Abstract
The pathogenesis of Parkinson’s disease (PD) is complex, multilayered, and not fully understood, resulting in a lack of effective disease-modifying treatments for this prevalent neurodegenerative condition. Symptoms of PD are heterogenous, including motor impairment as well as non-motor symptoms such as depression, cognitive impairment, and circadian disruption. Aging and stress are important risk factors for PD, leading us to explore pathways that may either accelerate or protect against cellular aging and the detrimental effects of stress. Cortisol is a much-studied hormone that can disrupt mitochondrial function and increase oxidative stress and neuroinflammation, which are recognized as key underlying disease mechanisms in PD. The more recently discovered klotho protein, considered a general aging-suppressor, has a similarly wide range of actions but in the opposite direction to cortisol: promoting mitochondrial function while reducing oxidative stress and inflammation. Both hormones also converge on pathways of vitamin D metabolism and insulin resistance, also implicated to play a role in PD. Interestingly, aging, stress and PD associate with an increase in cortisol and decrease in klotho, while physical exercise and certain genetic variations lead to a decrease in cortisol response and increased klotho. Here, we review the interrelated opposite actions of cortisol and klotho in the pathogenesis of PD. Together they impact powerful and divergent mechanisms that may go on to influence PD-related symptoms. Better understanding of these hormones in PD would facilitate the design of effective interventions that can simultaneously impact the multiple systems involved in the pathogenesis of PD.
1. Introduction
Parkinson’s disease (PD) is the fastest growing neurological disorder globally [,]. Fueled by aging, the number of people with PD is expected to exceed 12 million by 2040 []. PD is characterized as a movement disorder, resultant from progressive neurodegeneration of dopamine neurons in the substantia nigra pars compacta and its projections. However, it is increasingly apparent that other brain regions are affected, e.g., decreased metabolism in the prefrontal, occipital, and parietal cortices as well as changes in the lentiform nucleus, thalamus, pons and cerebellum []. Dopaminergic and non-dopaminergic pathology contribute to a wide range of non-motor symptoms (NMS), including cognitive impairment, mood disorder, circadian disruption, autonomic dysfunction, fatigue, and apathy [,,]. Despite the heterogeneity in pathogenesis and symptomatology, aging remains the single most important risk factor for development of PD and of NMSs that disrupt the quality of life [,,].
Mechanisms of aging and neurodegeneration are thought to be interrelated with chronic stress. Both aging and chronic stress are pertinent to the pathogenesis of PD and impact mitochondrial dysfunction, oxidative stress, inflammation, and changes in metabolism []. These pathways result in changes in cellular function such as decreased ATP synthesis, increase production of reactive oxygen species (ROS) and Ca+ accumulation, microglia response, and increased proinflammatory cytokines and infiltrating immune cells, all contributing to cell apoptosis. These processes occur in vulnerable brain regions and interplay with genetic and environmental risk factors to contribute to the development and progression of PD [,,]. Here, we review the actions of two hormones known to be implicated in the aging and stress processes and discuss their potential roles in PD pathophysiology. Cortisol is a critical hormone involved in normal stress responsivity, physiological homeostasis, and circadian function. Excess secretion is associated with a wide range of maladaptive correlates of aging and chronic stress [,,,]. Relatively newly discovered, klotho is a longevity hormone that delays aging and enhances cognition [,,]. In this review, we highlight the yin-yang roles of cortisol and klotho in key aging and stress pathways and how this may associate with progression and symptoms of PD.
2. Function of Cortisol and Klotho
2.1. Cortisol
Cortisol secretion is the product of hypothalamic-pituitary-adrenal (HPA) axis activation. It is a powerful steroid hormone that can pass into every cell of the body, with genomic and non-genomic actions affecting mitochondrial, immune, and metabolic function []. The brain is a prominent target for cortisol and thus a central structure for adaptation to stress. A wide brain network involving the hippocampus, amygdala, prefrontal cortex and brainstem nuclei are involved in HPA axis activation in response to acute or chronic stress []. The underlying basal secretory activity of the HPA axis is regulated by the hypothalamic central pacemaker: the suprachiasmatic nucleus (SCN). The SCN transmits its circadian signal to peripheral clock genes via neural and hormonal mechanisms, with cortisol secretion playing a significant role, synchronizing circadian oscillations throughout the body [].
