Characterization of Resting-State Striatal Differences in First-Episode Depression and Recurrent Depression
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Scan Acquisition
2.3. Image Processing
2.3.1. fMRI Data Preprocessing
2.3.2. Seed-Based Functional Connectivity
2.4. Statistical Analyses
2.4.1. Clinical Data Analysis
2.4.2. fMRI Data Analysis
3. Results
3.1. Characteristics of Research Samples
3.2. Among the Three Group Differences in Striatal FC and Post Hoc t-Test Analysis
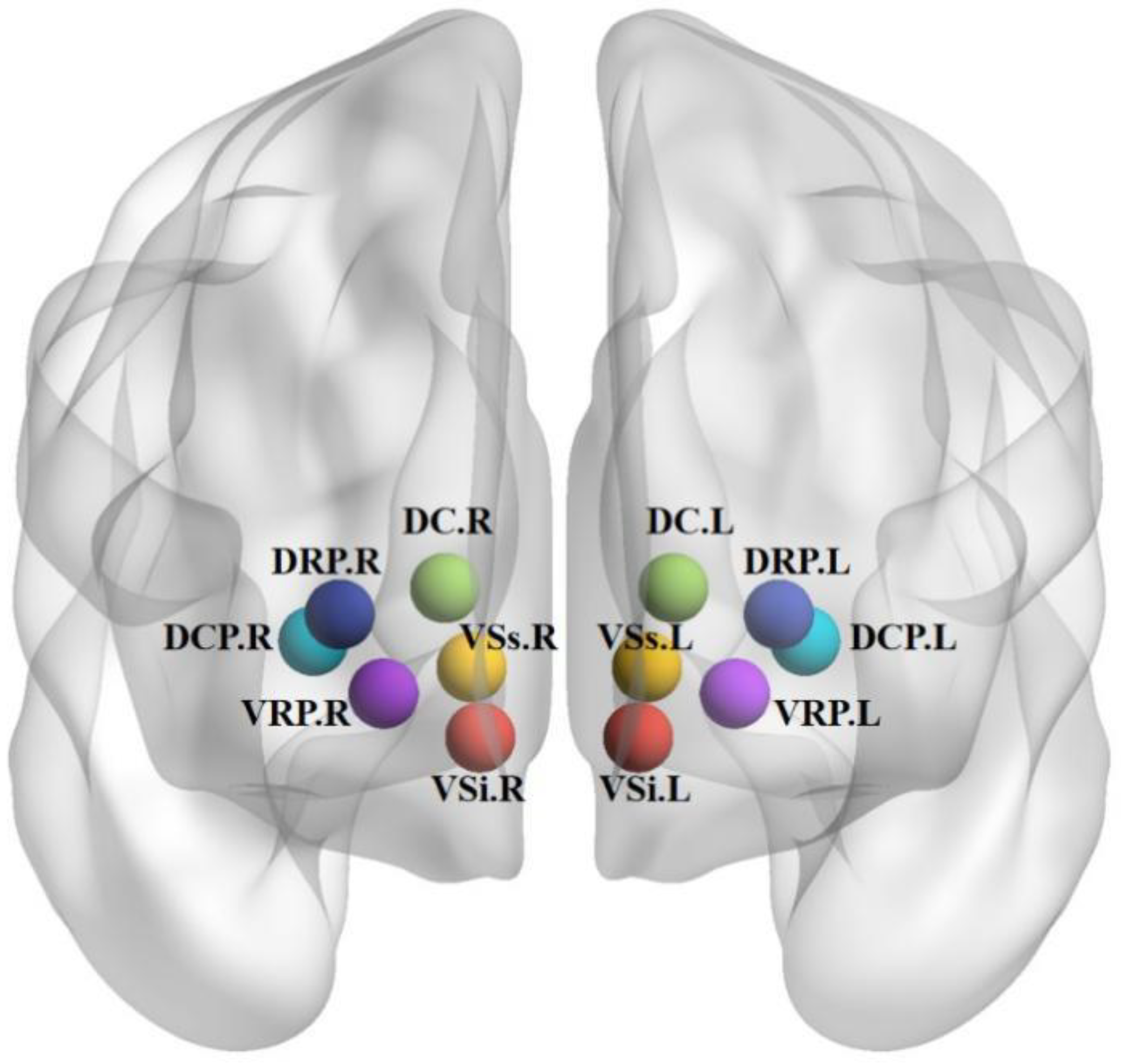
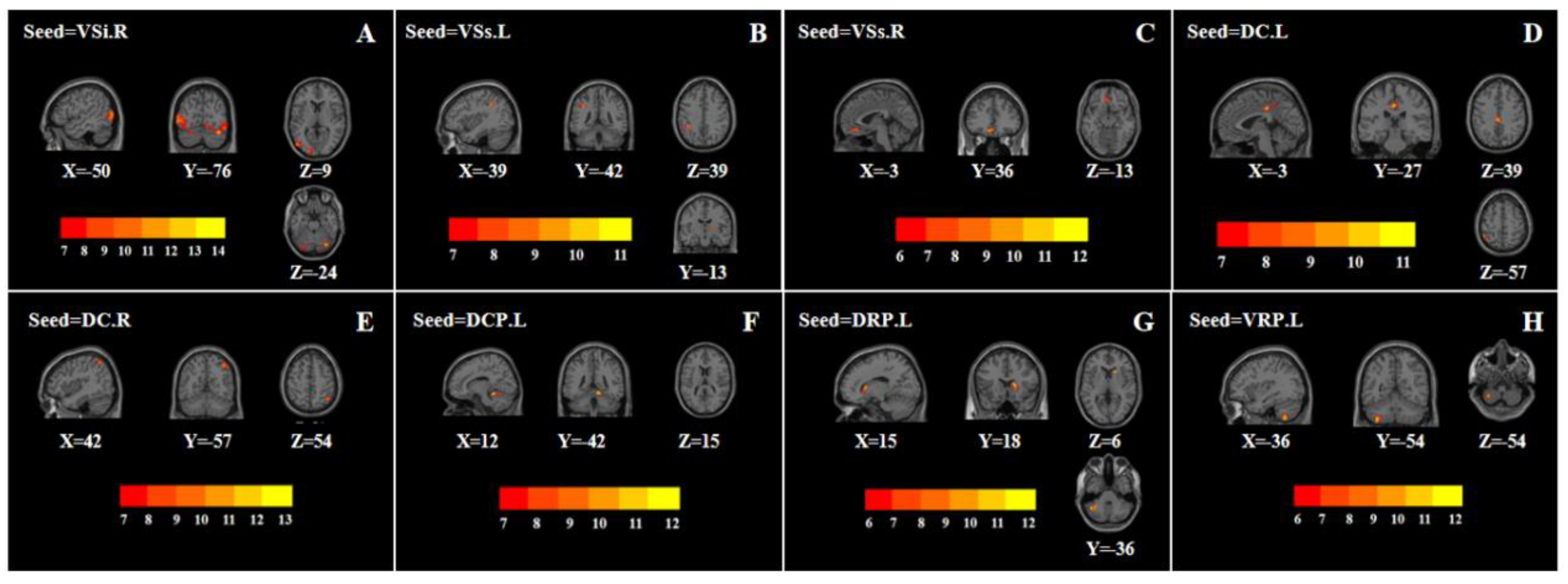
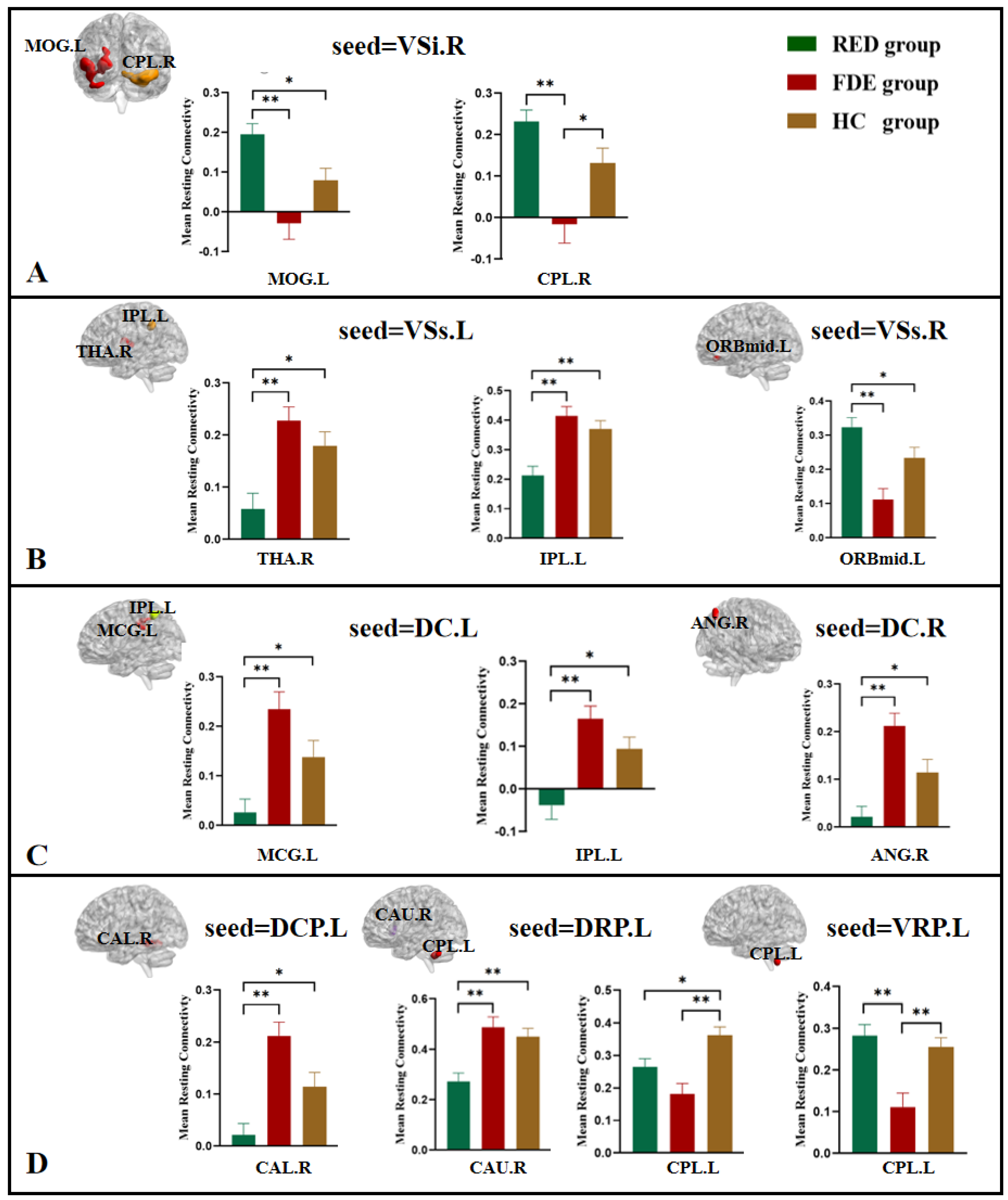
3.2.1. VSi, VSs
3.2.2. DC
3.2.3. DCP, DRP, VRP
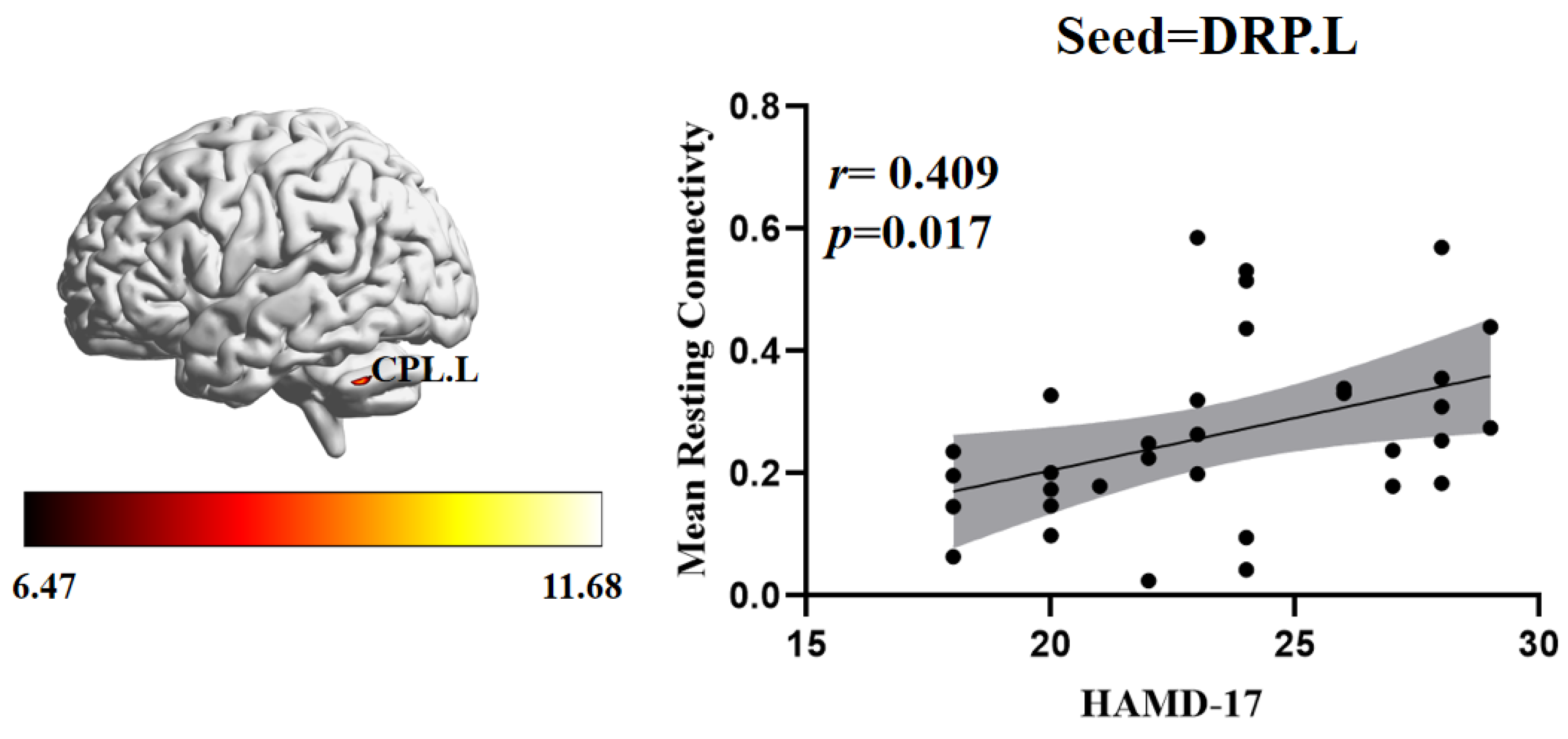
3.3. Relationship between FC and Clinical Symptoms
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 180–181. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Peng, W.; Sweeney, J.A.; Jia, Z.-Y.; Gong, Q.-Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Hiller, W.; Dichtl, G.; Hecht, H.; Hundt, W.; Mombour, W.; von Zerssen, D. Evaluating the new ICD-10 categories of depressive episode and recurrent depressive disorder. J. Affect. Disord. 1994, 31, 49–60. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Goodman, S.H.; Humphreys, K.L. Studying the Intergenerational Transmission of Risk for Depression: Current Status and Future Directions. Curr. Dir. Psychol. Sci. 2020, 29, 174–179. [Google Scholar] [CrossRef]
- Kessler, R.C.; Zhao, S.; Blazer, D.G.; Swartz, M. Prevalence, correlates, and course of minor depression and major depression in the national comorbidity survey. J. Affect. Disord. 1997, 45, 19–30. [Google Scholar] [CrossRef]
- Roca, M.; Armengol, S.; García-García, M.; Rodriguez-Bayón, A.; Ballesta, I.; Serrano, M.J.; Comas, A.; Gili, M. Clinical differences between first and recurrent episodes in depressive patients. Compr. Psychiatry 2011, 52, 26–32. [Google Scholar] [CrossRef]
- Varghese, S.; Frey, B.N.; Schneider, M.A.; Kapczinski, F.; Cardoso, T.D.A. Functional and cognitive impairment in the first episode of depression: A systematic review. Acta Psychiatr. Scand. 2022, 145, 156–185. [Google Scholar] [CrossRef]
- Zu, S.; Wang, D.; Fang, J.; Xiao, L.; Zhu, X.; Wu, W.; Wang, G.; Hu, Y. Comparison of Residual Depressive Symptoms, Functioning, and Quality of Life Between Patients with Recurrent Depression and First Episode Depression After Acute Treatment in China. Neuropsychiatr. Dis. Treat. 2021, 17, 3039–3051. [Google Scholar] [CrossRef]
