Interpretation for Individual Brain Age Prediction Based on Gray Matter Volume
Abstract
:1. Introduction
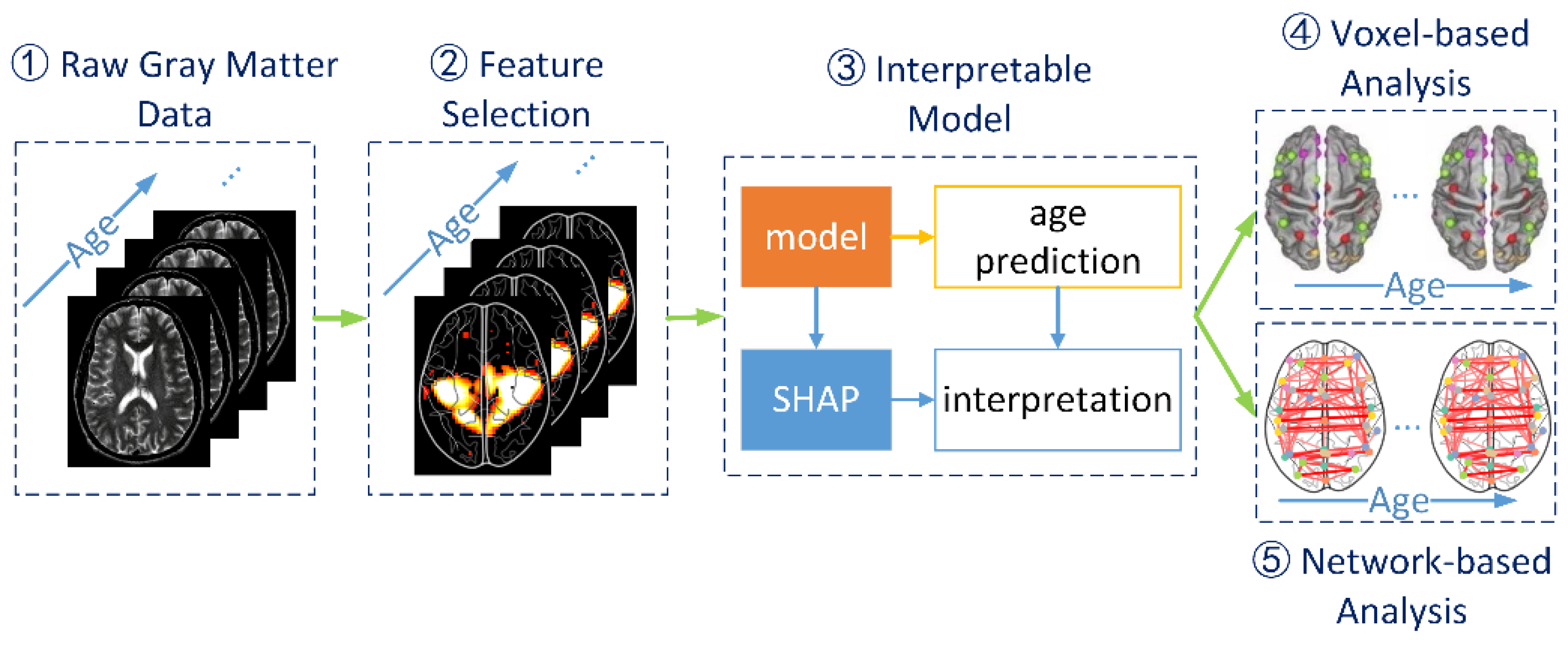
2. Materials and Methods
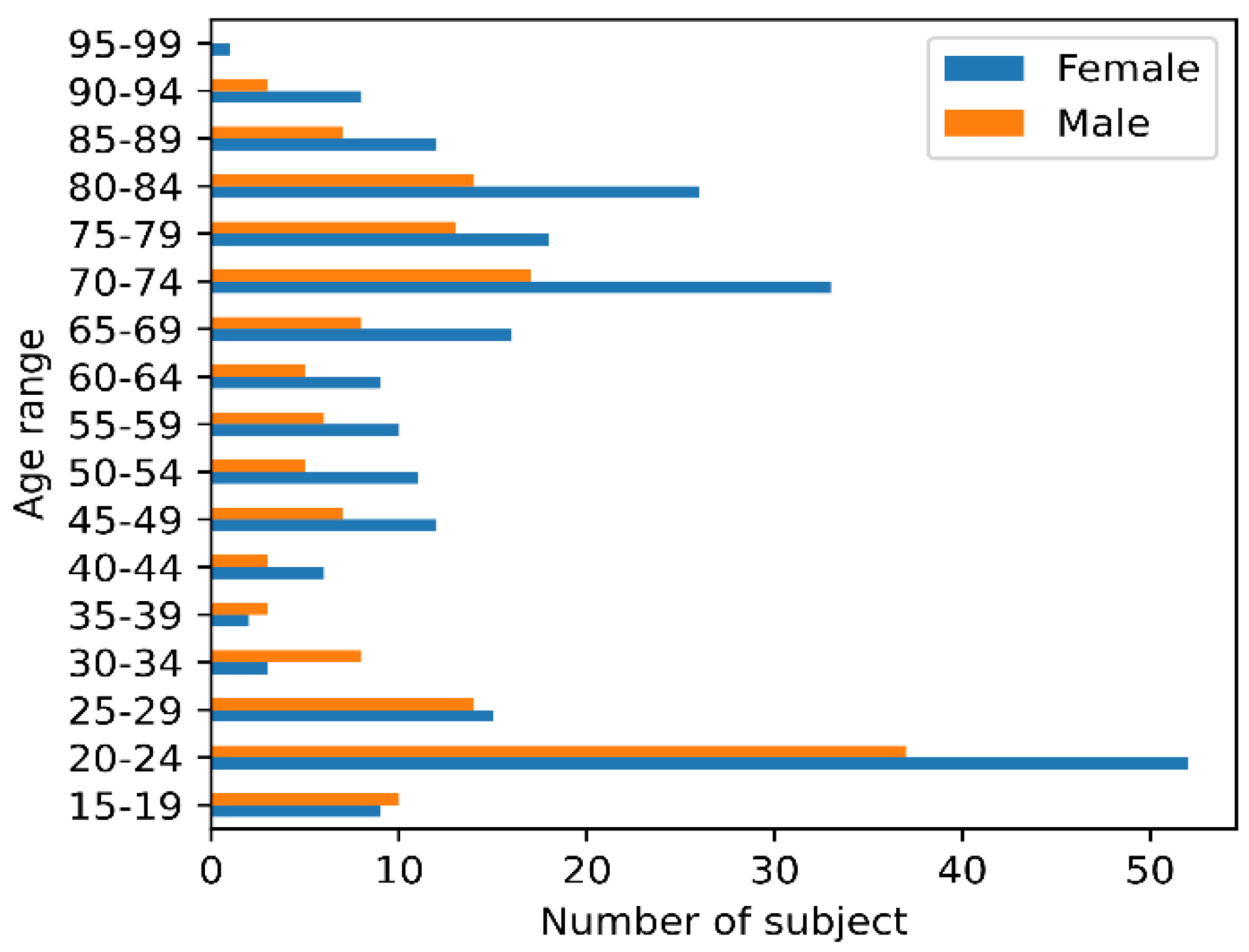
2.1. Data Sets
2.2. Data Preparation and Feature Selection
2.3. Interpretable Machine Learning Model
2.3.1. Formulation of Age Prediction
2.3.2. SHAP Model for Interpretation
2.4. Voxel-Based and Network-Based Analyses
2.4.1. Voxel-Based Analyses
2.4.2. Network-Based Analyses
3. Experiments and Results
3.1. Age Prediction
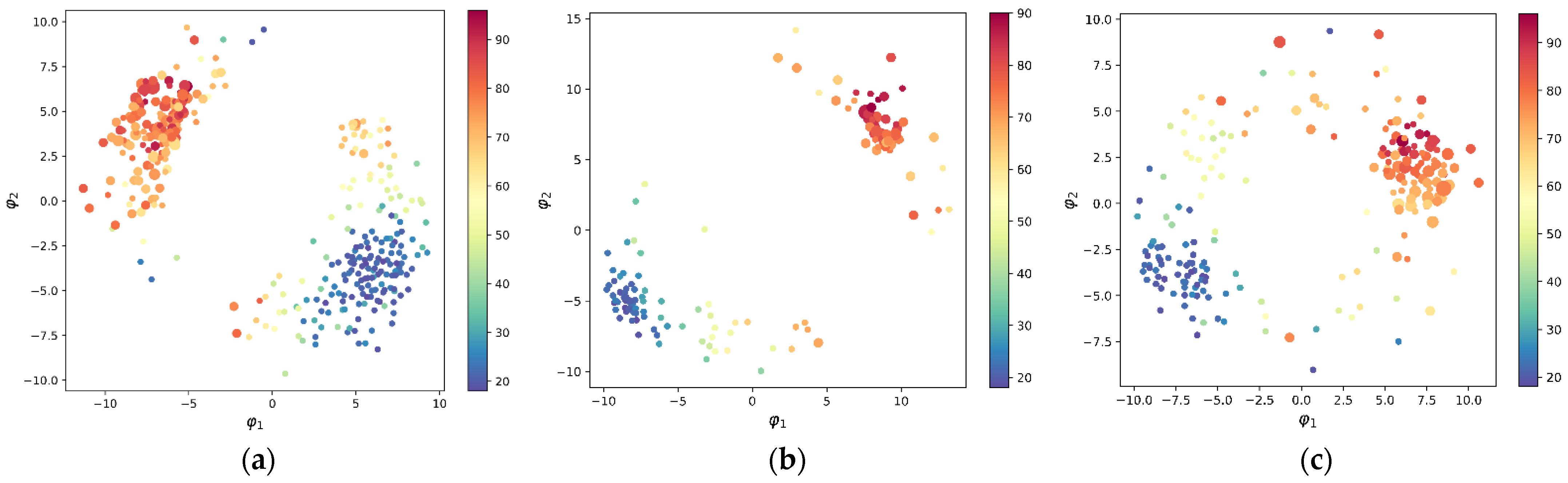
3.1.1. Relationship between Aging, Sex, and GMV
3.1.2. Age Prediction Using XGBoost Model
3.2. Voxel-Based Results
3.2.1. Individual Voxel-Based Analyses
3.2.2. Dynamic Analysis and Visualization of SHAP Values
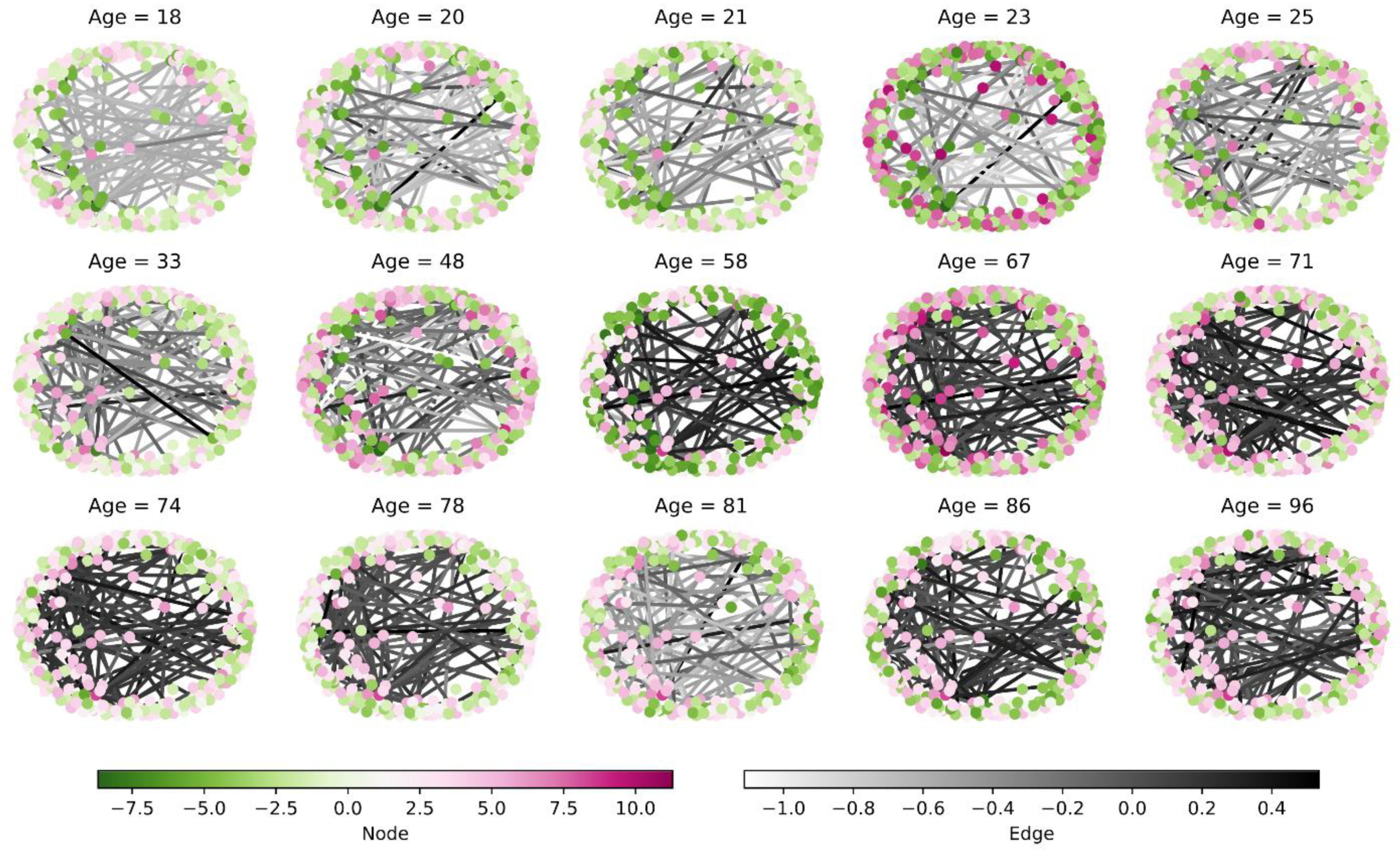
3.3. Network-Based Results
3.3.1. Individual Network-Based Analysis
3.3.2. Dynamic Network Analysis with Age
3.3.3. Network of SHAP Interaction Networks
4. Discussion
- For individuals, we give quantitative interpretations of the relationship between aging and GMV. For example, it can find a positive contribution of GMV of some specific locations to age.
- In addition to the GMVs themselves, we consider the interactions between GMVs that construct a complex network for each individual. The effects of these networks on age-related changes are then investigated.
- Based on dimensional reduction and network similarity, we investigate the dynamic properties of GMV as well as brain network changes with age.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennatas, E.D.; Avants, B.B.; Wolf, D.H.; Satterthwaite, T.D.; Ruparel, K.; Ciric, R.; Hakonarson, H.; Gur, R.E.; Gur, R.C. Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adulthood. J. Neurosci. 