Acute Effects of High-Frequency Insular Stimulation on Interictal Epileptiform Discharge Rates in Patients with Refractory Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Surgical Implantation
2.2. Stimulation Parameters
2.3. Data Acquisition and Spike Detection
2.4. Statistical Analysis
3. Results
- Group 1: HF-DBS-aINS in Patients with an Anterior Insular Epileptic Focus
- Group 2: HFS-pINS in Patients with a Posterior Insular Epileptic Focus
- Group 3: HFS-pINS in Patients with an Anterior Insular Epileptic Focus
- Group 4: HFS-pINS in Patients without an Insular Epileptic Focus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.C.H.; Cook, M.J. Deep brain stimulation for drug-resistant epilepsy. Epilepsia 2018, 59, 273–290. [Google Scholar] [CrossRef]
- Filipescu, C.; Lagarde, S.; Lambert, I.; Pizzo, F.; Trébuchon, A.; McGonigal, A.; Scavarda, D.; Carron, R.; Bartolomei, F. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia 2019, 60, e25–e30. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Salanova, V.; Witt, T.; Worth, R.; Henry, T.; Gross, R.; Oommen, K.; Osorio, I.; Nazzaro, J.; Labar, D.; et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010, 51, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Salanova, V.; Witt, T.; Worth, R.; Henry, T.R.; Gross, R.E.; Nazzaro, J.M.; Labar, D.; Sperling, M.R.; Sharan, A.; Sandok, E.; et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015, 84, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Dalic, L.J.; Warren, A.E.L.; Bulluss, K.J.; Thevathasan, W.; Roten, A.; Churilov, L.; Archer, J.S. DBS of Thalamic Centromedian Nucleus for Lennox-Gastaut Syndrome (ESTEL Trial). Ann. Neurol. 2022, 91, 253–267. [Google Scholar] [CrossRef]
- Hodaie, M.; Wennberg, R.A.; Dostrovsky, J.O.; Lozano, A.M. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia 2002, 43, 603–608. [Google Scholar] [CrossRef]
