dev. Disord. 2013, 43, 1717–1724. [Google Scholar] [CrossRef]
- Persico, A.M.; Cucinotta, F.; Ricciardello, A.; Turriziani, L. Autisms. In Neurodevelopmental Disorders; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–77. [Google Scholar] [CrossRef]
- Ellis Weismer, S.; Kover, S.T. Preschool Language Variation, Growth, and Predictors in Children on the Autism Spectrum. J. Child Psychol. Psychiatry 2015, 56, 1327–1337. [Google Scholar] [CrossRef]
- Bal, V.H.; Fok, M.; Lord, C.; Smith, I.M.; Mirenda, P.; Szatmari, P.; Vaillancourt, T.; Volden, J.; Waddell, C.; Zwaigenbaum, L.; et al. Predictors of Longer-term Development of Expressive Language in Two Independent Longitudinal Cohorts of Language-delayed Preschoolers with Autism Spectrum Disorder. J. Child Psychol. Psychiatry 2020, 61, 826–835. [Google Scholar] [CrossRef]
- Sherer, M.R.; Schreibman, L. Individual Behavioral Profiles and Predictors of Treatment Effectiveness for Children With Autism. J. Consult. Clin. Psychol. 2005, 73, 525–538. [Google Scholar] [CrossRef]
- Schreibman, L.; Stahmer, A.C.; Barlett, V.C.; Dufek, S. Brief Report: Toward Refinement of a Predictive Behavioral Profile for Treatment Outcome in Children with Autism. Res. Autism Spectr. Disord. 2009, 3, 163–172. [Google Scholar] [CrossRef][Green Version]
- Eikeseth, S.; Smith, T.; Jahr, E.; Eldevik, S. Intensive Behavioral Treatment at School for 4- to 7-Year-Old Children with Autism: A 1-Year Comparison Controlled Study. Behav. Modif. 2002, 26, 49–68. [Google Scholar] [CrossRef]
- Eikeseth, S.; Smith, T.; Jahr, E.; Eldevik, S. Outcome for Children with Autism Who Began Intensive Behavioral Treatment Between Ages 4 and 7: A Comparison Controlled Study. Behav. Modif. 2007, 31, 264–278. [Google Scholar] [CrossRef]
- Perry, A.; Cummings, A.; Geier, J.D.; Freeman, N.L.; Hughes, S.; Managhan, T.; Reitzel, J.-A.; Williams, J. Predictors of Outcome for Children Receiving Intensive Behavioral Intervention in a Large, Community-Based Program. Res. Autism Spectr. Disord. 2011, 5, 592–603. [Google Scholar] [CrossRef]
- Klintwall, L.; Eldevik, S.; Eikeseth, S. Narrowing the Gap: Effects of Intervention on Developmental Trajectories in Autism. Autism 2015, 19, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zwaigenbaum, L.; Bauman, M.L.; Fein, D.; Pierce, K.; Buie, T.; Davis, P.A.; Newschaffer, C.; Robins, D.L.; Wetherby, A.; Choueiri, R.; et al. Early Screening of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics 2015, 136 (Suppl. 1), S41–S59. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.L.; Handleman, J.S. Age and IQ at Intake as Predictors of Placement for Young Children with Autism: A Four- to Six-Year Follow-Up. J. Autism Dev. Disord. 2000, 30, 137–142. [Google Scholar] [CrossRef]
- Robain, F.; Franchini, M.; Kojovic, N.; Wood de Wilde, H.; Schaer, M. Predictors of Treatment Outcome in Preschoolers with Autism Spectrum Disorder: An Observational Study in the Greater Geneva Area, Switzerland. J. Autism Dev. Disord. 2020, 50, 3815–3830. [Google Scholar] [CrossRef]
- Smith, D.P.; Hayward, D.W.; Gale, C.M.; Eikeseth, S.; Klintwall, L. Treatment Gains from Early and Intensive Behavioral Intervention (EIBI) Are Maintained 10 Years Later. Behav. Modif. 2021, 45, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Magiati, I.; Charman, T.; Howlin, P. A Two-Year Prospective Follow-up Study of Community-Based Early Intensive Behavioural Intervention and Specialist Nursery Provision for Children with Autism Spectrum Disorders. J. Child Psychol. Psychiatry 2007, 48, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Magiati, I.; Moss, J.; Charman, T.; Howlin, P. Patterns of Change in Children with Autism Spectrum Disorders Who Received Community Based Comprehensive Interventions in Their Pre-School Years: A Seven Year Follow-up Study. Res. Autism Spectr. Disord. 2011, 5, 1016–1027. [Google Scholar] [CrossRef]