2017, 37, 5065–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, K.; Ziegler, G.; Klöppel, S.; Gaser, C. Estimating the Age of Healthy Subjects from T1-Weighted MRI Scans Using Kernel Methods: Exploring the Influence of Various Parameters. Neuroimage 2010, 50, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Dosenbach, N.U.F.; Nardos, B.; Cohen, A.L.; Fair, D.A.; Power, J.D.; Church, J.A.; Nelson, S.M.; Wig, G.S.; Vogel, A.C.; Lessov-Schlaggar, C.N.; et al. Prediction of Individual Brain Maturity Using FMRI. Science 2010, 329, 1358–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.H.; Franke, K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 2017, 40, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Orta, C.; Mendiola-Santibañez, J.D.; Ibrahimi, D.; Rodríguez-Reséndiz, J.; Díaz-Florez, G.; Olvera-Olvera, C.A. Hyperconnected Openings Codified in a Max Tree Structure: An Application for Skull-Stripping in Brain MRI T1. Sensors 2022, 22, 1378. [Google Scholar] [CrossRef] [PubMed]
- Schnack, H.G.; van Haren, N.E.M.; Nieuwenhuis, M.; Hulshoff Pol, H.E.; Cahn, W.; Kahn, R.S. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am. J. Psychiatry 2016, 173, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Jin, C.; Fu, Z.; Zhang, B.; Bin, G.; Wu, S. Predicting Healthy Older Adult’s Brain Age Based on Structural Connectivity Networks Using Artificial Neural Networks. Comput. Methods Programs Biomed. 2016, 125, 8–17. [Google Scholar] [CrossRef]
- Liem, F.; Varoquaux, G.; Kynast, J.; Beyer, F.; Kharabian Masouleh, S.; Huntenburg, J.M.; Lampe, L.; Rahim, M.; Abraham, A.; Craddock, R.C.; et al. Predicting Brain-Age from Multimodal Imaging Data Captures Cognitive Impairment. Neuroimage 2017, 148, 179–188. [Google Scholar] [CrossRef]
- Sokolova, K.; Barker, G.; Montana, G. Convolutional Neural Network-Based Ordinal Regression for Brain Age Prediction from MRI Scans. In Proceedings of the Medical Imaging 2020: Image Processing, Houston, TX, USA, 15–20 February 2020; Landman, B.A., Išgum, I., Eds.; SPIE: Houston, TX, USA, 2020; p. 82. [Google Scholar]