- Gili, M.; Armengol, S.; Soriano, J.B.; Roca, M.; Garcia-Toro, M.; Garcia-Campayo, J.; Vives, M. Medical comorbidity in recurrent versus first-episode depressive patients. Acta Psychiatr. Scand. 2011, 123, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Wang, Y.; Liu, C.; Mou, J.; Yuan, Y.; Qiu, L.; Gong, Q. Study of Sex Differences in Unmedicated Patients with Major Depressive Disorder by Using Resting State Brain Functional Magnetic Resonance Imaging. Front. Neurosci. 2022, 16, 814410. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Chen, Y.; Jiang, Y.; Wen, M.; Zhou, B.; Li, S.; Wei, Y.; Yang, Z.; Wang, C.; Cheng, J.; et al. Dynamic Altered Amplitude of Low-Frequency Fluctuations in Patients with Major Depressive Disorder. Front. Psychiatry 2021, 12, 683610. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, M.; Xue, K.; Ye, C.; Man, W.; Cheng, M.; Liu, Z.; Zhu, D.; Liu, F.; Wang, J. Abnormal Regional Spontaneous Brain Activities in White Matter in Patients with Autism Spectrum Disorder. Neuroscience 2022, 490, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, J.; Liu, X.; Pu, C.; Lai, Y.; Chen, L.; Yu, X.; Hong, N. Structural and functional brain abnormalities in schizophrenia: A cross-sectional study at different stages of the disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 83, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Palaniyappan, L.; Liddle, P.F.; Rangaprakash, D.; Wei, W.; Deshpande, G. Characterization of Hemodynamic Alterations in Schizophrenia and Bipolar Disorder and Their Effect on Resting-State fMRI Functional Connectivity. Schizophr. Bull. 2022, 48, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-B.; Liu, F.; Xue, Z.-M.; Xu, X.-J.; Wu, R.-R.; Ma, C.-Q.; Wooderson, S.C.; Tan, C.-L.; Sun, X.-L.; Chen, J.-D.; et al. Alterations of the amplitude of low-frequency fluctuations in treatment-resistant and treatment-response depression: A resting-state fMRI study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 37, 153–160. [Google Scholar] [CrossRef]
- Sun, J.; Chen, L.; He, J.; Du, Z.; Ma, Y.; Wang, Z.; Guo, C.; Luo, Y.; Gao, D.; Hong, Y.; et al. Altered Brain Function in First-Episode and Recurrent Depression: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Neurosci. 2022, 16, 876121. [Google Scholar] [CrossRef]