Cortisol has pleiotropic effects on the brain, affecting mood, behavior, cognition, and programming of the stress response []. Cortisol is necessary for neuronal differentiation, integrity, and growth, as well as synaptic and dendritic plasticity [,]. These processes in turn support brain functions such as decision-making, reward-based behavior, motor control, visual information processing, learning and memory, and energy regulation. Cortisol actions are mediated by the glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs). In addition to rapid non-genomic mechanisms, cortisol influences brain functions by activating GR-mediated gene transcription []. Some of these target genes code for neurotrophic factors and their receptors, anti- and pro-inflammatory markers, signal transduction, neurotransmitter catabolism, energy metabolism, and cell adhesion [,,]. Stress-induced shifts in cortisol associate with time- and region-dependent changes in neuronal activity to promote the brain’s adaptation to the continuously changing environment in the short and long-term []. A dysfunctional HPA axis is thought to occur in PD, leading to GR desensitization and high circulating cortisol concentrations [].
2.2. Klotho
KLOTHO (KL) is a serendipitously discovered gene on chromosome 13 found to have profound effects on lifespan []. Deficiency of klotho protein in mice severely shortens lifespan and prompts signs of premature aging while overexpression of klotho increases lifespan by ~30%. Since its initial discovery, increased levels of klotho have been associated with longevity in several populations and decreased levels of klotho have been associated with aging-related diseases including cancer, cardiovascular disease, kidney disease, and recently neurodegenerative diseases [,,,,,,].
Klotho is expressed primarily in the kidneys and choroid plexus [,,]. Klotho mRNA is also detectible in many other brain regions, including cortex, hippocampus, cerebellum, striatum, substantia nigra, olfactory bulb, and medulla [,,,]. Klotho’s actions are complex and multidimensional—it suppresses insulin and Wnt signaling [,], regulates ion channel clustering and transport [], modulates N-methyl-d-aspartate receptor (NMDAR) signaling [] and promotes fibroblast growth factor (FGF) function []. Klotho, linked with FGF23, is important for regulation of calcium, phosphate, and vitamin D homeostasis [,,,,,]. Klotho linked with FGF21 is involved in stimulation of the starvation response, activating the HPA axis and sympathetic nervous system, as well as increasing intracellular klotho expression in the SCN within the hypothalamus [].
3. Cortisol and Klotho in Neurodegenerative Disease
Cortisol and klotho have relevance to various neuropsychiatric and neurodegenerative disorders. Cortisol levels are increased in individuals with depression, sleep disturbances, and neurodegenerative diseases like AD and PD [,,,,,]. Conversely, lower circulating klotho levels are reported in bipolar disorder, depression, multiple sclerosis, temporal lobe epilepsy, AD and recently, PD [,,,,,].
Accumulating evidence suggests that the HPA axis is dysregulated in PD. Cortisol levels are found to be increased in toxin animal models of PD []. Elevated cortisol levels also induce impairment of motor function and accelerate nigral neuronal loss in rats exposed to chronic stress and subsequent increase in cortisol []. Individuals with PD have elevated cortisol secretion in blood and saliva, especially in the morning [,,]. More recently, glucocorticoid concentrations measured in the hair of PD patients showed an excess of cortisone, the main cortisol metabolite, but not cortisol itself [].
Klotho was initially linked to neurodegenerative diseases in studies of AD. Klotho is decreased in the CSF of AD patients []. Higher klotho is also associated with reduced amyloid-beta (Aβ) burden and improved cognition in populations at risk for AD []. Emerging studies are now connecting klotho with PD. Klotho-insufficient mice develop neurodegeneration of mesencephalic dopaminergic neurons in substantia nigra and ventral tegmentum area, while klotho overexpression protects dopaminergic neurons against oxidative injury [,,]. Exogenous klotho administration demonstrates neuroprotective potential in toxin rat models of PD through alleviation of astrogliosis, apoptosis, and oxidative stress []. One study reported that while plasma klotho levels were not significantly different between people with PD and healthy controls, klotho levels were lower in men compared to women with PD []. This is interesting since sex is an important biological factor in development and phenotype of PD as well as hormonal regulation in general. Another study looking at two independent cohorts of people with PD found that CSF levels of klotho were lower in people with PD compared to healthy controls []. A recent perspective by Grillo et al. (2022) also points out that enteric cells express klotho, and both blood and enteric levels of klotho are altered in the setting of gut disease or inflammation []. The authors go on to suggest that since PD pathology is hypothesized to start in the enteric nervous system, this poses an important need to assess klotho in the gastrointestinal tract of people with PD and evaluate whether modulation of klotho in the gut may serve as a disease-modifying strategy.