- Jonsson, B.A.; Bjornsdottir, G.; Thorgeirsson, T.E.; Ellingsen, L.M.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Stefansson, K.; Ulfarsson, M.O. Brain Age Prediction Using Deep Learning Uncovers Associated Sequence Variants. Nat. Commun. 2019, 10, 5409. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, N.; Chen, K.; Yao, L.; Li, K.; Zhang, J.; Guo, X. Predicting Brain Age of Healthy Adults Based on Structural MRI Parcellation Using Convolutional Neural Networks. Front. Neurol. 2020, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, D.P.; Genon, S.; Sotiras, A.; Schwender, H.; Hoffstaedter, F.; Patil, K.R.; Jockwitz, C.; Caspers, S.; Moebus, S.; Amunts, K.; et al. Evaluation of Non-Negative Matrix Factorization of Grey Matter in Age Prediction. Neuroimage 2018, 173, 394–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.H.; Poudel, R.P.; Tsagkrasoulis, D.; Caan, M.W.; Steves, C.; Spector, T.D.; Montana, G. Predicting Brain Age with Deep Learning from Raw Imaging Data Results in a Reliable and Heritable Biomarker. Neuroimage 2017, 163, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, K.; Gaser, C.; Manor, B.; Novak, V. Advanced BrainAGE in Older Adults with Type 2 Diabetes Mellitus. Front. Aging Neurosci. 2013, 5, 90. [Google Scholar] [CrossRef]
- Wang, J.; Knol, M.J.; Tiulpin, A.; Dubost, F.; de Bruijne, M.; Vernooij, M.W.; Adams, H.H.H.; Ikram, M.A.; Niessen, W.J.; Roshchupkin, G.V. Gray Matter Age Prediction as a Biomarker for Risk of Dementia. Proc. Natl. Acad. Sci. USA 2019, 116, 21213–21218. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, T.; van der Meer, D.; Doan, N.T.; Schwarz, E.; Lund, M.J.; Agartz, I.; Alnæs, D.; Barch, D.M.; Baur-Streubel, R.; Bertolino, A.; et al. Common Brain Disorders Are Associated with Heritable Patterns of Apparent Aging of the Brain. Nat. Neurosci. 2019, 22, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Mohajer, B.; Abbasi, N.; Mohammadi, E.; Khazaie, H.; Osorio, R.S.; Rosenzweig, I.; Eickhoff, C.R.; Zarei, M.; Tahmasian, M.; Eickhoff, S.B. Gray Matter Volume and Estimated Brain Age Gapare Not Linked with Sleep-Disordered Breathing. Hum. Brain Mapp. 2020, 41, 3034–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrup, K. Reimagining Alzheimer’s Disease—An Age-Based Hypothesis. J. Neurosci. 2010, 30, 16755–16762. [Google Scholar] [CrossRef] [Green Version]