- Flanagan, H.E.; Perry, A.; Freeman, N.L. Effectiveness of Large-Scale Community-Based Intensive Behavioral Intervention: A Waitlist Comparison Study Exploring Outcomes and Predictors. Res. Autism Spectr. Disord. 2012, 6, 673–682. [Google Scholar] [CrossRef]
- Lewon, A.B.; Ghezzi, P.M. An Evaluation of the Early Learning Measure as a Predictor of Outcomes in Early Intensive Behavioral Intervention. Behav. Interv. 2021, 36, 388–406. [Google Scholar] [CrossRef]
- Fulton, E.; Eapen, V.; Crnčec, R.; Walter, A.; Rogers, S. Reducing Maladaptive Behaviors in Preschool-Aged Children with Autism Spectrum Disorder Using the Early Start Denver Model. Front. Pediatr. 2014, 2, 40s. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, G.; Prior, M.; Williams, K.; Dissanayake, C. Predictors of Outcomes in Autism Early Intervention: Why Don’t We Know More? Front. Pediatr. 2014, 2, 58. [Google Scholar] [CrossRef]
- Sallows, G.O.; Graupner, T.D. Intensive Behavioral Treatment for Children With Autism: Four-Year Outcome and Predictors. Am. J. Ment. Retard. 2005, 110, 417–438. [Google Scholar] [CrossRef]
- Kasari, C.; Paparella, T.; Freeman, S.; Jahromi, L.B. Language Outcome in Autism: Randomized Comparison of Joint Attention and Play Interventions. J. Consult. Clin. Psychol. 2008, 76, 125–137. [Google Scholar] [CrossRef]
- Kasari, C.; Gulsrud, A.; Freeman, S.; Paparella, T.; Hellemann, G. Longitudinal Follow-Up of Children With Autism Receiving Targeted Interventions on Joint Attention and Play. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 487–495. [Google Scholar] [CrossRef]
- Contaldo, A.; Colombi, C.; Pierotti, C.; Masoni, P.; Muratori, F. Outcomes and Moderators of Early Start Denver Model Intervention in Young Children with Autism Spectrum Disorder Delivered in a Mixed Individual and Group Setting. Autism 2020, 24, 718–729. [Google Scholar] [CrossRef]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorder: DSM III, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- World Health Organization (WHO) (Ed.) The ICD-10 Classification of Mental and Behavioural Disorders; World Health Organization: Genève, Switzerland, 1993. [Google Scholar]
- Fisher, R.A. Statistical Methods for Research Workers, 4th ed.; Oliver and Boyd: London, UK, 1932. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Cinar, O.; Viechtbauer, W. The Poolr Package for Combining Independent and Dependent p Values. J. Stat. Softw. 2022, 101, 1–42. [Google Scholar] [CrossRef]
- Zachor, D.A.; Ben Itzchak, E. Treatment Approach, Autism Severity and Intervention Outcomes in Young Children. Res. Autism Spectr. Disord. 2010, 4, 425–432. [Google Scholar] [CrossRef]
- Ben-Itzchak, E.; Zachor, D.A. The Effects of Intellectual Functioning and Autism Severity on Outcome of Early Behavioral Intervention for Children with Autism. Res. Dev. Disabil. 2007, 28, 287–303. [Google Scholar] [CrossRef]
- Ben-Itzchak, E.; Watson, L.R.; Zachor, D.A. Cognitive Ability Is Associated with Different Outcome Trajectories in Autism Spectrum Disorders. J. Autism Dev. Disord. 2014, 44, 2221–2229. [Google Scholar] [CrossRef]
- MacDonald, R.; Parry-Cruwys, D.; Dupere, S.; Ahearn, W. Assessing Progress and Outcome of Early Intensive Behavioral Intervention for Toddlers with Autism. Res. Dev. Disabil. 2014, 35, 3632–3644. [Google Scholar] [CrossRef]
- Kovshoff, H.; Hastings, R.P.; Remington, B. Two-Year Outcomes for Children With Autism After the Cessation of Early Intensive Behavioral Intervention. Behav. Modif. 2011, 35, 427–450. [Google Scholar] [CrossRef]
- Ben Itzchak, E.; Zachor, D.A. Change in Autism Classification with Early Intervention: Predictors and Outcomes. Res. Autism Spectr. Disord. 2009, 3, 967–976. [Google Scholar] [CrossRef]
