Exogenous Melatonin Regulates Puberty and the Hypothalamic GnRH-GnIH System in Female Mice
Abstract
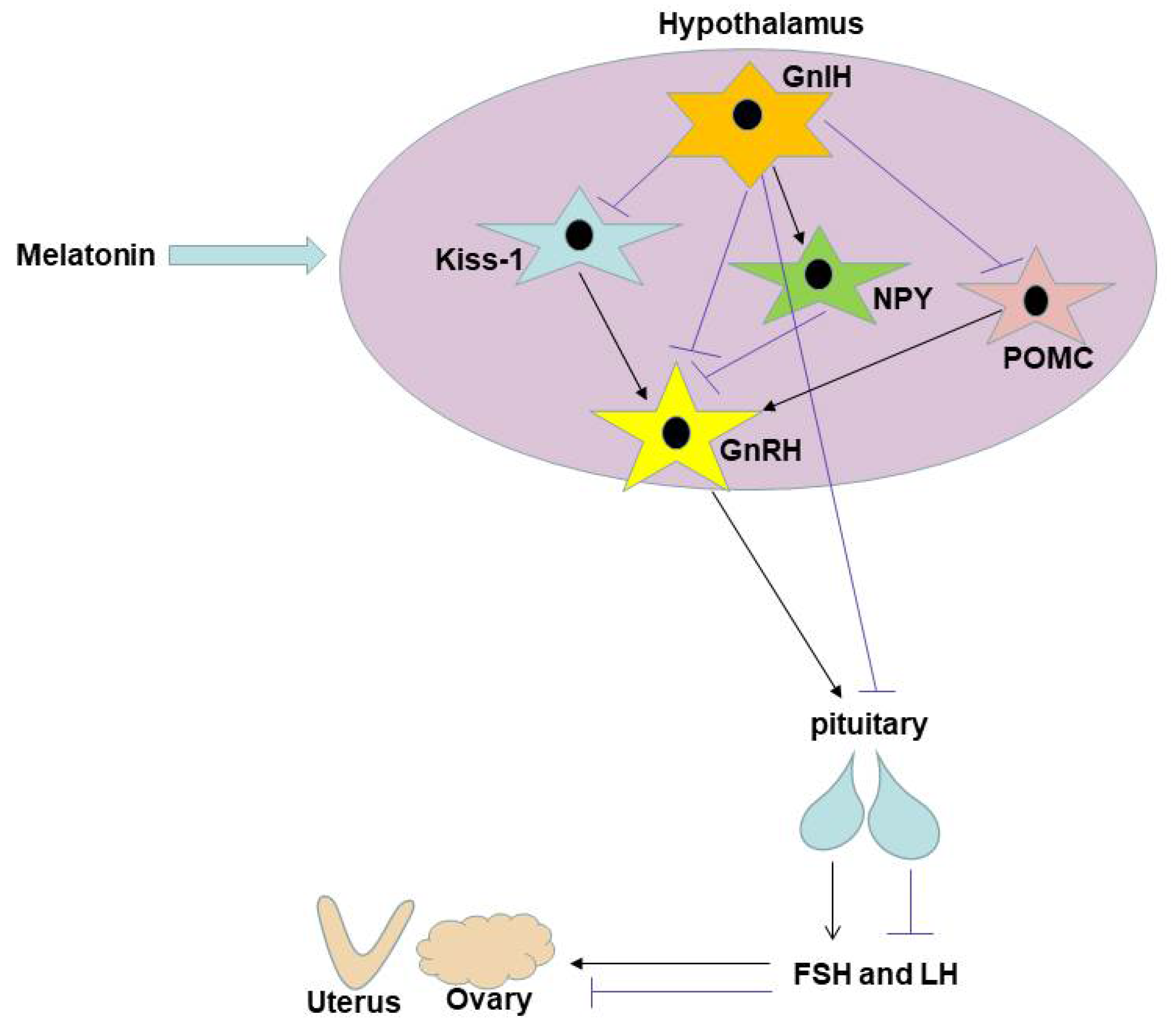
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Treatment and Grouping
2.3. Puberty Onset in Female Mice
2.4. Pathological Examination of the Uterus and Ovary
2.5. Hormonal Analysis of Serum
2.6. Real-Time Fluorescence Quantitative Polymerase Chain Reaction (RT-PCR) Assay
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of Melatonin on Vaginal Opening Rate in Female Mice
3.2. Effects of Melatonin on Body Weight in Female Mice
3.3. Effects of Melatonin on Ovarian and Uterine Development
3.4. Effects of Melatonin on the Levels of the Female Mouse Serum Hormones GnRH, FSH, and GnIH
3.5. Effects of Melatonin on the Expression of Reproductive-Related Neuronal mRNA in Female Mice’s Hypothalamus
3.6. Effects of Melatonin on the Expression of Reproductive-Related Neuronal Protein in Female Mice’s Hypothalamus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebling, F.J.; Cronin, A.S. The neurobiology of reproductive development. NeuroReport 2000, 11, R23–R33. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016, 12, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Burgus, R.; Butcher, M.; Amoss, M.; Ling, N.; Monahan, M.; Rivier, J.; Fellows, R.; Blackwell, R.; Vale, W.; Guillemin, R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) (LH-hypothalamus-LRF-gas chromatography-mass spectrometry-decapeptide-Edman degradation). Proc. Natl. Acad. Sci. USA 1972, 69, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, H.; Baba, Y.; Nair, R.M.; Arimura, A.; Schally, A.V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem. Biophys. Res. Commun. 1971, 43, 1334–1339. [Google Scholar] [CrossRef]
- Diaz, L.B.; Diaz, R.E.; Urquijo, C.; Fernandez, A.C. Melatonin influences on the neuroendocrine-reproductive axis. Ann. N. Y. Acad. Sci. 2005, 1057, 337–364. [Google Scholar] [CrossRef]
- Clarke, I.J.; Smith, J.T.; Caraty, A.; Goodman, R.L.; Lehman, M.N. Kisspeptin and seasonality in sheep. Peptides 2009, 30, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Takase, K.; Uenoyama, Y.; Inoue, N.; Matsui, H.; Yamada, S.; Shimizu, M.; Homma, T.; Tomikawa, J.; Kanda, S.; Matsumoto, H.; et al. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J. Neuroendocrinol. 2009, 21, 527–537. [Google Scholar] [CrossRef]
- Crown, A.; Clifton, D.K.; Steiner, R.A. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 2007, 86, 175–182. [Google Scholar] [CrossRef]
- Tsutsui, K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Prog. Neurobiol. 2009, 88, 76–88. [Google Scholar] [CrossRef]