- Lee, K.J.; Shon, Y.M.; Cho, C.B. Long-term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotact. Funct. Neurosurg. 2012, 90, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-N.; Lee, S.-T.; Tsai, Y.-T.; Chen, I.-A.; Tu, P.-H.; Chen, J.-L.; Chang, H.-W.; Su, Y.-C.; Wu, T. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: A long-term follow-up study. Epilepsia 2007, 48, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-S.; Kim, H.J.; Lee, K.J.; Kim, Y.I.; Lim, S.-C.; Shon, Y.-M. Cognitive improvement after long-term electrical stimulation of bilateral anterior thalamic nucleus in refractory epilepsy patients. Seizure 2012, 21, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, G.F.; Sailer, A.; Gunraj, C.A.; Cunic, D.I.; Wennberg, R.A.; Lozano, A.M.; Chen, R. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology 2006, 66, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, C.; Malheiros, J.M.; Battapady, H.; Tannus, A.; Hamani, C.; Covolan, L. The neural response to deep brain stimulation of the anterior nucleus of the thalamus: A MEMRI and c-Fos study. Brain Res. Bull. 2019, 147, 133–139. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, R.S.; Pigott, S.; Tellez-Zenteno, J.F.; Wiebe, S.; Parrent, A. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: Impact on seizures and memory. Epilepsia 2010, 51, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Zenteno, J.F.; McLachlan, R.S.; Parrent, A.; Kubu, C.S.; Wiebe, S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology 2006, 66, 1490–1494. [Google Scholar] [CrossRef]
- Vázquez-Barrón, D.; Cuéllar-Herrera, M.; Velasco, F.; Velasco, A.L. Electrical Stimulation of Subiculum for the Treatment of Refractory Mesial Temporal Lobe Epilepsy with Hippocampal Sclerosis: A 2-Year Follow-Up Study. Stereotact. Funct. Neurosurg. 2021, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.L.; Velasco, F.; Velasco, M.; Jiménez, F.; Carrillo-Ruiz, J.D.; Castro, G. The role of neuromodulation of the hippocampus in the treatment of intractable complex partial seizures of the temporal lobe. Acta Neurochir. Suppl. 2007, 97, 329–332. [Google Scholar] [CrossRef]
- Velasco, M.; Velasco, F.; Velasco, A.L.; Boleaga, B.; Jimenez, F.; Brito, F.; Marquez, I. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia 2000, 41, 158–169. [Google Scholar] [CrossRef]