- Ben Itzchak, E.; Zachor, D.A. Who Benefits from Early Intervention in Autism Spectrum Disorders? Res. Autism Spectr. Disord. 2011, 5, 345–350. [Google Scholar] [CrossRef]
- Zachor, D.A.; Ben-Itzchak, E.; Rabinovich, A.-L.; Lahat, E. Change in Autism Core Symptoms with Intervention. Res. Autism Spectr. Disord. 2007, 1, 304–317. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DiLavore, P.C. Autism Diagnostic Observation Schedule-WPS (ADOS-WPS); Western Psychological Services: Los Angeles, CA, USA, 1999. [Google Scholar]
- Lord, C.; Luyster, R.J.; Gotham, K.; Guthrie, W. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part II): Toddler Module; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Leaf, R.; McEachin, J. A Work in Progress: Behavior Management Strategies and a Curriculum for Intensive Behavioral Treatment of Autism; DRL: New York, NY, USA, 1999. [Google Scholar]
- Maurice, C.; Green, G.; Luce, S.C. Behavioral Intervention for Young Children with Autism: A Manual for Parents and Professionals; Pro-Ed: Austin, TX, USA, 1996. [Google Scholar]
- Freeman, B.J.; Ritvo, E.R.; Needleman, R.; Yokota, A. The Stability of Cognitive and Linguistic Parameters in Autism: A Five-Year Prospective Study. J. Am. Acad. Child Psychiatry 1985, 24, 459–464. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant Development, 2nd ed.; Psychological Corp.: San Antonio, TX, USA, 1993. [Google Scholar]
- Thorndike, R.L. Manual for Stanford-Binet Intelligence Scale; Houghton Mifflin.: Boston, MA, USA, 1972. [Google Scholar]
- Thorndike, R.L. Stanford-Binet Intelligence Scale, 4th ed.; Riverside: Itasca, IL, USA, 1986. [Google Scholar]
- Wechsler, D. WPPSI-R: Wechsler Preschool and Primary Scale of Intelligence-Revised, Revised Edition; Psychological Corp.: San Antonio, TX, USA, 1989. [Google Scholar]
- Wechsler, D. Manual for the Wechsler Intelligence Scale for Children-Revised; Psychological Corporation: New York, NY, USA, 1974. [Google Scholar]
- Mullen, E. Mullen Scales of Early Learning: AGS Edition; American Guidance Service: Circle Pines, MN, USA, 1995. [Google Scholar]
- Cattel, P. The Measurement of Intelligence of Infants and Young Children; Psychological Corporation: New York, NY, USA, 1960. [Google Scholar]
- Gesell, A. Gesell Developmental Schedules; Psychological Corporation: New York, NY, USA, 1949. [Google Scholar]
- Doll, E.A. The Measurement of Social Competence: A Manual for the Vineland Social Maturity Scale; Educational Test Bureau, Educational Publishers: Minneapolis, MN, USA, 1953. [Google Scholar]
- Stutsman, R. Guide for Administering the Merrill-Palmer Scale of Mental Tests; Harcourt, Brace & World: New York, NY, USA, 1948. [Google Scholar]
- Berument, S.K.; Rutter, M.; Lord, C.; Pickles, A.; Bailey, A. Autism Screening Questionnaire: Diagnostic Validity. Br. J. Psychiatry 1999, 175, 444–451. [Google Scholar] [CrossRef]
- Edwards, S.; Fletcher, P.; Garman, M.; Hughes, A.; Letts, C.; Sinka, I. The Reynell Developmental Language Scales III: The University of Reading Edition, 3rd ed.; NFER Nelson: Windsor, UK, 1997. [Google Scholar]
- Fenson, L.; Marchman, V.; Thal, D.; Dale, P.; Reznick, S.; Bates, E. MacArthur Bates Communicative Development Inventories MB-CDI: The MacArthur-Bates Communicative Development Inventories, User’s Guide and Technical Manual, 2nd ed.; Brookes Publishing: Washington, DC, USA, 2006. [Google Scholar]
- Sparrow, S.S.; Bella, D.A.; Cicchetti, D.V. The Vineland Adaptive Behavior Scale; American Guidance Service: Circle River, MN, USA, 1984. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Bella, D.A. Vineland-II Adaptive Behavior Scales, 2nd ed.; NCS Pearson: Eagan, MN, USA, 2005. [Google Scholar]
- Smith, T.; Eikeseth, S.; Buch, G.; Lovaas, I. Early Learning Measure. Unpublished test. 1995. [Google Scholar]