- Kriegsfeld, L.J.; Ubuka, T.; Bentley, G.E.; Tsutsui, K. Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front. Neuroendocrinol. 2015, 37, 65–75. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Vance, G.; Maywood, E. Some reflections on the phylogeny and function of the pineal. Experientia 1989, 45, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in peripheral nerve. Nature 1959, 183, 1821. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Wu, Y.H.; Swaab, D.F. The human pineal gland and melatonin in aging and Alzheimer’s disease. J. Pineal. Res. 2005, 38, 145–152. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the immune system in aging. Neuroimmunomodulat 2008, 15, 272–278. [Google Scholar] [CrossRef]
- Inoue, Y. Anti-hypertensive action of melatoninergic compounds. Nihon rinsho. Jpn. J. Clin. Med. 2014, 72, 1386–1394. [Google Scholar]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Day, D.; Burgess, C.M.; Kircik, L.H. Assessing the Potential Role for Topical Melatonin in an Antiaging Skin Regimen. J. Drugs Dermatol. 2018, 17, 966–969. [Google Scholar] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef]
- Aleandri, V.; Spina, V.; Ciardo, A. The role of the pineal body in the endocrine control of puberty. Minerva Ginecol. 1997, 49, 43–48. [Google Scholar] [PubMed]
- Venugopal, S.P. Effect of melatonin on the onset of puberty in male juvenile rats. Anat. Cell. Biol. 2019, 52, 286–295. [Google Scholar] [CrossRef]
- Pool, K.R.; Rickard, J.P.; de Graaf, S.P. Overcoming neuroendocrine and metabolic barriers to puberty: The role of melatonin in advancing puberty in ewe lambs. Domest. Anim. Endocrinol. 2020, 72, 106457. [Google Scholar] [CrossRef]
- Yang, C.; Ran, Z.; Liu, G.; Hou, R.; He, C.; Liu, Q.; Chen, Y.; Liu, Y.; Wang, X.; Ling, C.; et al. Melatonin Administration Accelerates Puberty Onset in Mice by Promoting FSH Synthesis. Molecules 2021, 26, 1474. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Peek, J.C.; Gilmore, T.A.; Royles, P. Pituitary response to LHRH, LH pulsatility and plasma melatonin and prolactin changes in ewe lambs treated with melatonin implants to delay puberty. J. Reprod. Fertil. 1986, 78, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, K.L.; Yellon, S.M. Delayed puberty in the male Djungarian hamster: Effect of short photoperiod or melatonin treatment on the GnRH neuronal system. Neuroendocrinology 1991, 54, 96–102. [Google Scholar] [CrossRef]
- Shi, L.; Li, N.; Bo, L.; Xu, Z. Melatonin and hypothalamic-pituitary-gonadal axis. Curr. Med. Chem. 2013, 20, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomova, A.; Robeva, R.; Kumanov, P. Influence of the body weight on the onset and progression of puberty in boys. J. Pediatr. Endocrinol. Metab. 2015, 28, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Plant, T.M. Neuroendocrine control of the onset of puberty. Front. Neuroendocrinol. 2015, 38, 73–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, K.; Ubuka, T. GnIH Control of Feeding and Reproductive Behaviors. Front. Endocrinol. 2016, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, K.; Ubuka, T.; Son, Y.L.; Bentley, G.E.; Kriegsfeld, L.J. Contribution of GnIH Research to the Progress of Reproductive Neuroendocrinology. Front. Endocrinol. 2015, 6, 179. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.Z.; Poling, M.C.; Corr, M.; Cornes, P.A.; Augustine, R.A.; Quennell, J.H.; Kauffman, A.S.; Anderson, G.M. RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology 2012, 153, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, S.; Si, L.; Wei, M.; Guo, S.; Chen, Z.; Wang, S.; Qiao, Y. Direct effect of RFRP-3 microinjection into the lateral ventricle on the hypothalamic kisspeptin neurons in ovariectomized estrogen-primed rats. Exp. Ther. Med. 2022, 23, 24. [Google Scholar] [CrossRef]
- Clarke, I.J.; Smith, J.T.; Henry, B.A.; Oldfield, B.J.; Stefanidis, A.; Millar, R.P.; Sari, I.P.; Chng, K.; Fabre-Nys, C.; Caraty, A.; et al. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 2012, 95, 305–316. [Google Scholar] [CrossRef]
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. Kiss-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef]
- Kuohung, W.; Kaiser, U.B. GPR54 and Kiss-1: Role in the regulation of puberty and reproduction. Rev. Endocr. Metab. Disord. 2006, 7, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Y.M.; Zhang, L.; Zhao, Z.M.; Zhao, J.; Peng, S.Q. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signaling. Mol. Cell. Endocrinol. 2016, 437, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez-Pascual, E.; Martinez-Fuentes, A.J.; Pinilla, L.; Tena-Sempere, M.; Malagon, M.M.; Castano, J.P. Direct pituitary effects of kisspeptin: Activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J. Neuroendocrinol. 2007, 19, 521–530. [Google Scholar] [CrossRef]
- Revel, F.G.; Saboureau, M.; Masson-Pevet, M.; Pevet, P.; Mikkelsen, J.D.; Simonneaux, V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr. Biol. 2006, 16, 1730–1735. [Google Scholar] [CrossRef] [Green Version]
- Kanasaki, H.; Tumurbaatar, T.; Oride, A.; Tumurgan, Z.; Okada, H.; Hara, T.; Tsutsui, K.; Kyo, S. Role of RFRP-3 in the Regulation of Kiss-1 Gene Expression in the AVPV Hypothalamic Cell Model mHypoA-50. Reprod. Sci. 2019, 26, 1249–1255. [Google Scholar] [CrossRef]
- Klenke, U.; Constantin, S.; Wray, S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology 2010, 151, 2736–2746. [Google Scholar] [CrossRef] [Green Version]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Xu, Y.; Faulkner, L.D.; Hill, J.W. Cross-Talk between Metabolism and Reproduction: The Role of POMC and SF1 Neurons. Front. Endocrinol. 2011, 2, 98. [Google Scholar] [CrossRef] [Green Version]
- Ronnekleiv, O.K.; Qiu, J.; Kelly, M.J. Arcuate Kisspeptin Neurons Coordinate Reproductive Activities with Metabolism. Semin. Reprod. Med. 2019, 37, 131–140. [Google Scholar] [CrossRef]