- Lou, W.; Wang, D.; Wong, A.; Chu, W.C.W.; Mok, V.C.T.; Shi, L. Frequency-specific Age-related Decreased Brain Network Diversity in Cognitively Healthy Elderly: A Whole-brain Data-driven Analysis. Hum. Brain Mapp. 2019, 40, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Löwe, L.C.; Gaser, C.; Franke, K. The Effect of the APOE Genotype on Individual BrainAGE in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. PLoS ONE 2016, 11, e0157514. [Google Scholar] [CrossRef]
- Jo, T.; Nho, K.; Saykin, A.J. Deep Learning in Alzheimer’s Disease: Diagnostic Classification and Prognostic Prediction Using Neuroimaging Data. Front. Aging Neurosci. 2019, 11, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, S.; Joshi, P.S.; Miller, M.I.; Xue, C.; Zhou, X.; Karjadi, C.; Chang, G.H.; Joshi, A.S.; Dwyer, B.; Zhu, S.; et al. Development and Validation of an Interpretable Deep Learning Framework for Alzheimer’s Disease Classification. Brain 2020, 143, 1920–1933. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, Y.; Wu, Z.; Niu, H.; Chen, S. Task-Oriented Snapshot Network Construction of Stock Market. In Proceedings of the International Conference on Intelligent Computing, ICIC 2021, Shenzhen, China, 12–15 August 2021; Springer: Shenzhen, China, 2021; pp. 3–11. [Google Scholar]
- Marcus, D.S.; Wang, T.H.; Parker, J.; Csernansky, J.G.; Morris, J.C.; Buckner, R.L. Open Access Series of Imaging Studies (OASIS): Cross-Sectional MRI Data in Young, Middle Aged, Nondemented, and Demented Older Adults. J. Cogn. Neurosci. 2007, 19, 1498–1507. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine Learning for Neuroimaging with Scikit-Learn. Front. Neuroinform. 2014, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4766–4775. [Google Scholar]