- Yi, L.; Wang, J.; Jia, L.; Zhao, Z.; Lu, J.; Li, K.; Jia, J.; He, Y.; Jiang, C.; Han, Y. Structural and Functional Changes in Subcortical Vascular Mild Cognitive Impairment: A Combined Voxel-Based Morphometry and Resting-State fMRI Study. PLoS ONE 2012, 7, e44758. [Google Scholar] [CrossRef]
- Kelly, C.; Castellanos, F. Strengthening Connections: Functional Connectivity and Brain Plasticity. Neuropsychol. Rev. 2014, 24, 63–76. [Google Scholar] [CrossRef]
- Peng, X.; Wu, X.; Gong, R.; Yang, R.; Wang, X.; Zhu, W.; Lin, P. Sub-regional anterior cingulate cortex functional connectivity revealed default network subsystem dysfunction in patients with major depressive disorder. Psychol. Med. 2021, 51, 1687–1695. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, J.; Wang, X.; Chen, Z.; Liu, H.; Pei, C.; Zhang, S.; Yao, Z.; Lu, Q. Functional impairment-based segmentation of anterior cingulate cortex in depression and its relationship with treatment effects. Hum. Brain Mapp. 2021, 42, 4035–4047. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, H.; Xu, J.; Chen, F.; Wu, F.; Wang, C.; Wang, J. Abnormal large-scale resting-state functional networks in drug-free major depressive disorder. Brain Imaging Behav. 2021, 15, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-G.; Chen, X.; Li, L.; Castellanos, F.X.; Bai, T.-J.; Bo, Q.-J.; Cao, J.; Chen, G.-M.; Chen, N.-X.; Chen, W.; et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. USA 2019, 116, 9078–9083. [Google Scholar] [CrossRef]
- Roberts, H.; Jacobs, R.H.; Bessette, K.L.; Crowell, S.E.; Westlund-Schreiner, M.; Thomas, L.; Easter, R.E.; Pocius, S.L.; Dillahunt, A.; Frandsen, S.; et al. Mechanisms of rumination change in adolescent depression (RuMeChange): Study protocol for a randomised controlled trial of rumination-focused cognitive behavioural therapy to reduce ruminative habit and risk of depressive relapse in high-ruminating adolescents. BMC Psychiatry 2021, 21, 206. [Google Scholar] [CrossRef]
- Jacobs, R.H.; Barba, A.; Gowins, J.R.; Klumpp, H.; Jenkins, L.M.; Mickey, B.J.; Ajilore, O.; Peciña, M.; Sikora, M.; Ryan, K.A.; et al. Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol. Med. 2016, 46, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E. CSPT circuity in affective disorders. Biol. Psychiatry 1994, 36, 208–209. [Google Scholar] [CrossRef]
