Approaching the Gut and Nasal Microbiota in Parkinson’s Disease in the Era of the Seed Amplification Assays
Abstract
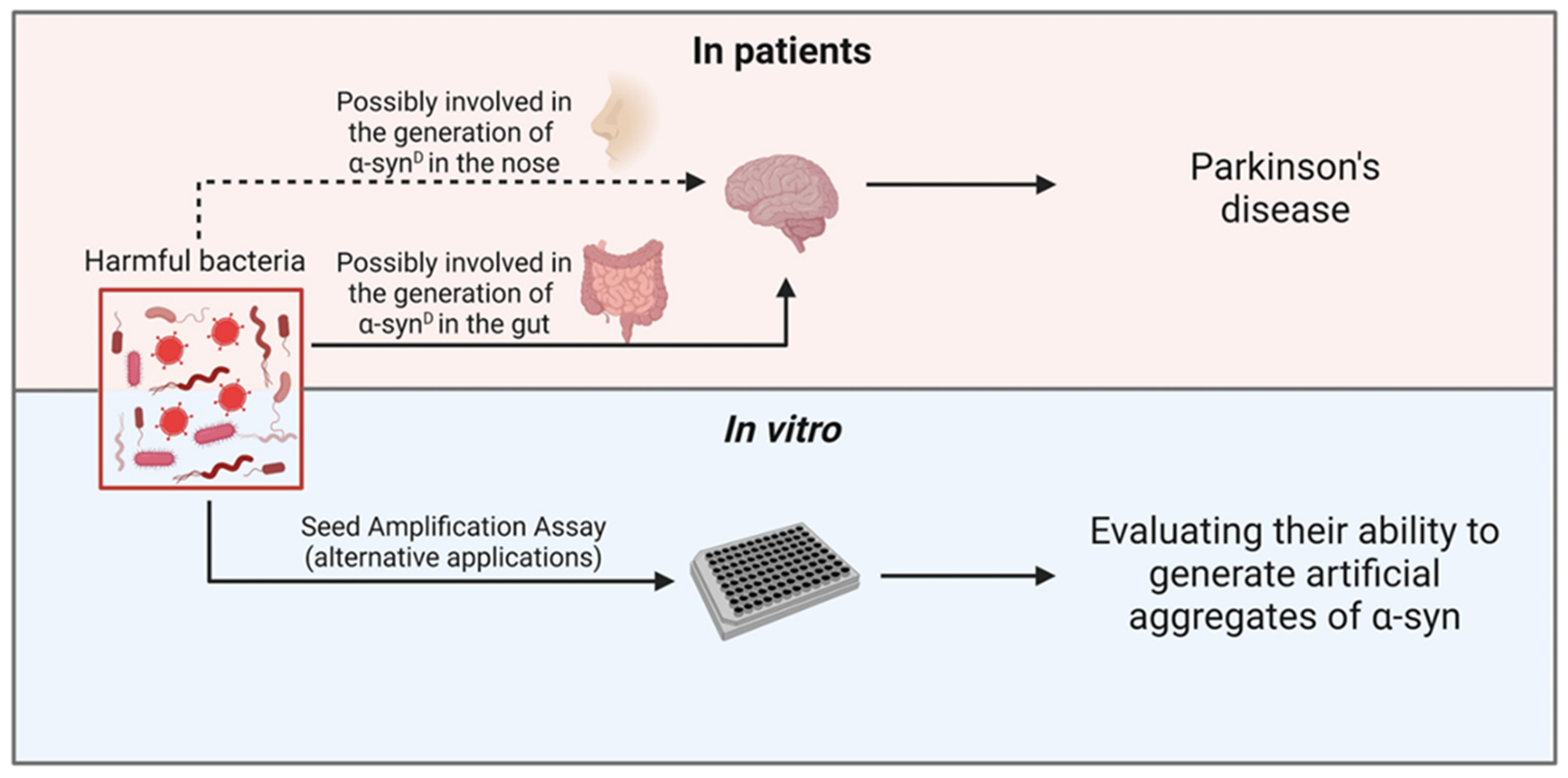
:1. Introduction
2. Gut Microbiota
The Gut-Brain Axis: Gut Microbiota and Central Nervous System Interaction
3. Nasal Microbiota
The Nose-Brain Axis: Nasal Microbiota and Central Nervous System Interaction
4. Parkinson’s Disease
4.1. Neuroinflammation in PD
4.2. Gut Microbiota and PD
4.3. Nasal Microbiota and PD
5. SAA Analyses of Olfactory Mucosa and Gut of PD Patients
6. Treatments for PD
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederberg, J.; McCray, A. ‘Ome Sweet’ Omics—A Genealogical Treasury of Words. The Scientist 2001, 15, 8. [Google Scholar]
- Fung, T.C.; Artis, D.; Sonnenberg, G.F. Anatomical Localization of Commensal Bacteria in Immune Cell Homeostasis and Disease. Immunol. Rev. 2014, 260, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. A Cross Talk between Dysbiosis and Gut-Associated Immune System Governs the Development of Inflammatory Arthropathies. Semin. Arthritis Rheum. 2019, 49, 474–484. [Google Scholar] [CrossRef]
- Biragyn, A.; Ferrucci, L. Gut Dysbiosis: A Potential Link between Increased Cancer Risk in Ageing and Inflammaging. Lancet Oncol. 2018, 19, e295–e304. [Google Scholar] [CrossRef]
- Fang, P.; Kazmi, S.A.; Jameson, K.G.; Hsiao, E.Y. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host Microbe 2020, 28, 201–222. [Google Scholar] [CrossRef]
- Kolbert, C.P.; Persing, D.H. Ribosomal DNA Sequencing as a Tool for Identification of Bacterial Pathogens. Curr. Opin. Microbiol. 1999, 2, 299–305. [Google Scholar] [CrossRef]
- Srinivasan, R.; Karaoz, U.; Volegova, M.; MacKichan, J.; Kato-Maeda, M.; Miller, S.; Nadarajan, R.; Brodie, E.L.; Lynch, S.V. Use of 16S RRNA Gene for Identification of a Broad Range of Clinically Relevant Bacterial Pathogens. PLoS ONE 2015, 10, e0117617. [Google Scholar] [CrossRef]
- Church, D.L.; Cerutti, L.; Gürtler, A.; Griener, T.; Zelazny, A.; Emler, S. Performance and Application of 16S RRNA Gene Cycle Sequencing for Routine Identification of Bacteria in the Clinical Microbiology Laboratory. Clin. Microbiol. Rev. 2020, 33, e00053-19. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Zarei, K.; Guseva, N.V.; Mangalam, A.K. Microbiota Analysis Using Two-Step Pcr and next-Generation 16s Rrna Gene Sequencing. J. Vis. Exp. 2019, 152, e59980. [Google Scholar] [CrossRef] [PubMed]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-Generation Sequencing: Insights to Advance Clinical Investigations of the Microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, G.; De Luca, C.M.G.; Paolini Paoletti, F.; Gaetani, L.; Moda, F.; Parnetti, L. α-Synuclein Seed Amplification Assays for Diagnosing Synucleinopathies: The Way Forward. Neurology 2022, 99, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 56, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Morgan, X.C.; Segata, N.; Huttenhower, C. Biodiversity and Functional Genomics in the Human Microbiome. Trends Genet. 2013, 29, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The Role of the Gut Microbiota in Nutrition and Health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk- and Solid-Feeding Practices and Daycare Attendance Are Associated with Differences in Bacterial Diversity, Predominant Communities, and Metabolic and Immune Function of the Infant Gut Microbiome. Front. Cell. Infect. Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, M.J.; Sharp, R.; Macfarlane, G.T. Age and Disease Related Changes in Intestinal Bacterial Populations Assessed by Cell Culture, 16S RRNA Abundance, and Community Cellular Fatty Acid Profiles. Gut 2001, 48, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarǎes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, K.; Meyer, K.M.; Aagaard, K.M.; Wilmes, P. The Human Gut Microbiome in Health: Establishment and Resilience of Microbiota over a Lifetime. Environ. Microbiol. 2016, 18, 2103–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and Dysbiosis: The Two Sides of the Microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar] [PubMed]