- Mundy, P.; Hogan, A.; Doehring, P. A Preliminary Manual for the Abridged Early Social Communication Scales (ESCS); University of Miami: Coral Gables, FL, USA, 1996. [Google Scholar]
- MacDonald, R.; Anderson, J.; Dube, W.V.; Geckeler, A.; Green, G.; Holcomb, W.; Mansfield, R.; Sanchez, J. Behavioral Assessment of Joint Attention: A Methodological Report. Res. Dev. Disabil. 2006, 27, 138–150. [Google Scholar] [CrossRef]
- Tasse, M. The Nisonger Child Behavior Rating Form: Age and Gender Effects and Norms*1. Res. Dev. Disabil. 1996, 17, 59–75. [Google Scholar] [CrossRef]
- Einfeld, S.L.; Tonge, B.J. The Developmental Behavior Checklist: The Development and Validation of an Instrument to Assess Behavioral and Emotional Disturbance in Children and Adolescents with Mental Retardation. J. Autism Dev. Disord. 1995, 25, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.N. Child Behavior Checklist; University of Vermont Department of Psychiatry: Burlington, VT, USA, 1991. [Google Scholar]
- Sulek, R.; Smith, J.; Bent, C.A.; Hudry, K.; Trembath, D.; Vivanti, G.; Dissanayake, C. The Utility of LENA as an Indicator of Developmental Outcomes for Young Children with Autism. Int. J. Lang. Commun. Disord. 2022, 57, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zitter, A.; Rinn, H.; Szapuova, Z.; Avila-Pons, V.M.; Coulter, K.L.; Stahmer, A.C.; Robins, D.L.; Vivanti, G. Does Treatment Fidelity of the Early Start Denver Model Impact Skill Acquisition in Young Children with Autism? J. Autism Dev. Disord. 2021; Advance online publication. [Google Scholar] [CrossRef]
- Godel, M.; Robain, F.; Kojovic, N.; Franchini, M.; Wood de Wilde, H.; Schaer, M. Distinct Patterns of Cognitive Outcome in Young Children With Autism Spectrum Disorder Receiving the Early Start Denver Model. Front. Psychiatry 2022, 13, 835580. [Google Scholar] [CrossRef] [PubMed]
- Devescovi, R.; Monasta, L.; Mancini, A.; Bin, M.; Vellante, V.; Carrozzi, M.; Colombi, C. Early Diagnosis and Early Start Denver Model Intervention in Autism Spectrum Disorders Delivered in an Italian Public Health System Service. Neuropsychiatr. Dis. Treat. 2016, 12, 1379–1384. [Google Scholar] [CrossRef]
- Vivanti, G.; Dissanayake, C.; Duncan, E.; Feary, J.; Capes, K.; Upson, S.; Bent, C.A.; Rogers, S.J.; Hudry, K.; Jones, C.; et al. Outcomes of Children Receiving Group-Early Start Denver Model in an Inclusive versus Autism-Specific Setting: A Pilot Randomized Controlled Trial. Autism 2019, 23, 1165–1175. [Google Scholar] [CrossRef]
- Vivanti, G.; Dissanayake, C.; The Victorian ASELCC Team. Outcome for Children Receiving the Early Start Denver Model Before and After 48 Months. J. Autism Dev. Disord. 2016, 46, 2441–2449. [Google Scholar] [CrossRef]
- Latrèche, K.; Kojovic, N.; Franchini, M.; Schaer, M. Attention to Face as a Predictor of Developmental Change and Treatment Outcome in Young Children with Autism Spectrum Disorder. Biomedicines 2021, 9, 942. [Google Scholar] [CrossRef]
- Wang, S.-H.; Zhang, H.-T.; Zou, Y.-Y.; Cheng, S.-M.; Zou, X.-B.; Chen, K.-Y. Efficacy and Moderating Factors of the Early Start Denver Model in Chinese Toddlers with Autism Spectrum Disorder: A Longitudinal Study. World J. Pediatr. 2022; Onlined ahead of print. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development: Bayley-III, 3rd ed.; Harcourt Assessment: San Antonio, TX, USA, 2006. [Google Scholar]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence Third Edition WPPSI III, 3rd ed.; Giunti O.S.: Florence, Italy, 2008. [Google Scholar]
- Gesell Institute of Child Development. Gesell Developmental Observation–Revised and Gesell Early Screener. Technical Report: Ages 3–6. 2012. Available online: https://nanopdf.com/download/gdo-r-gesell-institute_pdf (accessed on 26 October 2022).