- Bora, E.; Harrison, B.; Davey, C.G.; Yücel, M.; Pantelis, C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 2012, 42, 671–681. [Google Scholar] [CrossRef]
- Epstein, J.; Pan, H.; Kocsis, J.H.; Yang, Y.; Butler, T.; Chusid, J.; Hochberg, H.; Murrough, J.; Strohmayer, E.; Stern, E.; et al. Lack of Ventral Striatal Response to Positive Stimuli in Depressed Versus Normal Subjects. Am. J. Psychiatry 2006, 163, 1784–1790. [Google Scholar] [CrossRef]
- Gabbay, V.; Mao, X.; Klein, R.G.; Ely, B.A.; Babb, J.; Panzer, A.M.; Alonso, C.M.; Shungu, D.C. Anterior Cingulate Cortexγ-Aminobutyric Acid in Depressed Adolescents: Relationship to anhedonia. Arch. Gen. Psychiatry 2012, 69, 139–149. [Google Scholar] [CrossRef]
- Zhu, X.; Helpman, L.; Papini, S.; Schneier, F.; Markowitz, J.C.; Van Meter, P.E.; Lindquist, M.A.; Wager, T.D.; Neria, Y. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress. Anxiety 2017, 34, 641–650. [Google Scholar] [CrossRef]
- Gong, L.; Yin, Y.; He, C.; Ye, Q.; Bai, F.; Yuan, Y.; Zhang, H.; Lv, L.; Zhang, H.; Xie, C.; et al. Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J. Psychiatr. Res. 2017, 84, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; Ely, B.A.; Li, Q.; Bangaru, S.D.; Panzer, A.M.; Alonso, C.M.; Castellanos, F.X.; Milham, M.P. Striatum-Based Circuitry of Adolescent Depression and Anhedonia. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 628–641.e13. [Google Scholar] [CrossRef]
- Rutledge, R.B.; Moutoussis, M.; Smittenaar, P.; Zeidman, P.; Taylor, T.; Hrynkiewicz, L.; Lam, J.; Skandali, N.; Siegel, J.Z.; Ousdal, O.T.; et al. Association of Neural and Emotional Impacts of Reward Prediction Errors with Major Depression. JAMA Psychiatry 2017, 74, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Sacchet, M.D.; Hjørnevik, T.; Chin, F.T.; Bin Shen, B.; Kämpe, R.; Park, J.H.; Knutson, B.D.; Williams, L.M.; Borg, N.; et al. Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: A concurrent 11C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl. Psychiatry 2018, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Berretta, S.; Wooten, D.; Goer, F.; Pilobello, K.T.; Kumar, P.; Murray, L.; Beltzer, M.; Boyer-Boiteau, A.; Alpert, N.; et al. Assessment of Striatal Dopamine Transporter Binding in Individuals with Major Depressive Disorder: In Vivo Positron Emission Tomography and Postmortem Evidence. JAMA Psychiatry 2019, 76, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Peciña, M.; Sikora, M.; Avery, E.T.; Heffernan, J.; Peciña, S.; Mickey, B.J.; Zubieta, J.