- Alshehri, D.; Saadah, O.; Mosli, M.; Edris, S.; Alhindi, R.; Bahieldin, A. Dysbiosis of Gut Microbiota in Inflammatory Bowel Disease: Current Therapies and Potential for Microbiota-Modulating Therapeutic Approaches. Bosn. J. Basic Med. Sci. 2021, 21, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Leonard, M.M.; Valitutti, F.; Karathia, H.; Pujolassos, M.; Kenyon, V.; Fanelli, B.; Troisi, J.; Subramanian, P.; Camhi, S.; Colucci, A.; et al. Microbiome Signatures of Progression toward Celiac Disease Onset in At-Risk Children in a Longitudinal Prospective Cohort Study. Proc. Natl. Acad. Sci. USA 2021, 118, e2020322118. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef]
- Chen, X.; D’Souza, R.; Hong, S.T. The Role of Gut Microbiota in the Gut-Brain Axis: Current Challenges and Perspectives. Protein Cell 2013, 4, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-Gut-Brain Axis and the Central Nervous System. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef] [Green Version]
- Sudo, N. Microbiome, HPA Axis and Production of Endocrine Hormones in the Gut. Adv. Exp. Med. Biol. 2014, 817, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Guan, N.L.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 9, eaah6888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A Dural Lymphatic Vascular System That Drains Brain Interstitial Fluid and Macromolecules. J. Exp. Med. 2015, 115, 627. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-Producing Bifidobacterium Dentium Modulates Visceral Sensitivity in the Intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [Green Version]
- Gershon, M.D. 5-Hydroxytryptamine (Serotonin) in the Gastrointestinal Tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Glebov, K.; Löchner, M.; Jabs, R.; Lau, T.; Merkel, O.; Schloss, P.; Steinhäuser, C.; Walter, J. Serotonin Stimulates Secretion of Exosomes from Microglia Cells. Glia 2015, 63, 626–634. [Google Scholar] [CrossRef]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.C.; Ardura-Fabregat, A.; De Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial Control of Astrocytes in Response to Microbial Metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. (Lausanne) 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of Microbiota-Derived Short-Chain Fatty Acids in Nervous System Disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Pulikkan, J.; Mazumder, A.; Grace, T. Role of the Gut Microbiome in Autism Spectrum Disorders. Adv Exp Med Biol. 2019, 1118, 253–269. [Google Scholar] [PubMed]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.F.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sci. 2020, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The Gut Microbiome: A Key Player in the Complexity of Amyotrophic Lateral Sclerosis (ALS). BMC Med. 2021, 19, 1–14. [Google Scholar] [CrossRef]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef]
- Limbana, T.; Khan, F.; Eskander, N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus 2020, 12, e9966. [Google Scholar] [CrossRef]