- Griffiths, R. The Abilities of Young Children: A Comprehensive System of Mental Measurement for the First Eight Years of Life, Revised Edition; A.R.C.I.D. Test Agency Limited: Bukcs, UK, 1984. [Google Scholar]
- Schopler, E.; Lansing, M.D.; Reichler, R.J.; Marcus, L.M. Psychoeducational Profile: TEACCH Individualized Psychoeducational Assessment for Children with Autism Spectrum Disorders (PEP-3) 3; Pro-Ed: Austin, TX, USA, 2005. [Google Scholar]
- Schopler, E.; Reichler, R.J.; Renner, B.R. The Childhood Autism Rating Scale (CARS); Western Psychological Services: Los Angeles, CA, USA, 2002. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Saulnier, C.A. Vineland Adaptive Behavior Scales, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 2016. [Google Scholar]
- Caselli, M.C.; Pasqualetti, P.; Stefanini, S. Parole e Frasi Nel «Primo Vocabolario Del Bambino». Nuovi Dati Normativi Fra i 18 e 36 Mesi e Forma Breve Del Questionario [Words and Sentences in the First Vocabulary of the Child: New Normative Data from 18 to 36 Months and Short Form of the Questionnaire]; Franco Angeli: Milan, Italy, 2007. [Google Scholar]
- Lena Research Foundation. User Guide LENA Pro, 2015. Available online: https://docplayer.net/44645642-User-guide-lena-pro-lena-research-foundation.html (accessed on 8 June 2022).
- Lamb, Y.N.; McKay, N.S.; Thompson, C.S.; Hamm, J.P.; Waldie, K.E.; Kirk, I.J. Brain-Derived Neurotrophic Factor Val66Met Polymorphism, Human Memory, and Synaptic Neuroplasticity: BDNF, Human Memory, and Synaptic Plasticity. Wiley Interdiscip. Rev. Cogn. Sci. 2015, 6, 97–108. [Google Scholar] [CrossRef]
- Law, M.; Stewart, D.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Critical Review Form–Quantitative Studies; McMaster University: Hamilton, ON, Canada, 1998. [Google Scholar]
- Laister, D.; Stammler, M.; Vivanti, G.; Holzinger, D. Social-Communicative Gestures at Baseline Predict Verbal and Nonverbal Gains for Children with Autism Receiving the Early Start Denver Model. Autism 2021, 25, 1640–1652. [Google Scholar] [CrossRef]
- Smith, T.; Klorman, R.; Mruzek, D.W. Predicting Outcome of Community-Based Early Intensive Behavioral Intervention for Children with Autism. J. Abnorm. Child Psychol. 2015, 43, 1271–1282. [Google Scholar] [CrossRef]
- Goin-Kochel, R.P.; Myers, B.J.; Hendricks, D.R.; Carr, S.E.; Wiley, S.B. Early Responsiveness to Intensive Behavioural Intervention Predicts Outcomes Among Preschool Children with Autism. Int. J. Disabil. Dev. Educ. 2007, 54, 151–175. [Google Scholar] [CrossRef]
- Virues-Ortega, J.; Rodríguez, V.; Yu, C.T. Prediction of Treatment Outcomes and Longitudinal Analysis in Children with Autism Undergoing Intensive Behavioral Intervention. Int. J. Clin. Health Psychol. 2013, 13, 91–100. [Google Scholar] [CrossRef]
- Weiss, M.J. Differential Rates of Skill Acquisition and Outcomes of Early Intensive Behavioral Intervention for Autism. Behav. Interv. 1999, 14, 3–22. [Google Scholar] [CrossRef]
- Préfontaine, I.; Morizot, J.; Lanovaz, M.J.; Rivard, M. A Person-Centered Perspective on Differential Efficacy of Early Behavioral Intervention in Children with Autism: A Latent Profile Analysis. Res. Autism Spectr. Disord. 2022, 97, 102017. [Google Scholar] [CrossRef]
- Howlin, P.; Magiati, I.; Charman, T. Systematic Review of Early Intensive Behavioral Interventions for Children With Autism. Am. J. Intellect. Dev. Disabil. 2009, 114, 19. [Google Scholar] [CrossRef]