- Chowdhury, V.S.; Ubuka, T.; Tsutsui, K. Review: Melatonin stimulates the synthesis and release of gonadotropin-inhibitory hormone in birds. Gen. Comp. Endocrinol. 2013, 181, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, V.S.; Yamamoto, K.; Ubuka, T.; Bentley, G.E.; Hattori, A.; Tsutsui, K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology 2010, 151, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, P.J.; Ross, A.W.; Mercer, J.G.; Barrett, P. Photoperiodic programming of body weight through the neuroendocrine hypothalamus. J. Endocrinol. 2003, 177, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revel, F.G.; Saboureau, M.; Pevet, P.; Simonneaux, V.; Mikkelsen, J.D. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology 2008, 149, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Inoue, K.; Fukuda, Y.; Mizuno, T.; Ukena, K.; Kriegsfeld, L.J.; Tsutsui, K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 2012, 153, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.; Lee, Y.S.; Yu, J. Basal serum luteinizing hormone value as the screening biomarker in female central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Montalbano, G.; Mania, M.; Abbate, F.; Navarra, M.; Guerrera, M.C.; Laura, R.; Vega, J.A.; Levanti, M.; Germana, A. Melatonin treatment suppresses appetite genes and improves adipose tissue plasticity in diet-induced obese zebrafish. Endocrine 2018, 62, 381–393. [Google Scholar] [CrossRef]







| Name of the Gene | Primer Sequence (5′-3′) | Amplification Fraction (bp) |
|---|---|---|
| Forward: | ||
| GnRH | ACTGCTGACTGTGTGTTTGGAAGG | 136 |
| Reverse: TTCTGCCATTTGATCCACCTCCTTG | ||
| Forward: | ||
| GnIH | CCCCAAGACACCCGCTGATTTG | 123 |
| Reverse: CTCCTCGTTCGCTTTCCACCAG | ||
| Forward: | ||
| GPR147 | CGAGTCTGAACGAGAGTGATGCTG | 80 |
| Reverse: CGGAGAGGAGTGCTGGTAGTAGG | ||
| Forward: | ||
| Kiss-1 | GCTGCTGCTTCTCCTCTGTGTC | 119 |
| Reverse: GCGATTCCTTTTCCCAGGCATTAAC | ||
| Forward: | ||
| POMC | TAGAGTTCAAGAGGGAGCTGGAAGG | 141 |
| Reverse: CACCGTAACGCTTGTCCTTGGG | ||
| Forward: | ||
| NPY | TGTGTTTGGGCATTCTGGCTGAG | 117 |
| Reverse: TGAGATTGATGTAGTGTCGCAGAGC | ||
| Forward: | ||
| GAPDH | GGTTGTCTCCTGCGACTTCA | 183 |
| Reverse: TGGTCCAGGGTTTCTTACTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Si, L.; Shu, W.; Zhang, X.; Wei, C.; Wei, M.; Cheng, L.; Chen, Z.; Qiao, Y.; Yang, S. Exogenous Melatonin Regulates Puberty and the Hypothalamic GnRH-GnIH System in Female Mice. Brain Sci. 2022, 12, 1550. https://doi.org/10.3390/brainsci12111550
Chen Z, Si L, Shu W, Zhang X, Wei C, Wei M, Cheng L, Chen Z, Qiao Y, Yang S. Exogenous Melatonin Regulates Puberty and the Hypothalamic GnRH-GnIH System in Female Mice. Brain Sciences. 2022; 12(11):1550. https://doi.org/10.3390/brainsci12111550
Chicago/Turabian StyleChen, Zixuan, Lina Si, Weihan Shu, Xin Zhang, Chenyang Wei, Meng Wei, Luyang Cheng, Zhihong Chen, Yuebing Qiao, and Songhe Yang. 2022. "Exogenous Melatonin Regulates Puberty and the Hypothalamic GnRH-GnIH System in Female Mice" Brain Sciences 12, no. 11: 1550. https://doi.org/10.3390/brainsci12111550
APA StyleChen, Z., Si, L., Shu, W., Zhang, X., Wei, C., Wei, M., Cheng, L., Chen, Z., Qiao, Y., & Yang, S. (2022). Exogenous Melatonin Regulates Puberty and the Hypothalamic GnRH-GnIH System in Female Mice. Brain Sciences, 12(11), 1550. https://doi.org/10.3390/brainsci12111550