- D’Argenio, V.; Sarnataro, D. Microbiome Influence in the Pathogenesis of Prion and Alzheimer’s Diseases. Int. J. Mol. Sci. 2019, 20, 4704. [Google Scholar] [CrossRef] [Green Version]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and Its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.M.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.F.; Bogaert, D. Early Respiratory Microbiota Composition Determines Bacterial Succession Patterns and Respiratory Health in Children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- van den Bergh, M.R.; Biesbroek, G.; Rossen, J.W.A.; de Steenhuijsen Piters, W.A.A.; Bosch, A.A.T.M.; van Gils, E.J.M.; Wang, X.; Boonacker, C.W.B.; Veenhoven, R.H.; Bruin, J.P.; et al. Associations between Pathogens in the Upper Respiratory Tract of Young Children: Interplay between Viruses and Bacteria. PLoS ONE 2012, 7, e47711. [Google Scholar] [CrossRef] [PubMed]
- Wos-Oxley, M.L.; Chaves-Moreno, D.; Jáuregui, R.; Oxley, A.P.A.; Kaspar, U.; Plumeier, I.; Kahl, S.; Rudack, C.; Becker, K.; Pieper, D.H. Exploring the Bacterial Assemblages along the Human Nasal Passage. Environ. Microbiol. 2016, 18, 2259–2271. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus Epidermidis Esp Inhibits Staphylococcus Aureus Biofilm Formation and Nasal Colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, E.S.; Clarke, T.B.; Shchepetov, M.; Ratner, A.J.; Roper, D.I.; Dowson, C.G.; Weiser, J.N. Nod1 Signaling Overcomes Resistance of S. Pneumoniae to Opsonophagocytic Killing. PLoS Pathog. 2007, 3, e118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, C.; Thornton, D.J. Mucins: The Frontline Defence of the Lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Mellert, T.K.; Getchell, M.L.; Sparks, L.; Getchell, T.V. Characterization of the Immune Barrier in Human Olfactory Mucosa. Otolaryngol.-Head Neck Surg. 1992, 106, 181–188. [Google Scholar] [CrossRef]
- Kristensson, K. Microbes’ Roadmap to Neurons. Nat. Rev. Neurosci. 2011, 12, 345–357. [Google Scholar] [CrossRef]
- Doty, R.L. The Olfactory Vector Hypothesis of Neurodegenerative Disease: Is It Viable? Ann. Neurol. 2008, 63, 7–15. [Google Scholar] [CrossRef]
- Rey, N.L.; Wesson, D.W.; Brundin, P. The Olfactory Bulb as the Entry Site for Prion-like Propagation in Neurodegenerative Diseases. Neurobiol. Dis. 2018, 109, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Rey, N.L.; Petit, G.H.; Bousset, L.; Melki, R.; Brundin, P. Transfer of Human α-Synuclein from the Olfactory Bulb to Interconnected Brain Regions in Mice. Acta Neuropathol. 2013, 126, 555–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Zheng, M.; Liu, Q.; Shi, Z.; Long, S.; Lu, X.; Pei, Z.; Yuan, T.F.; Su, H.; Yao, X. Injected Amyloid Beta in the Olfactory Bulb Transfers to Other Brain Regions via Neural Connections in Mice. Mol. Neurobiol. 2018, 55, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Lalancette-Hbert, M.; Phaneuf, D.; Soucy, G.; Weng, Y.C.; Kriz, J. Live Imaging of Toll-like Receptor 2 Response in Cerebral Ischaemia Reveals a Role of Olfactory Bulb Microglia as Modulators of Inflammation. Brain 2009, 132, 940–954. [Google Scholar] [CrossRef] [Green Version]
- Filippidis, A.; Fountas, K.N. Nasal Lymphatics as a Novel Invasion and Dissemination Route of Bacterial Meningitis. Med. Hypotheses 2009, 72, 694–697. [Google Scholar] [CrossRef]