- Strauss, K.; Vicari, S.; Valeri, G.; D’Elia, L.; Arima, S.; Fava, L. Parent Inclusion in Early Intensive Behavioral Intervention: The Influence of Parental Stress, Parent Treatment Fidelity and Parent-Mediated Generalization of Behavior Targets on Child Outcomes. Res. Dev. Disabil. 2012, 33, 688–703. [Google Scholar] [CrossRef]
| Study | Cases | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| N (M:F) | Age at Intake in Months (Mean) | Diagnosis | Exclusion Criteria | Control Intervention | N (M:F) | Age at Intake in Months (Mean) | Diagnosis | |
| [59] Ben-Itzchak and Zachor, 2007 | 29 (25:4) | 20–32 (27) | DSM-IV; ADOS; ADI-R |
| --- | --- | --- | --- |
| [60] Ben-Itzchak et al., 2014 | 46 (39:7) | 17–33 (25.5) | DSM-IV; ADOS |
| --- | --- | --- | --- |
| [13] Cohen et al., 2006 | 21 (18:3) | 20–41 (30) | ADI-R |
| TAU | 21 (17:4) | 20–41 (33) | ASD |
| [15] Hayward et al., 2009 | 23 (19:4) | 24–42 (36) | ICD-10; ADI-R |
| Parent-commissioned EIBI | 21 (15:6) | 24–42 (34) | ASD |
| [11] Lovaas, 1987 | 19 (16:3) | <46 (35) | DSM-III |
| C1: low intensity EIBI; C2: none | C1: 19 (11:8); C2: 21 (n.r.) | C1: <42 (41); C2: <42 (n.r.) | C1: ASD; C2:ASD |
| [61] MacDonald et al., 2014 | 83 (n.r.) | 17–48 (n.r.) | DSM-IV | n.r. | None | 58 (n.r.) | 18–59 | TD |
| [26] Remington et al., 2007; [62] Kovshoff et al., 2011 | 23 (n.r.) | 30–42 (36) | DSM-IV; ADI-R |
| TAU | 21 (n.r.) | 30–42 (38) | ASD |
| [23] Rogers et al., 2021 | 45 (34:11) | 12–30 (23) | DSM-5; ADOS-2 |
| ESDM | 42 (32:10) | 12–30 (24) | ASD |
| [48] Sallows and Graupner, 2005 | 13 (11:2) | 24–42 (35) | DSM-IV; ADI-R |
| P-EIBI | 10 (8:2) | 24–42 (37) | ASD |
| [16] Smith et al., 2000 | 15 (12:3) | 18–42 (36) | n.r. |
| P-EIBI | 13 (11:2) | 18–42 (36) | ASD |
| [58] Zachor and Ben-Itzchak, 2010 | 45 (n.r.) | 17–35 (25) | DSM-IV; ADOS; ADI-R |
| Eclectic | 33 (n.r.) | 15–33 (26) | ASD |
| [65] Zachor et al., 2007 | 20 (19:1) | 22–34 (28) | DSM-IV; ADOS; ADI-R |
| Eclectic | 19 (18:1) | 23–33 (29) | ASD |
| Study | Country | Study Design | Intervention Type | Setting | Intensity | Duration |
|---|---|---|---|---|---|---|
| [59] Ben-Itzchak and Zachor, 2007 | Israel | One group pre-test–post-test | ABA | Autism-specific preschool programs | 35 h/week | 12 mo |
| [60] Ben-Itzchak et al., 2014 | Israel | One group pre-test–post-test | ABA | Centre-based | 20 h/week | 24 mo |
| [13] Cohen et al., 2006 | USA | Case–control trial | UCLA EIBI | Home-based | 35–40 h/week | 36 mo |
| [15] Hayward et al., 2009 | UK | Non-concurrent multiple baseline design | UCLA EIBI | Home-based | 37 h/week | 12 mo |
| [11] Lovaas, 1987 | USA | Case–control trial | UCLA EIBI | Home/School | ≥40 h/week | ≥24 mo |
| [61] MacDonald et al., 2014 | USA | Case–control trial | ABA | Home/School | 20–30 h/week | 12 mo |
| [26] Remington et al., 2007; [62] Kovshoff et al., 2011 | UK | Case–control trial; 2-year follow-up | ABA | Home-based | 18–34 h/week (mean = 26) | 12 mo |
| [23] Rogers et al., 2021 | USA | RCT | UCLA EIBI | Home/Childcare setting | 12 vs. 20 h/week | 12 mo |
| [48] Sallows and Graupner, 2005 | USA | Case–control trial | UCLA EIBI | Not reported | 38 h/week (gradually decreasing when children entered school) | 48 mo |
| [16] Smith et al., 2000 | USA | RCT | UCLA EIBI | Home/Preschool | 24 h/week (gradually decreasing after the first year) | 24–36 mo (mean = 33) |