- Ingwer BorgPatrick, J.F. Groenen Modern Multidimensional Scaling: Theory and Applications, 2nd ed.; Springer: New York, NY, USA, 1997; ISBN 978-0-387-25150-9. [Google Scholar]
- Higham, N.J. Computing a Nearest Symmetric Positive Semidefinite Matrix. Linear Algebra Appl. 1988, 103, 103–118. [Google Scholar] [CrossRef]
- Pennec, X.; Fillard, P.; Ayache, N. A Riemannian Framework for Tensor Computing. Int. J. Comput. Vis. 2006, 66, 41–66. [Google Scholar] [CrossRef] [Green Version]
- Sanchis-Segura, C.; Ibañez-Gual, M.V.; Adrián-Ventura, J.; Aguirre, N.; Gómez-Cruz, Á.J.; Avila, C.; Forn, C. Sex Differences in Gray Matter Volume: How Many and How Large Are They Really? Biol. Sex Differ. 2019, 10, 32. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Nair, B.; Vavilala, M.S.; Horibe, M.; Eisses, M.J.; Adams, T.; Liston, D.E.; Low, D.K.-W.; Newman, S.-F.; Kim, J.; et al. Explainable Machine-Learning Predictions for the Prevention of Hypoxaemia during Surgery. Nat. Biomed. Eng. 2018, 2, 749–760. [Google Scholar] [CrossRef]
- Taki, Y.; Goto, R.; Evans, A.; Zijdenbos, A.; Neelin, P.; Lerch, J.; Sato, K.; Ono, S.; Kinomura, S.; Nakagawa, M.; et al. Voxel-Based Morphometry of Human Brain with Age and Cerebrovascular Risk Factors. Neurobiol. Aging 2004, 25, 455–463. [Google Scholar] [CrossRef]
- Grieve, S.M.; Clark, C.R.; Williams, L.M.; Peduto, A.J.; Gordon, E. Preservation of Limbic and Paralimbic Structures in Aging. Hum. Brain Mapp. 2005, 25, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Erickson, K.I.; Rodrigue, K.M.; Voss, M.W.; Colcombe, S.J.; Kramer, A.F.; Acker, J.D.; Raz, N. Age-Related Differences in Regional Brain Volumes: A Comparison of Optimized Voxel-Based Morphometry to Manual Volumetry. Neurobiol. Aging 2009, 30, 1657–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terribilli, D.; Schaufelberger, M.S.; Duran, F.L.S.; Zanetti, M.V.; Curiati, P.K.; Menezes, P.R.; Scazufca, M.; Amaro, E.; Leite, C.C.; Busatto, G.F. Age-Related Gray Matter Volume Changes in the Brain during Non-Elderly Adulthood. Neurobiol. Aging 2011, 32, 354–368. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Y.A.; De Bellis, M.D. The Relationship of Age, Gender, and IQ with the Brainstem and Thalamus in Healthy Children and Adolescents: A Magnetic Resonance Imaging Volumetric Study. J. Child Neurol. 2012, 27, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Bouhrara, M.; Cortina, L.E.; Rejimon, A.C.; Khattar, N.; Bergeron, C.; Bergeron, J.; Melvin, D.; Zukley, L.; Spencer, R.G. Quantitative Age-Dependent Differences in Human Brainstem Myelination Assessed Using High-Resolution Magnetic Resonance Mapping. Neuroimage 2020, 206, 116307. [Google Scholar] [CrossRef]
- Anderson, M.J.; Robinson, J. Permutation Tests for Linear Models. Aust. N. Z. J. Stat. 2001, 43, 75–88. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [Green Version]
- Honey, C.J.; Sporns, O.; Cammoun, L.; Gigandet, X.; Thiran, J.P.; Meuli, R.; Hagmann, P. Predicting Human Resting-State Functional Connectivity from Structural Connectivity. Proc. Natl. Acad. Sci. USA 2009, 106, 2035–2040. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, A.A.; Schult, D.A.; Swart, P.J. Exploring Network Structure, Dynamics, and Function Using NetworkX. In Proceedings of the 7th Python in Science Conference (SciPy 2008), Pasadena, CA, USA, 19–24 August 2008; pp. 11–15. [Google Scholar]
- Haufe, S.; Meinecke, F.; Görgen, K.; Dähne, S.; Haynes, J.D.; Blankertz, B.; Bießmann, F. On the Interpretation of Weight Vectors of Linear Models in Multivariate Neuroimaging. Neuroimage 2014, 87, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Parra, L.C.; Spence, C.D.; Gerson, A.D.; Sajda, P. Recipes for the Linear Analysis of EEG. Neuroimage 2005, 28, 326–341. [Google Scholar] [CrossRef]