- Emery, D.C.; Shoemark, D.K.; Batstone, T.E.; Waterfall, C.M.; Coghill, J.A.; Cerajewska, T.L.; Davies, M.; West, N.X.; Allen, S.J. 16S RRNA next Generation Sequencing Analysis Shows Bacteria in Alzheimer’s Post-Mortem Brain. Front. Aging Neurosci. 2017, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Little, C.S.; Hammond, C.J.; MacIntyre, A.; Balin, B.J.; Appelt, D.M. Chlamydia Pneumoniae Induces Alzheimer-like Amyloid Plaques in Brains of BALB/c Mice. Neurobiol. Aging 2004, 25, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Hoggard, M.; Nocera, A.; Biswas, K.; Taylor, M.W.; Douglas, R.G.; Bleier, B.S. The Sinonasal Microbiota, Neural Signaling, and Depression in Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2018, 8, 394–405. [Google Scholar] [CrossRef]
- Mazzoni, P.; Shabbott, B.; Cortés, J.C. Motor Control Abnormalities in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009282. [Google Scholar] [CrossRef] [Green Version]
- Goldman, J.G.; Postuma, R. Premotor and Nonmotor Features of Parkinson’s Disease. Curr. Opin. Neurol. 2014, 27, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 28, 375–381. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Di Fonzo, A.; Monfrini, E.; Erro, R. Genetics of Movement Disorders and the Practicing Clinician; Who and What to Test For? Curr. Neurol. Neurosci. Rep. 2018, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, F.A.; De Luca, C.M.G.; Bistaffa, E.; Consonni, A.; Legname, G.; Giaccone, G.; Moda, F. Cell-Free Amplification of Prions: Where Do We Stand? Prog. Mol. Biol. Transl. Sci. 2020, 175, 325–358, ISBN 9780128200025. [Google Scholar] [PubMed]
- Yan, F.; Chen, Y.; Li, M.; Wang, Y.; Zhang, W.; Chen, X.; Ye, Q. Gastrointestinal Nervous System A-Synuclein as a Potential Biomarker of Parkinson Disease. Medicine 2018, 97, e11337. [Google Scholar] [CrossRef]
- Schaeffer, E.; Kluge, A.; Böttner, M.; Zunke, F.; Cossais, F.; Berg, D.; Arnold, P. Alpha Synuclein Connects the Gut-Brain Axis in Parkinson’s Disease Patients-A View on Clinical Aspects, Cellular Pathology and Analytical Methodology. Front. Cell Dev. Biol. 2020, 8, 573696. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s Disease: A Dual-Hit Hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s Disease: Its Role in Neuronal Death and Implications for Therapeutic Intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef] [Green Version]
- McGeer, P.L.; McGeer, E.G. Glial Cell Reactions in Neurodegenerative Diseases Pathophysiology and Therapeutic Interventions. Alzheimer Dis. Assoc. Disord. 1998, 12, S1–S6. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of Cytokines in Inflammatory Process in Parkinson’s Disease. J Neural Transm Suppl. 2006, 70, 373–381. [Google Scholar]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In Vivo Imaging of Microglial Activation with [11C](R)-PK11195 PET in Idiopathic Parkinson’s Disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Rayaprolu, S.; Mullen, B.; Baker, M.; Lynch, T.; Finger, E.; Seeley, W.W.; Hatanpaa, K.J.; Lomen-Hoerth, C.; Kertesz, A.; Bigio, E.H.; et al. TREM2 in Neurodegeneration: Evidence for Association of the p.R47H Variant with Frontotemporal Dementia and Parkinson’s Disease. Mol. Neurodegener. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, T.H.; Zabetian, C.P.; Tenesa, A.; Laederach, A.; Montimurro, J.; Yearout, D.; Kay, D.M.; Doheny, K.F.; Paschall, J.; Pugh, E.; et al. Common Genetic Variation in the HLA Region Is Associated with Late-Onset Sporadic Parkinson’s Disease. Nat. Genet. 2010, 42, 781–785. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive Microglia Are Positive for HLA-DR in the: Substantia Nigra of Parkinson’s and Alzheimer’s Disease Brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Gao, H.M.; Kotzbauer, P.T.; Uryu, K.; Leight, S.; Trojanowski, J.Q.; Lee, V.M.Y. Neuroinflammation and Oxidation/Nitration of α-Synuclein Linked to Dopaminergic Neurodegeneration. J. Neurosci. 2008, 28, 7687–7698. [Google Scholar] [CrossRef] [Green Version]