| [58] Zachor and Ben-Itzchak, 2010 | Israel | Case–control trial | ABA | Autism-specific preschool programs | 20 h/week | 12 mo |
| [65] Zachor et al., 2007 | Israel | Case–control trial | ABA | Autism-specific preschool programs | 35 h/week | 12 mo |
| Study | Predictors of Better Outcome | Improved Functions Correlated with Predictors | Non-Predictors |
|---|---|---|---|
| [65] Ben-Itzchak and Zachor, 2007 |
|
|
|
| [60] Ben-Itzchak et al., 2014 |
|
|
|
| [13] Cohen et al., 2006 |
|
| |
| [15] Hayward et al., 2009 |
|
|
|
| [11] Lovaas, 1987 |
|
|
|
| [61] MacDonald et al., 2014 |
|
|
|
| [26] Remington et al., 2007 [62] Kovshoff et al., 2011 |
|
|
|
| [23] Rogers et al., 2021 |
|
|
|
| [48] Sallows and Graupner, 2005 |
|
|
|
| [16] Smith et al., 2000 |
|
|
|
| [58] Zachor and Ben-Itzchak, 2010 |
|
|
|
| [65] Zachor et al., 2007 |
|
|
|
| Study | Cases | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| N (M:F) | Age at Intake in Months (Mean) | Diagnosis | Exclusion Criteria | Control Intervention | N (M:F) | Age at Intake in Months (Mean) | Diagnosis | |
| [51] Contaldo et al., 2019 | 32 (26:6) | 18–39 (29) | ADOS-2 |
| --- | --- | --- | --- |
| [96] Devescovi et al., 2016 | 21 (18:3) | 20–36 (27) | DSM-5; ADOS-2 |
| --- | --- | --- | --- |
| [95] Godel et al., 2022 | 55 (48:7) | 15–42 (28.7) | DSM-5 ADOS-2 |
| --- | --- | --- | --- |
| [99] Latrèche et al., 2021 | 51 (45:6) | 17–48 (34) | ADOS-2 | n.r. | C1 = CT; C2 = None | C1: 30 (25:5) C2: 16 (12:4) | C1: 17–48 (34); C2: 17–48 (30) | C1: ASD C2: None |
| [22] Rogers et al., 2019 | 55 (41:14) | 14–29 (21) | DSM-IV; ADOS-2 |
| CT | 63 (51:12) | 14–29 (21) | ASD |
| [23] Rogers et al., 2021 | 42 (32:10) | 12–30 (24) | DSM-5; ADOS-2 |
| EIBI | 45 (34:11) | 12–30 (23) | ASD |
| [93] Sulek et al., 2022 | 99 (70:29) | 14–47 (32) | ADOS-2 | n.r. | --- | --- | --- | --- |
| [98] Vivanti et al., 2016 | 32 (26:6) | 18–48 (33) | DSM-5; ADOS |
| ESDM | 28 (25:3) | 48–62 (49.5) | ASD |
| [97] Vivanti et al., 2019 | 44 (27:17) | 15–32 (26) | DSM-5; ADOS-2 | No exclusion criteria based on child behavior or cognition | --- | --- | --- | --- |
| [100] Wang et al., 2022 | 21 (17:4) | 18–36 (21) | DSM-5 ADOS |
| None (Waitlist for ESDM) | 24 (18:6) | 18–36 (22) | ASD |
| [94] Zitter et al., 2021 | 16 (11:5) | 20–39 (29) | ADOS-2 | n.r. | --- | --- | --- | --- |
| Study | Country | Study Design | Setting | Intensity | Duration |
|---|---|---|---|---|---|
| [51] Contaldo et al., 2019 | Italy | One group pretest-posttest | Community-based (GS) | 4 h/week (2 h GS and 2 h 1:1) | 8–16 mo (mean 12 mo) |
| [96] Devescovi et al., 2016 | Italy | Retrospective study | Community-based | 3 h/week | 11–19 mo (mean 15 mo) |
| [95] Godel et al., 2022 | Switzerland | One group pretest-posttest | Center-based | 20 h/week | 24 mo |
| [99] Latrèche et al., 2021 | Switzerland | Case–control trial | n.r. | 20 h/week | 24 mo |
| [22] Rogers et al., 2019 | USA | RCT | Home/Preschool/Daycare | 20 h/week | 24 mo |
| [23] Rogers et al., 2021 | USA | RCT | Home/Daycare | 12 vs. 20 h/week | 12 mo |
| [93] Sulek et al., 2022 | Australia | One group pretest-posttest | Childcare setting (GS) | ~15 h/week | 12 mo |
| [98] Vivanti et al., 2016 | Australia | Case–control trial | University-based (GS) | 15–25 h/week | 12 mo |
| [97] Vivanti et al., 2019 | Australia | RCT | School-based (GS) | 15 h/week | 10 mo |