-K. Striatal dopamine D2/3 receptor-mediated neurotransmission in major depression: Implications for anhedonia, anxiety and treatment response. Eur. Neuropsychopharmacol. 2017, 27, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Scheres, A.; Margulies, D.; Kelly, C.; Uddin, L.; Shehzad, Z.; Biswal, B.; Walters, J.R.; Castellanos, F.; Milham, M.P. Functional Connectivity of Human Striatum: A Resting State fMRI Study. Cereb. Cortex 2008, 18, 2735–2747. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, F.; Mitchell, P.B.; Wang, C.-Y.; Si, T.-M. Striatal Resting-State Connectivity Abnormalities Associated with Different Clinical Stages of Major Depressive Disorder. J. Clin. Psychiatry 2020, 81, 19m12790. [Google Scholar] [CrossRef]
- Admon, R.; Holsen, L.M.; Aizley, H.; Remington, A.; Whitfield-Gabrieli, S.; Goldstein, J.M.; Pizzagalli, D.A. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biol. Psychiatry 2015, 78, 67–76. [Google Scholar] [CrossRef]
- Hammar, Å.; Neto, E.; Clemo, L.; Hjetland, G.J.; Hugdahl, K.; Elliott, R. Striatal hypoactivation and cognitive slowing in patients with partially remitted and remitted major depression. PsyCh J. 2016, 5, 191–205. [Google Scholar] [CrossRef]
- Chao-Gan, Y.; Yu-Feng, Z. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, K.; Liu, J.-H.; Wang, Y.-P. Altered Default Mode and Sensorimotor Network Connectivity with Striatal Subregions in Primary Insomnia: A Resting-State Multi-Band fMRI Study. Front. Neurosci. 2018, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, E.J.R.; Schactera, D.L. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Yamamura, T.; Okamoto, Y.; Okada, G.; Takaishi, Y.; Takamura, M.; Mantani, A.; Kurata, A.; Otagaki, Y.; Yamashita, H.; Yamawaki, S. Association of thalamic hyperactivity with treatment-resistant depression and poor response in early treatment for major depression: A resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Transl. Psychiatry 2016, 6, e754. [Google Scholar] [CrossRef] [PubMed]