- Vonck, K.; Sprengers, M.; Carrette, E.; Dauwe, I.; Miatton, M.; Meurs, A.; Goossens, L.; DE Herdt, V.; Achten, R.; Thiery, E.; et al. A decade of experience with deep brain stimulation for patients with refractory medial temporal lobe epilepsy. Int. J. Neural Syst. 2013, 23, 1250034. [Google Scholar] [CrossRef] [Green Version]
- Ghaziri, J.; Tucholka, A.; Girard, G.; Boucher, O.; Houde, J.-C.; Descoteaux, M.; Obaid, S.; Gilbert, G.; Rouleau, I.; Nguyen, D.K. Subcortical structural connectivity of insular subregions. Sci. Rep. 2018, 8, 8596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergeron, D.; Obaid, S.; Fournier-Gosselin, M.-P.; Bouthillier, A.; Nguyen, D.K. Deep Brain Stimulation of the Posterior Insula in Chronic Pain: A Theoretical Framework. Brain Sci. 2021, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hollunder, B.; Baldermann, J.C.; Kibleur, A.; Treu, S.; Akram, H.; Al-Fatly, B.; Strange, B.A.; Barcia, J.A.; Zrinzo, L.; et al. A Unified Functional Network Target for Deep Brain Stimulation in Obsessive-Compulsive Disorder. Biol. Psychiatry 2021, 90, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, C.; Rubin-Kahana, D.S.; Pushparaj, A.; Musiol, M.; Blumberger, D.M.; Daskalakis, Z.J.; Zangen, A.; Le Foll, B. The Insula: A Brain Stimulation Target for the Treatment of Addiction. Front. Pharmacol. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhao, M.; Li, T.; Zhang, C.; Zhou, J.; Wang, M.; Wang, X.; Ma, K.; Luan, G.; Guan, Y. Long-term efficacy and cognitive effects of bilateral hippocampal deep brain stimulation in patients with drug-resistant temporal lobe epilepsy. Neurol. Sci. 2021, 42, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, Z.; Guo, Z.; Zhou, W.; Cai, Z.; Durand, D.M. High frequency stimulation of afferent fibers generates asynchronous firing in the downstream neurons in hippocampus through partial block of axonal conduction. Brain Res. 2017, 1661, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Mohan, U.R.; Watrous, A.J.; Miller, J.F.; Lega, B.C.; Sperling, M.R.; Worrell, G.A.; Gross, R.E.; Zaghloul, K.A.; Jobst, B.C.; Davis, K.A.; et al. The effects of direct brain stimulation in humans depend on frequency, amplitude, and white-matter proximity. Brain Stimul. 2020, 13, 1183–1195. [Google Scholar] [CrossRef]