- Blankertz, B.; Lemm, S.; Treder, M.; Haufe, S.; Müller, K.-R. Single-Trial Analysis and Classification of ERP Components—A Tutorial. Neuroimage 2011, 56, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Naselaris, T.; Kay, K.N.; Nishimoto, S.; Gallant, J.L. Encoding and Decoding in FMRI. Neuroimage 2011, 56, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bießmann, F.; Murayama, Y.; Logothetis, N.K.; Müller, K.-R.; Meinecke, F.C. Improved Decoding of Neural Activity from FMRI Signals Using Non-Separable Spatiotemporal Deconvolutions. Neuroimage 2012, 61, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Huth, A.G.; Nishimoto, S.; Vu, A.T.; Gallant, J.L. A Continuous Semantic Space Describes the Representation of Thousands of Object and Action Categories across the Human Brain. Neuron 2012, 76, 1210–1224. [Google Scholar] [CrossRef] [Green Version]
- Raz, N.; Ghisletta, P.; Rodrigue, K.M.; Kennedy, K.M.; Lindenberger, U. Trajectories of Brain Aging in Middle-Aged and Older Adults: Regional and Individual Differences. Neuroimage 2010, 51, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Storsve, A.B.; Fjell, A.M.; Tamnes, C.K.; Westlye, L.T.; Overbye, K.; Aasland, H.W.; Walhovd, K.B. Differential Longitudinal Changes in Cortical Thickness, Surface Area and Volume across the Adult Life Span: Regions of Accelerating and Decelerating Change. J. Neurosci. 2014, 34, 8488–8498. [Google Scholar] [CrossRef] [Green Version]
- Fjell, A.M.; Westlye, L.T.; Grydeland, H.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Holland, D.; Dale, A.M.; Walhovd, K.B. Critical Ages in the Life Course of the Adult Brain: Nonlinear Subcortical Aging. Neurobiol. Aging 2013, 34, 2239–2247. [Google Scholar] [CrossRef] [Green Version]
- Drysdale, A.T.; Grosenick, L.; Downar, J.; Dunlop, K.; Mansouri, F.; Meng, Y.; Fetcho, R.N.; Zebley, B.; Oathes, D.J.; Etkin, A.; et al. Resting-State Connectivity Biomarkers Define Neurophysiological Subtypes of Depression. Nat. Med. 2017, 23, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Szabó, C.Á.; Lancaster, J.L.; Lee, S.; Xiong, J.H.; Cook, C.; Mayes, B.N.; Fox, P.T. MR Imaging Volumetry of Subcortical Structures and Cerebellar Hemispheres in Temporal Lobe Epilepsy. Am. J. Neuroradiol. 2006, 27, 2155–2160. [Google Scholar]