- Smeyne, R.J.; Breckenridge, C.B.; Beck, M.; Jiao, Y.; Butt, M.T.; Wolf, J.C.; Zadory, D.; Minnema, D.J.; Sturgess, N.C.; Travis, K.Z.; et al. Assessment of the Effects of MPTP and Paraquat on Dopaminergic Neurons and Microglia in the Substantia Nigra Pars Compacta of C57BL/6 Mice. PLoS ONE 2016, 11, e0164094. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Federoff, H.J.; Maguire-Zeiss, K.A. Mutant α-Synuclein Overexpression Mediates Early Proinflammatory Activity. Neurotox. Res. 2009, 16, 238–254. [Google Scholar] [CrossRef] [Green Version]
- Hewett, S.J.; Corbett, J.A.; McDaniel, M.L.; Choi, D.W. Interferon-γ and Interleukin-1β Induce Nitric Oxide Formation from Primary Mouse Astrocytes. Neurosci. Lett. 1993, 164, 229–232. [Google Scholar] [CrossRef]
- Consonni, A.; Morara, S.; Codazzi, F.; Grohovaz, F.; Zacchetti, D. Inhibition of Lipopolysaccharide-Induced Microglia Activation by Calcitonin Gene Related Peptide and Adrenomedullin. Mol. Cell. Neurosci. 2011, 48, 151–160. [Google Scholar] [CrossRef]
- Paxinou, E.; Chen, Q.; Weisse, M.; Giasson, B.I.; Norris, E.H.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.Y.; Ischiropoulos, H. Induction of α-Synuclein Aggregation by Intracellular Nitrative Insult. J. Neurosci. 2001, 21, 8053–8061. [Google Scholar] [CrossRef] [PubMed]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D.; Friedman, A. Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio May Reflect Differences in PD and MSA-P Neuroinflammation Patterns. Polish J. Neurol. Neurosurg. 2022, 56, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Grathwohl, S.A.; Steiner, J.A.; Britschgi, M.; Brundin, P. Mind the Gut: Secretion of α-Synuclein by Enteric Neurons. J. Neurochem. 2013, 125, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferro, Á.; Rábano, A.; Catalán, M.J.; Rodríguez-Valcárcel, F.C.; Díez, S.F.; Herreros-Rodríguez, J.; García-Cobos, E.; Álvarez-Santullano, M.M.; López-Manzanares, L.; Mosqueira, A.J.; et al. In Vivo Gastric Detection of α-Synuclein Inclusions in Parkinson’s Disease. Mov. Disord. 2015, 30, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Fayyad, M.; Salim, S.; Majbour, N.; Erskine, D.; Stoops, E.; Mollenhauer, B.; El-Agnaf, O.M.A. Parkinson’s Disease Biomarkers Based on α-Synuclein. J. Neurochem. 2019, 150, 626–636. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s Disease: Possible Routes by Which Vulnerable Neuronal Types May Be Subject to Neuroinvasion by an Unknown Pathogen. J. Neural. Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Musgrove, R.E.; Helwig, M.; Bae, E.J.; Aboutalebi, H.; Lee, S.J.; Ulusoy, A.; Di Monte, D.A. Oxidative Stress in Vagal Neurons Promotes Parkinsonian Pathology and Intercellular α-Synuclein Transfer. J. Clin. Investig. 2019, 130, 3738–3753. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-Seeded α-Synuclein Fibrils Promote Gut Dysfunction and Brain Pathology Specifically in Aged Mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Lv, G.; Lee, J.S.; Jung, B.C.; Masuda-Suzukake, M.; Hong, C.S.; Valera, E.; Lee, H.J.; Paik, S.R.; Hasegawa, M.; et al. Exposure to Bacterial Endotoxin Generates a Distinct Strain of α-Synuclein Fibril. Sci. Rep. 2016, 6, 30891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota Regulation of the Mammalian Gut-Brain Axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A Gut Bacterial Amyloid Promotes A-Synuclein Aggregation and Motor Impairment in Mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B.; et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis Elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haikal, C.; Pascual, L.O.; Najarzadeh, Z.; Bernfur, K.; Svanbergsson, A.; Otzen, D.E.; Linse, S.; Li, J.Y. The Bacterial Amyloids Phenol Soluble Modulins from Staphylococcus Aureus Catalyze Alpha-Synuclein Aggregation. Int. J. Mol. Sci. 2021, 22, 11594. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic Bacterial Composition in Parkinson’s Disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov. Disord. 2020, 35, 1626–1635. [Google Scholar] [CrossRef]
- Jin, M.; Li, J.; Liu, F.; Lyu, N.; Wang, K.; Wang, L.; Liang, S.; Tao, H.; Zhu, B.; Alkasir, R. Analysis of the Gut Microflora in Patients With Parkinson’s Disease. Front. Neurosci. 2019, 13, 1184. [Google Scholar] [CrossRef] [Green Version]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Rawls, M.; Ellis, A.K. The Microbiome of the Nose. Ann. Allergy Asthma Immunol. 2019, 122, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Leboucq, N.; Menjot De Champfleur, N.; Menjot De Champfleur, S.; Bonafé, A. The Olfactory System. Diagn. Interv. Imaging 2013, 94, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; LoSavio, P.S.; Engen, P.A.; Naqib, A.; Mehta, A.; Kota, R.; Khan, R.J.; Tobin, M.C.; Green, S.J.; Schleimer, R.P.; et al. Association of Nasal Microbiome and Asthma Control in Patients with Chronic Rhinosinusitis. Clin. Exp. Allergy 2018, 48, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.A.B.; Aho, V.T.E.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Oral and Nasal Microbiota in Parkinson’s Disease. Park. Relat. Disord. 2017, 38, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The Nasal and Gut Microbiome in Parkinson’s Disease and Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, G.; Ramirez, V.; Engen, P.A.; Naqib, A.; Forsyth, C.B.; Green, S.J.; Mahdavinia, M.; Batra, P.S.; Tajudeen, B.A.; Keshavarzian, A. Deep Nasal Sinus Cavity Microbiota Dysbiosis in Parkinson’s Disease. NPJ Park. Dis. 2021, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Luo, E.; Wu, B.; Li, Z.; Guo, J.; Xia, Z.; Zheng, C.; Su, Q.; Zeng, Y.; et al. Oral, Nasal, and Gut Microbiota in Parkinson’s Disease. Neuroscience 2022, 480, 65–78. [Google Scholar] [CrossRef]
- Bargar, C.; De Luca, C.M.G.; Devigili, G.; Elia, A.E.; Cilia, R.; Portaleone, S.M.; Wang, W.; Tramacere, I.; Bistaffa, E.; Cazzaniga, F.A.; et al. Discrimination of MSA-P and MSA-C by RT-QuIC Analysis of Olfactory Mucosa: The First Assessment of Assay Reproducibility between Two Specialized Laboratories. Mol. Neurodegener. 2021, in press. [CrossRef]
- Shahnawaz, M.; Tokuda, T.; Waragai, M.; Mendez, N.; Ishii, R.; Trenkwalder, C.; Mollenhauer, B.; Soto, C. Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid. JAMA Neurol. 2017, 74, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-Synuclein RT-QuIC in the CSF of Patients with Alpha-Synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and Ultra-Sensitive Quantitation of Disease-Associated α-Synuclein Seeds in Brain and Cerebrospinal Fluid by ASyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.J.; Orru, C.D.; Concha-Marambio, L.; Giaisi, S.; Groveman, B.R.; Farris, C.M.; Holguin, B.; Hughson, A.G.; LaFontant, D.E.; Caspell-Garcia, C.; et al. High Diagnostic Performance of Independent Alpha-Synuclein Seed Amplification Assays for Detection of Early Parkinson’s Disease. Acta Neuropathol. Commun. 2021, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. A-Synuclein RT-QuIC Assay in Cerebrospinal Fluid of Patients with Dementia with Lewy Bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126. [Google Scholar] [CrossRef]