- Seghier, M.L. The Angular Gyrus: Multiple functions and multiple subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Leaver, A.M.; Espinoza, R.; Joshi, S.H.; Vasavada, M.; Njau, S.; Woods, R.P.; Narr, K.L. Desynchronization and Plasticity of Striato-frontal Connectivity in Major Depressive Disorder. Cereb. Cortex 2016, 26, 4337–4346. [Google Scholar] [CrossRef]
- Ho, T.C.; Connolly, C.G.; Blom, E.H.; LeWinn, K.Z.; Strigo, I.A.; Paulus, M.P.; Frank, G.; Max, J.E.; Wu, J.; Chan, M.; et al. Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biol. Psychiatry 2015, 78, 635–646. [Google Scholar] [CrossRef]
- Ding, Y.-D.; Chen, X.; Chen, Z.-B.; Le Li, L.; Li, X.-Y.; Castellanos, F.X.; Bai, T.-J.; Bo, Q.-J.; Cao, J.; Chang, Z.-K.; et al. Reduced nucleus accumbens functional connectivity in reward network and default mode network in patients with recurrent major depressive disorder. Transl. Psychiatry 2022, 12, 236. [Google Scholar] [CrossRef]
- Renier, L.A.; Anurova, I.; De Volder, A.G.; Carlson, S.; VanMeter, J.; Rauschecker, J.P. Preserved Functional Specialization for Spatial Processing in the Middle Occipital Gyrus of the Early Blind. Neuron 2010, 68, 138–148. [Google Scholar] [CrossRef]
- Geng, J.; Yan, R.; Shi, J.; Chen, Y.; Mo, Z.; Shao, J.; Wang, X.; Yao, Z.; Lu, Q. Altered regional homogeneity in patients with somatic depression: A resting-state fMRI study. J. Affect. Disord. 2019, 246, 498–505. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, J.; Ma, H.; Tan, Y.; Wu, X.; Guan, C.; Qiao, H.; Li, J.; Zhong, Y.; Wang, C.; et al. Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry 2018, 18, 370. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, J.; Liu, J.; Rong, P.; Jorgenson, K.; Park, J.; Lang, C.; Hong, Y.; Zhu, B.; Kong, J. Frequency-dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J. Psychiatr. Res. 2018, 102, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Liu, J.; Chen, J.; Liu, X.; Nie, G.; Jorgenson, K.; Sohn, K.C.; Huang, R.; Liu, M.; et al. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J. Psychiatr. Res. 2017, 84, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Manto, M.; Cattaneo, Z.; Clausi, S.; Ferrari, C.; Gabrieli, J.D.E.; Guell, X.; Heleven, E.; Lupo, M.; Ma, Q.; et al. Consensus Paper: Cerebellum and Social Cognition. Cerebellum 2020, 19, 833–868. [Google Scholar] [CrossRef] [PubMed]
- Olivito, G.; Lupo, M.; Iacobacci, C.; Clausi, S.; Romano, S.; Masciullo, M.; Molinari, M.; Cercignani, M.; Bozzali, M.; Leggio, M. Structural cerebellar correlates of cognitive functions in spinocerebellar ataxia type 2. J. Neurol. 2018, 265, 597–606. [Google Scholar] [CrossRef]