- Maller, J.J.; Anstey, K.J.; Réglade-Meslin, C.; Christensen, H.; Wen, W.; Sachdev, P. Hippocampus and Amygdala Volumes in a Random Community-Based Sample of 60–64 Year Olds and Their Relationship to Cognition. Psychiatry Res. Neuroimaging 2007, 156, 185–197. [Google Scholar] [CrossRef]
- Levita, L.; Bois, C.; Healey, A.; Smyllie, E.; Papakonstantinou, E.; Hartley, T.; Lever, C. The Behavioural Inhibition System, Anxiety and Hippocampal Volume in a Non-Clinical Population. Biol. Mood Anxiety Disord. 2014, 4, 4. [Google Scholar] [CrossRef]
- LaMontagne, P.; Benzinger, T.L.; Morris, J.; Keefe, S.; Hornbeck, R.; Xiong, C.; Grant, E.; Hassenstab, J.; Moulder, K.; Vlassenko, A.; et al. OASIS-3: Longitudinal Neuroimaging, Clinical, and Cognitive Dataset for Normal Aging and Alzheimer Disease. medRxiv 2019. [Google Scholar] [CrossRef]







| Articles | Method | MAE (Years) |
|---|---|---|
| [12] | CNN * (FPN *) | 5.55 |
| GPR * (FPN) | 7.47 | |
| RVR * (FPN) | 7.76 | |
| [14] | CNN (GM *) | 4.16 |
| GPR (GM) | 4.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Tu, Z.; Meng, D.; Gong, Y.; Zhang, M.; Xu, J. Interpretation for Individual Brain Age Prediction Based on Gray Matter Volume. Brain Sci. 2022, 12, 1517. https://doi.org/10.3390/brainsci12111517
Sun J, Tu Z, Meng D, Gong Y, Zhang M, Xu J. Interpretation for Individual Brain Age Prediction Based on Gray Matter Volume. Brain Sciences. 2022; 12(11):1517. https://doi.org/10.3390/brainsci12111517
Chicago/Turabian StyleSun, Jiancheng, Zongqing Tu, Deqi Meng, Yizhou Gong, Mengmeng Zhang, and Jinsong Xu. 2022. "Interpretation for Individual Brain Age Prediction Based on Gray Matter Volume" Brain Sciences 12, no. 11: 1517. https://doi.org/10.3390/brainsci12111517
APA StyleSun, J., Tu, Z., Meng, D., Gong, Y., Zhang, M., & Xu, J. (2022). Interpretation for Individual Brain Age Prediction Based on Gray Matter Volume. Brain Sciences, 12(11), 1517. https://doi.org/10.3390/brainsci12111517