- De Luca, C.M.G.; Elia, A.E.; Portaleone, S.M.; Cazzaniga, F.A.; Rossi, M.; Bistaffa, E.; De Cecco, E.; Narkiewicz, J.; Salzano, G.; Carletta, O.; et al. Efficient RT-QuIC Seeding Activity for α-Synuclein in Olfactory Mucosa Samples of Patients with Parkinson’s Disease and Multiple System Atrophy. Transl. Neurodegener. 2019, 8, 24. [Google Scholar] [CrossRef]
- Stefani, A.; Iranzo, A.; Holzknecht, E.; Perra, D.; Bongianni, M.; Gaig, C.; Heim, B.; Serradell, M.; Sacchetto, L.; Garrido, A.; et al. Alpha-Synuclein Seeds in Olfactory Mucosa of Patients with Isolated REM Sleep Behaviour Disorder. Brain 2021, 144, 1118–1126. [Google Scholar] [CrossRef]
- Perra, D.; Bongianni, M.; Novi, G.; Janes, F.; Bessi, V.; Capaldi, S.; Sacchetto, L.; Tagliapietra, M.; Schenone, G.; Morbelli, S.; et al. Alpha-Synuclein Seeds in Olfactory Mucosa and Cerebrospinal Fluid of Patients with Dementia with Lewy Bodies. Brain Commun. 2021, 3, fcab045. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Catalan, M.; Perra, D.; Fontana, E.; Janes, F.; Bertolotti, C.; Sacchetto, L.; Capaldi, S.; Tagliapietra, M.; Polverino, P.; et al. Olfactory Swab Sampling Optimization for α-Synuclein Aggregate Detection in Patients with Parkinson’s Disease. Transl. Neurodegener. 2022, 11, 37. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Jin, H.; Anantharam, V.; Huang, X.; Kanthasamy, A.; Kanthasamy, A.G. A-Synuclein Real-Time Quaking-Induced Conversion in the Submandibular Glands of Parkinson’s Disease Patients. Mov. Disord. 2020, 35, 268–278. [Google Scholar] [CrossRef]
- Bargar, C.; Wang, W.; Gunzler, S.A.; LeFevre, A.; Wang, Z.; Lerner, A.J.; Singh, N.; Tatsuoka, C.; Appleby, B.; Zhu, X.; et al. Streamlined Alpha-Synuclein RT-QuIC Assay for Various Biospecimens in Parkinson’s Disease and Dementia with Lewy Bodies. Acta Neuropathol. Commun. 2021, 9, 62. [Google Scholar] [CrossRef]
- Wang, Z.; Becker, K.; Donadio, V.; Siedlak, S.; Yuan, J.; Rezaee, M.; Incensi, A.; Kuzkina, A.; Orrú, C.D.; Tatsuoka, C.; et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 2021, 78, 30. [Google Scholar] [CrossRef]
- Kuzkina, A.; Bargar, C.; Schmitt, D.; Rößle, J.; Wang, W.; Schubert, A.L.; Tatsuoka, C.; Gunzler, S.A.; Zou, W.Q.; Volkmann, J.; et al. Diagnostic Value of Skin RT-QuIC in Parkinson’s Disease: A Two-Laboratory Study. NPJ Park. Dis. 2021, 7, 99. [Google Scholar] [CrossRef]
- Donadio, V.; Wang, Z.; Incensi, A.; Rizzo, G.; Fileccia, E.; Vacchiano, V.; Capellari, S.; Magnani, M.; Scaglione, C.; Stanzani Maserati, M.; et al. In Vivo Diagnosis of Synucleinopathies: A Comparative Study of Skin Biopsy and RT-QuIC. Neurology 2021, 96, e2513–e2524. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Sun, Y.; Chen, J.; Jiang, Y.; Li, F.; Wei, L.; Sun, W.; Ma, J.; Song, L.; Liu, J.; et al. Diagnostic Value of Salivary Real-Time Quaking-Induced Conversion in Parkinson’s Disease and Multiple System Atrophy. Mov. Disord. 2022, 37, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Bunk, J.; Schaeffer, E.; Drobny, A.; Xiang, W.; Knacke, H.; Bub, S.; Lückstädt, W.; Arnold, P.; Lucius, R.; et al. Detection of Neuron-Derived Pathological α-Synuclein in Blood. Brain 2022, 145, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Fenyi, A.; Leclair-Visonneau, L.; Clairembault, T.; Coron, E.; Neunlist, M.; Melki, R.; Derkinderen, P.; Bousset, L. Detection of Alpha-Synuclein Aggregates in Gastrointestinal Biopsies by Protein Misfolding Cyclic Amplification. Neurobiol. Dis. 2019, 129, 38–43. [Google Scholar] [CrossRef]