- Depping, M.S.; Schmitgen, M.M.; Kubera, K.M.; Wolf, R.C. Cerebellar Contributions to Major Depression. Front. Psychiatry 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Chantiluke, K.; Halari, R.; Simic, M.; Pariante, C.M.; Papadopoulos, A.; Giampietro, V.; Rubia, K. Fronto-Striato-Cerebellar Dysregulation in Adolescents with Depression during Motivated Attention. Biol. Psychiatry 2012, 71, 59–67. [Google Scholar] [CrossRef]
- Sahib, A.K.; Loureiro, J.R.; Vasavada, M.; Anderson, C.; Kubicki, A.; Wade, B.; Joshi, S.H.; Woods, R.P.; Congdon, E.; Espinoza, R.; et al. Modulation of the functional connectome in major depressive disorder by ketamine therapy. Psychol. Med. 2020, 52, 1–10. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for action, emotion, and memory. Handb. Clin. Neurol. 2019, 166, 23–37. [Google Scholar] [CrossRef]
- Heimer, L.; Van Hoesen, G.W. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev. 2006, 30, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dai, J.; Chen, Y.; Liu, C.; Xin, F.; Zhou, X.; Zhou, F.; Stamatakis, E.A.; Yao, S.; Luo, L.; et al. Intrinsic connectivity of the prefrontal cortex and striato-limbic system respectively differentiate major depressive from generalized anxiety disorder. Neuropsychopharmacology 2021, 46, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Hu, H. Reward and Aversion. Annu. Rev. Neurosci. 2016, 39, 297–324. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.L.; Keshavan, M.S.; Hardan, A.Y.; Yorbik, O.; Brambilla, P.; Sassi, R.B.; Nicoletti, M.; Mallinger, A.G.; Frank, E.; Kupfer, D.J.; et al. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol. Psychiatry 2004, 55, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Fettes, P.; Schulze, L.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness. Front. Syst. Neurosci. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed]
| Variable | RDE (n = 33) | FDE (n = 27) | HCs (n = 35) | t(F)/χ2 | p-Value |
|---|---|---|---|---|---|
| Sex (M/F) | 8/25 | 5/22 | 9/26 | 0.477 | 0.788 a |
| Age (years) | 34.63 ± 12.80 | 33.92 ± 12.36 | 35.00 ± 12.50 | 0.056 | 0.945 b |
| Years of education | 14.39 ± 2.76 | 14.88 ± 2.37 | 14.65 ± 3.69 | 0.198 | 0.821 b |