| [100] Wang et al., 2022 | China | Case–control trial | Hospital-based | 1 h/week | 6 mo |
| [94] Zitter et al., 2021 | USA | One group pretest-posttest | Clinic-based | 4 h/week | 12 mo |
| Study | Predictors of Better Outcome | Improved Functions Correlated with Predictors | Non-Predictors |
|---|---|---|---|
| [51] Contaldo et al., 2019 |
|
|
|
| [96] Devescovi et al., 2016 |
|
|
|
| [95] Godel et al., 2022 |
|
|
|
| [99] Latrèche et al., 2021 |
|
|
|
| [22] Rogers et al., 2019 |
|
|
|
| [23] Rogers et al., 2021 |
|
| |
| [93] Sulek et al., 2022 |
|
|
|
| [98] Vivanti et al., 2016 |
|
|
|
| [97] Vivanti et al., 2019 |
|
| |
| [100] Wang et al., 2022 |
|
|
|
| [94] Zitter et al., 2021 |
|
|
|
| EIBI | ESDM | |||||
|---|---|---|---|---|---|---|
| Variable | N. (%) of Positive Studies | Fisher’s Statistics * | Variable | N. (%) of Positive Studies | Fisher’s Statistics * | |
| First-line predictors | Higher IQ/DQ at intake | 7/11 (63.6%) | χ2 = 83.968 (df = 20); p = 8.24 × 10−10 | Verbal and non-verbal intention to communicate, attention to faces | 5/6 (83.3%) | χ2 = 77.733 (df = 12); p = 1.12 × 10−11 |
| Second-line predictors | Better receptive language abilities | 2/4 (50%) | χ2 = 38.399 (df = 8); p = 6.35 × 10−10 | Higher IQ or DQ at intake, action with objects | 5/7 (71.4%) | χ2 = 61.444 (df = 14); p = 6.54 × 10−8 |
| Greater social skills | 3/3 (100%) | χ2 = 23.799 (df = 6); p = 5.69 × 10−4 | Younger age at intake | 3/5 (60%) | χ2 = 25.633 (df = 8); p = 0.0012 | |
| Communication skills | 2/3 (66.6%) | χ2 = 17.710 (df = 6); p = 0.007 | Less stereotyped and repetitive behaviors | 2/3 (66.7%) | χ2 = 14.854 (df = 6); p = 0.021 | |
| Weak or non-predictors | Adaptive behaviors | 2/4 (50%) | χ2 = 18.757 (df = 6); p = 0.0046 | Milder severity of autistic symptoms | 1/6 (16%) | χ2 = 22.565 (df = 12); p = 0.032 |
| Younger age at intake | 1/4 (25%) | χ2 = 24.048 (df = 4); p = 7.81 × 10−5 | ||||
| Milder severity of autistic symptoms | 1/3 (33.3%) | χ2 = 20.802 (df = 6); p = 0.002 | ||||
| Insufficient evidence | Imitation | 1/1 | --- | Adaptive behaviors | 1/2 | --- |
| Joint Attention | 0/1 | --- | Imitation | 0/1 | --- | |
| Joint attention | 0/1 | --- | ||||
| Play skills | 0/1 | --- | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asta, L.; Persico, A.M. Differential Predictors of Response to Early Start Denver Model vs. Early Intensive Behavioral Intervention in Young Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 1499. https://doi.org/10.3390/brainsci12111499
Asta L, Persico AM. Differential Predictors of Response to Early Start Denver Model vs. Early Intensive Behavioral Intervention in Young Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Brain Sciences. 2022; 12(11):1499. https://doi.org/10.3390/brainsci12111499
Chicago/Turabian StyleAsta, Lisa, and Antonio M. Persico. 2022. "Differential Predictors of Response to Early Start Denver Model vs. Early Intensive Behavioral Intervention in Young Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis" Brain Sciences 12, no. 11: 1499. https://doi.org/10.3390/brainsci12111499
APA StyleAsta, L., & Persico, A. M. (2022). Differential Predictors of Response to Early Start Denver Model vs. Early Intensive Behavioral Intervention in Young Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Brain Sciences, 12(11), 1499. https://doi.org/10.3390/brainsci12111499