- Iremonger, K.J.; Anderson, T.R.; Hu, B.; Kiss, Z.H.T. Cellular mechanisms preventing sustained activation of cortex during subcortical high-frequency stimulation. J. Neurophysiol. 2006, 96, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, A.L.; Durand, D.M. High frequency stimulation can block axonal conduction. Exp. Neurol. 2009, 220, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Surbeck, W.; Bouthillier, A.; Weil, A.G.; Crevier, L.; Carmant, L.; Lortie, A.; Major, P.; Nguyen, D.K. The combination of subdural and depth electrodes for intracranial EEG investigation of suspected insular (perisylvian) epilepsy. Epilepsia 2011, 52, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Bouthillier, A.; Surbeck, W.; Weil, A.G.; Tayah, T.; Nguyen, D.K. The hybrid operculo-insular electrode: A new electrode for intracranial investigation of perisylvian/insular refractory epilepsy. Neurosurgery 2012, 70, 1574–1580, discussion 1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaziri, J.; Tucholka, A.; Girard, G.; Houde, J.-C.; Boucher, O.; Gilbert, G.; Descoteaux, M.; Lippé, S.; Rainville, P.; Nguyen, D.K. The Corticocortical Structural Connectivity of the Human Insula. Cereb. Cortex 2017, 27, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Rønborg, S.N.; Esteller, R.; Tcheng, T.K.; Greene, D.A.; Morrell, M.J.; Wesenberg Kjaer, T.; Desai, S.A. Acute effects of brain-responsive neurostimulation in drug-resistant partial onset epilepsy. Clin. Neurophysiol. 2021, 132, 1209–1220. [Google Scholar] [CrossRef] [PubMed]

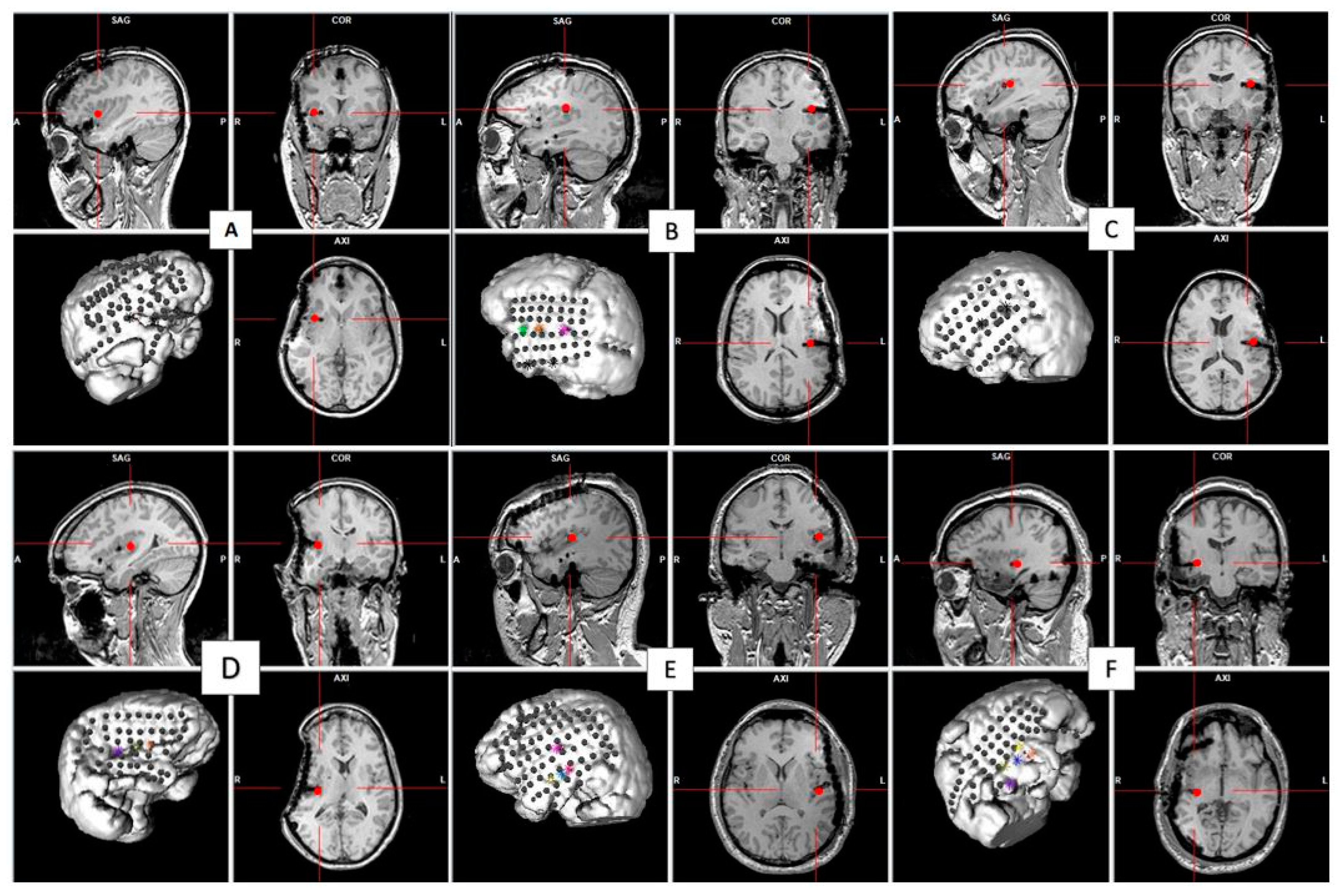
| Patient | Age at Onset of Epilepsy/Age at Invasive EEG | Gender/Dominance | Side and Location of Epileptic Focus | Stimulation Intensity (mA) | Location of Stimulated Insular Contacts |
|---|---|---|---|---|---|
| 1 | 9/25 | Fe/R | R, junction between OF and ant INS | 3.3 | R aINS |
| 2 | 16/47 | M/R | L, P operculum, pINS | 0.4 | L pINS |
| 3 | 5/38 | Fe/R | L, F operculum + mid-INS and aINS | 1.5 | L pINS |
| 4 | 22/32 | Fe/R | R, pINS | 7 | R INS |
| 5 | unknown/25 | M/R | L, FT | 7 | L pINS |
| 6 | 6 months/41 | M/R | R, OF | 6 | R pINS |
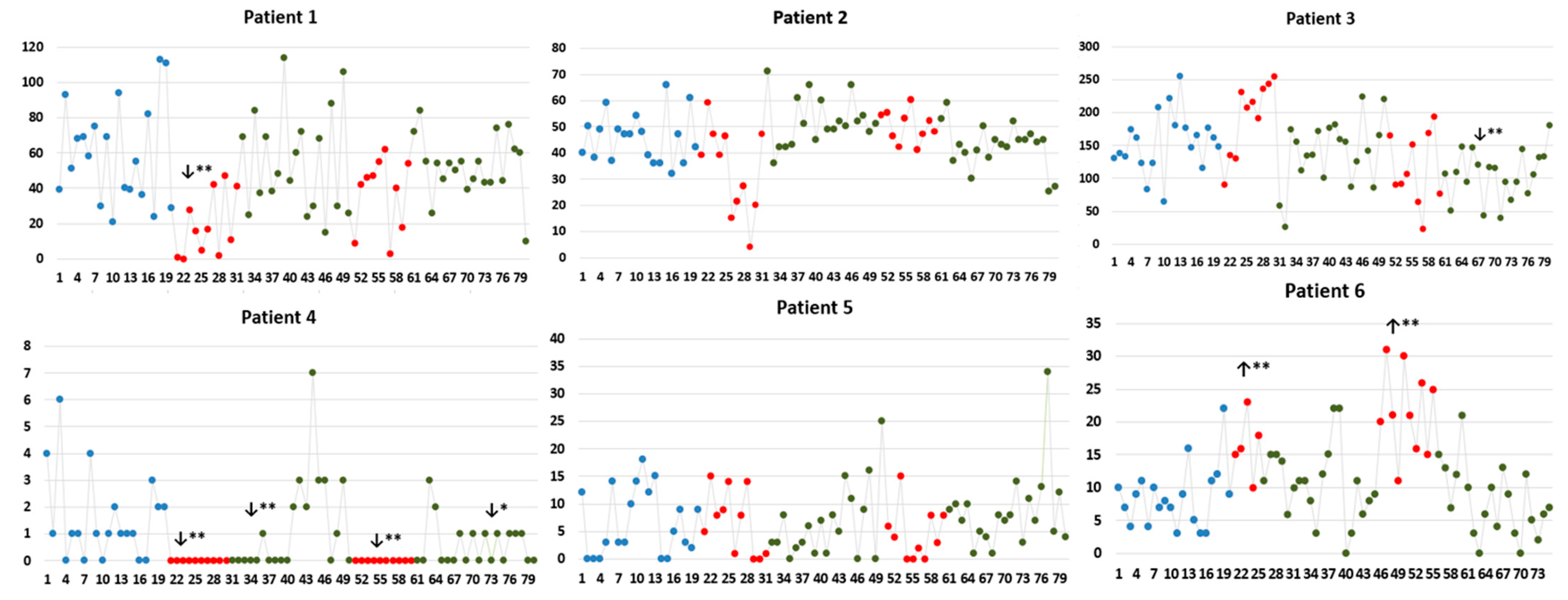