| Duration of illness (months) | 24.93 ± 12.27 | 2.37 ± 0.96 | NA | 9.513 | <0.001 c* |
| HAMD-17 score | 23.48 ± 3.51 | 22.96 ± 3.36 | NA | 0.583 | 0.562 c |
| Clusters | Brain Regions | MNI Peak | Cluster Size | F-Value (Peak) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| VSi.R | ||||||
| 1 | Left middle occipital gyrus | −50 | −76 | 9 | 548 | 10.056 |
| 2 | Right cerebellum posterior lobe | 36 | −75 | −24 | 314 | 14.957 |
| VSs.L | ||||||
| 1 | Right thalamus | 14 | −13 | 9 | 32 | 7.980 |
| 2 | Left inferior parietal lobule | −39 | −42 | 39 | 37 | 10.062 |
| VSs.R | ||||||
| 1 | Left orbital area of the middle frontal gyrus | −3 | 36 | −13 | 46 | 9.606 |
| DC.L | ||||||
| 1 | Left middle cingulate gyrus | −3 | −27 | 39 | 67 | 10.631 |
| 2 | Left inferior parietal lobule | −48 | −48 | 57 | 45 | 10.764 |
| DC.R | ||||||
| 1 | Right angular | 42 | −57 | 54 | 57 | 10.566 |
| DCP.L | ||||||
| 1 | Right cerebellum anterior lobe | 12 | −42 | 15 | 69 | 12.288 |
| DRP.L | ||||||
| 1 | Right caudate | 15 | 18 | 6 | 42 | 10.868 |
| 2 | Left cerebellum posterior lobe | −45 | −57 | −36 | 45 | 11.681 |
| VRP.L | ||||||
| 1 | Left cerebellum posterior lobe | −36 | −54 | −54 | 40 | 11.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Du, Z.; Ma, Y.; Guo, C.; Gao, S.; Luo, Y.; Chen, Q.; Hong, Y.; Xiao, X.; Yu, X.; et al. Characterization of Resting-State Striatal Differences in First-Episode Depression and Recurrent Depression. Brain Sci. 2022, 12, 1603. https://doi.org/10.3390/brainsci12121603
Sun J, Du Z, Ma Y, Guo C, Gao S, Luo Y, Chen Q, Hong Y, Xiao X, Yu X, et al. Characterization of Resting-State Striatal Differences in First-Episode Depression and Recurrent Depression. Brain Sciences. 2022; 12(12):1603. https://doi.org/10.3390/brainsci12121603
Chicago/Turabian StyleSun, Jifei, Zhongming Du, Yue Ma, Chunlei Guo, Shanshan Gao, Yi Luo, Qingyan Chen, Yang Hong, Xue Xiao, Xue Yu, and et al. 2022. "Characterization of Resting-State Striatal Differences in First-Episode Depression and Recurrent Depression" Brain Sciences 12, no. 12: 1603. https://doi.org/10.3390/brainsci12121603
APA StyleSun, J., Du, Z., Ma, Y., Guo, C., Gao, S., Luo, Y., Chen, Q., Hong, Y., Xiao, X., Yu, X., & Fang, J. (2022). Characterization of Resting-State Striatal Differences in First-Episode Depression and Recurrent Depression. Brain Sciences, 12(12), 1603. https://doi.org/10.3390/brainsci12121603