- Thomzig, A.; Wagenführ, K.; Pinder, P.; Joncic, M.; Schulz-Schaeffer, W.J.; Beekes, M. Transmissible α-Synuclein Seeding Activity in Brain and Stomach of Patients with Parkinson’s Disease. Acta Neuropathol. 2021, 141, 861–879. [Google Scholar] [CrossRef]
- Macleod, A.D.; Taylor, K.S.M.; Counsell, C.E. Mortality in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2014, 29, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA-J. Am. Med. Assoc. 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Müller, T. Catechol-O-Methyltransferase Inhibitors in Parkinson’s Disease. Drugs 2015, 75, 157–174. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Ravina, B.; Seppi, K.; Coelho, M.; Poewe, W.; Rascol, O.; Goetz, C.G.; Sampaio, C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the Motor Symptoms of Parkinson’s Disease. Mov. Disord. 2011, 26, S2–S41. [Google Scholar] [CrossRef] [PubMed]
- Bratsos, S.P.; Karponis, D.; Saleh, S.N. Efficacy and Safety of Deep Brain Stimulation in the Treatment of Parkinson’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus 2018, 10, e3474. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Rey, M.V.; Pavy-Le Traon, A.; Rascol, O. Emerging Drugs for Autonomic Dysfunction in Parkinson’s Disease. Expert Opin. Emerg. Drugs 2013, 18, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.; Fox, S.H. Treatment of Cognitive, Psychiatric, and Affective Disorders Associated with Parkinson’s Disease. Neurotherapeutics 2014, 11, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of Probiotics for the Treatment of Constipation in Parkinson’s Disease Patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Colombo, D.; Pnevmatikou, P.; Melloni, E.; Keywood, C. Therapeutic Innovation in Parkinson’s Disease: A 2020 Update on Disease-Modifying Approaches. Expert Rev. Neurother. 2020, 20, 1047–1064. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Azcarate-Peril, M.A. Emerging Technologies for Gut Microbiome Research. Trends Microbiol. 2016, 24, 887–901. [Google Scholar] [CrossRef] [Green Version]
- Galloway-Peña, J.; Hanson, B. Tools for Analysis of the Microbiome. Dig. Dis. Sci. 2020, 65, 674–685. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consonni, A.; Miglietti, M.; De Luca, C.M.G.; Cazzaniga, F.A.; Ciullini, A.; Dellarole, I.L.; Bufano, G.; Di Fonzo, A.; Giaccone, G.; Baggi, F.; et al. Approaching the Gut and Nasal Microbiota in Parkinson’s Disease in the Era of the Seed Amplification Assays. Brain Sci. 2022, 12, 1579. https://doi.org/10.3390/brainsci12111579
Consonni A, Miglietti M, De Luca CMG, Cazzaniga FA, Ciullini A, Dellarole IL, Bufano G, Di Fonzo A, Giaccone G, Baggi F, et al. Approaching the Gut and Nasal Microbiota in Parkinson’s Disease in the Era of the Seed Amplification Assays. Brain Sciences. 2022; 12(11):1579. https://doi.org/10.3390/brainsci12111579
Chicago/Turabian StyleConsonni, Alessandra, Martina Miglietti, Chiara Maria Giulia De Luca, Federico Angelo Cazzaniga, Arianna Ciullini, Ilaria Linda Dellarole, Giuseppe Bufano, Alessio Di Fonzo, Giorgio Giaccone, Fulvio Baggi, and et al. 2022. "Approaching the Gut and Nasal Microbiota in Parkinson’s Disease in the Era of the Seed Amplification Assays" Brain Sciences 12, no. 11: 1579. https://doi.org/10.3390/brainsci12111579
APA StyleConsonni, A., Miglietti, M., De Luca, C. M. G., Cazzaniga, F. A., Ciullini, A., Dellarole, I. L., Bufano, G., Di Fonzo, A., Giaccone, G., Baggi, F., & Moda, F. (2022). Approaching the Gut and Nasal Microbiota in Parkinson’s Disease in the Era of the Seed Amplification Assays. Brain Sciences, 12(11), 1579. https://doi.org/10.3390/brainsci12111579