| Patient 1 | IED rate (IED/minute); median (IQR) | ||||||
| IED localizations | B | S1 | PS1 | S2 | PS2 | ||
| 1st 10 min | 2nd 10 min | 1st 10 min | 2nd 10 min | ||||
| OF | 9 (15.75) | 0 (2.25) ↓ (p = 0.004) | 15.50 (9.75) | 20.50 (16) ↑ (p = 0.023) | 17 (19.17) | 22 (18) ↑ (p = 0.013) | 16.50 (18.50) |
| aINS | 1 (3.75) | 0 (0.25) ↓ (p = 0.042) | 0 (0) ↓ (p = 0.003) | 0 (0) ↓ (p = 0.003) | 0 (0) ↓ (p = 0.003) | 0 (0) ↓ (p = 0.003) | 0 (0) ↓ (p = 0.003) |
| OF-aINS | 31 (37.75) | 7 (21.75) ↓ (p = 0.025) | 35 (38.75) | 12.50 (60) | 22.5 (7.0) | 32.5 (17) | 36 (27.25) |
| Patient 2 | IED rate (IED/minute); median (IQR) | ||||||
| IED localizations | B | S1 | PS1 | S2 | PS2 | ||
| 1st 10 min | 2nd 10 min | 1st 10 min | 2nd 10 min | ||||
| T-P operculo-pINS | 46 (13.75) | 24 (15.75) ↓ (p = 0.006) | 45 (20.5) | 51 (7.50) ↑ (p = 0.007) | 49 (8.25) | 37 (14) | 41.50 (6.5) |
| lateral T | 0 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (2) | 0 (1) | 0 (0) |
| Synchronous | 2 (3.75) | 0 (1) ↓ (p = 0.03) | 0 (1) ↓ (p = 0.03) | 0 (2.25) | 0 (1) ↓ (p = 0.03) | 5 (9.25) ↑ (p = 0.038) | 2 (3.25) |
| Patient 3 | IED rate (IED/minute); median (IQR) | ||||||
| IED localizations | B | S1 | PS1 | S2 | PS2 | ||
| 1st 10 min | 2nd 10 min | 1st 10 min | 2nd 10 min | ||||
| aINS | 113 (54.75) | 126 (92.25) | 106.50 (8.25) | 123.5 (67.26) | 67 (86.5) | 94 (52.5) | 81.5 (60.6) |
| IFG-aINS | 32 (36.5) | 60 (40) | 20 (20.75) | 29.5 (34.75) | 29.5 (19) | 18 (14.25) ↓ (p = 0.022) | 22 (21.25) |
| STG-aINS | 0 (4.25) | 0 (2) | 0 (4.25) | 0 (1.75) | 0 (1.25) | 0 (0) | 0 (2.0) |
| synchronous | 1 (11.75) | 13 (41) | 0 (1) | 0 (2) | 0 (5.25) | 0.5 (3.25) | 0 (1.25) |
| Patient 4 | IED rate (IED/minute); median (IQR) | ||||||
| IED localizations | B | S1 | PS1 | S2 | PS2 | ||
| 1st 10 min | 2nd 10 min | 1st 10 min | 2nd 10 min | ||||
| T-P opercula-pINS | 1.6 (2.75) | 0 (0) ↓ (p < 0.001) | 0 (0) ↓ (p < 0.001) | 2 (3.0) | 0 (0) ↓ (p < 0.001) | 0 (1.25) | 0.5 (1.0) ↓ (p = 0.040) |
| Synchronous | 0 (0) | 0 (0) | 0 (0) | 0 (0.25) | 0 (0) | 0 (0) | 0 (0) |
| Patient 5 | IED rate (IED/minute); median (IQR) | ||||||
| IED localizations | B | S1 | PS1 | S2 | PS2 | ||
| 1st 10 min | 2nd 10 min | 1st 10 min | 2nd 10 min | ||||
| lateral T | 2.5 (4.5) | 1.5 (7.25) | 0 (1.25) ↓ (p = 0.010) | 3 (5.5) | 2.5 (5.75) | 2 (4.25) | 4.5 (5.0) |
| lateral prefrontal | 0 (1) | 0 (0.25) | 1.5 (2.25) ↑ (p = 0.005) | 0.5 (2.25) | 0 (1.0) | 0.5 (2.0) | 0.5 (2.0) |
| Synchronous | 1 (6.5) | 0 (1.25) | 0 (1.25) | 3 (5.5) | 0 (0.75) ↓ (p = 0.044) | 2 (4.25) | 1 (4) |
| Patient 6 | IED rate (IED/minute); median (IQR) | ||||||
| IED localizations | B | S1 | PS1 | S2 | PS2 | ||
| 1st 10 min | 2nd 10 min | 1st 10 min | 2nd 10 min | ||||
| OF | 6.50 (4.50) | 16 (7.0) ↑ (p = 0.001) | 8 (6.50) | 9 (10.75) | 21 (11.25) ↑ (p < 0.001) | 8 (8.25) | 5 (5) |
| pINS | 0 (1) | 0 (0) | 0 (1) | 0 (0) | 0 (0) | 0 (0.25) | 0 (0) |
| Posterior T | 1.5 (3) | 0 (3) | 3.5 (4.25) | 1.5 (3.25) | 0 (0.75) | 0.5 (3.5) | 0 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.P.Y.; Dionne, A.; Toffa, D.; Bergeron, D.; Obaid, S.; Robert, M.; Bouthillier, A.; Assi, E.B.; Nguyen, D.K. Acute Effects of High-Frequency Insular Stimulation on Interictal Epileptiform Discharge Rates in Patients with Refractory Epilepsy. Brain Sci. 2022, 12, 1616. https://doi.org/10.3390/brainsci12121616
Tran TPY, Dionne A, Toffa D, Bergeron D, Obaid S, Robert M, Bouthillier A, Assi EB, Nguyen DK. Acute Effects of High-Frequency Insular Stimulation on Interictal Epileptiform Discharge Rates in Patients with Refractory Epilepsy. Brain Sciences. 2022; 12(12):1616. https://doi.org/10.3390/brainsci12121616
Chicago/Turabian StyleTran, Thi Phuoc Yen, Antoine Dionne, Denahin Toffa, David Bergeron, Sami Obaid, Manon Robert, Alain Bouthillier, Elie Bou Assi, and Dang Khoa Nguyen. 2022. "Acute Effects of High-Frequency Insular Stimulation on Interictal Epileptiform Discharge Rates in Patients with Refractory Epilepsy" Brain Sciences 12, no. 12: 1616. https://doi.org/10.3390/brainsci12121616
APA StyleTran, T. P. Y., Dionne, A., Toffa, D., Bergeron, D., Obaid, S., Robert, M., Bouthillier, A., Assi, E. B., & Nguyen, D. K. (2022). Acute Effects of High-Frequency Insular Stimulation on Interictal Epileptiform Discharge Rates in Patients with Refractory Epilepsy. Brain Sciences, 12(12), 1616. https://doi.org/10.3390/brainsci12121616