Lastly, it is important to note how the main PD symptomatic treatment, levodopa, is associated with cortisol and klotho. Administration of levodopa decreases HPA axis activity, thereby decreasing cortisol levels [,]. It is currently unclear if levodopa affects klotho levels or vice versa and future studies should take this into account.
4. Factors Modulating Cortisol and Klotho Regulation
4.1. Aging
Aging is the most critical risk factor for PD [,], yet the relationship between molecular/cellular processes of healthy aging and those of PD pathogenesis remain unclear. It can be hypothesized that specific regions of the PD brain (e.g., dopaminergic neurons in substantia nigra pars compacta) undergo localized, accelerated aging []. Aging links together several pathological mechanisms known to play a significant role in PD—from increased inflammation and oxidative stress to mitochondrial dysfunction and dysregulation of lysosomal, proteasomal and autophagic functions– and all likely to contribute to neurodegeneration. The concept of “inflammaging” has been proposed as a principal mechanism in PD [] and describes the sustained systemic inflammatory state that develops with advanced age []. This chronic inflammation is thought to result from exposure to chronic stressors and/or imbalance between inflammatory and anti-inflammatory networks.
The convergence of cortisol and klotho along the pathways of aging has notable implications for PD. Cortisol levels decrease in decades 20s–30s, are relatively stable in 40-50s, and increase after age 60 []. Elevated cortisol has been reported in age-related illnesses such as cardiovascular disease, type II diabetes mellitus, osteoporosis, and cognitive impairment [,,,]. In contrast, klotho levels are highest at birth in humans, with levels 7-fold higher than adulthood levels, and decline after age 40 [,]. Several studies link klotho to increased lifespan and better health outcomes, including decreased risk for cardiovascular disease and stroke, decreased macrovascular complications in patients with type 2 diabetes, and improved grip strength [,,]. Klotho has also recently been included in a panel of biomarkers that may predict frailty in the elderly [].
4.2. Stress
The notion that chronic stress, in addition to aging, may play a role in the pathogenesis of PD has been controversial over the years but, despite some inconsistencies in the literature, is now generally recognized []. In one population-based cohort study of over 2 million males, higher job demands and expectations increased PD risk []. In another study, the risk of PD significantly increased with the number of exposures to stressful events []. Post-traumatic stress disorder and adjustment disorder, both indicating occurrence of significant stressors, also associated with increased risk of PD, independent of comorbid depression or anxiety [,].
Stress is known to affect functions of the limbic system such as learning, memory and emotions []. The hippocampus has extensive distribution of GRs and plays a crucial role in the biological effects of chronic stress [,]. Stress and hypercortisolemia also disrupt sleep [], which exerts powerful effects on the hippocampus and affects initial learning and memory consolidation []. Recent evidence shows that stress also modulates motor system function []. Since most parts of the motor system express GRs, their circuits are susceptible to the influence of cortisol. Stress can modulate movement through activation of the HPA axis and via stress-associated emotional changes. In PD mouse models, chronic stress exposure worsens motor deficits, aggravates the neurodegeneration of the nigrostriatal system, and completely blocks compensatory recovery of motor tasks []. A recent viewpoint by van der Heide et al. (2020) proposes a model of how chronic stress in patients with PD, resulting in higher cortisol levels, can lead to both higher susceptibility for depressive and anxiety disorders and a more rapid progression of the disease []. The authors review evidence on how chronic stress reduces levels of brain derived neurotrophic factor (BDNF), inducing atrophy in key brain regions of mood and behavior, and creates a proinflammatory environment that increases nigrostriatal cell loss.
Given klotho’s role in healthy aging, it is no wonder that it too has been linked to stress. Klotho levels are reported to be lower in caregivers with chronic high stress and show an age-related decline []. Klotho genetic variations that alter klotho levels influence the effects of stress on cellular aging, as evidenced by changes in multiple biomarkers of aging, including telomere length, CRP levels, metabolic dysfunction and white matter microstructural integrity []. Mice with chronic stress demonstrate downregulation of klotho in the nucleus accumbens (NAc) and depressive-like behavior [], responses modulated by Klotho regulation of NMDARs.
4.3. Genetics
PD pathogenesis is mediated by an interaction between multiple environmental and genetic factors. The role of cortisol and klotho in PD may also be dependent on genetic variations that dictate levels or function of these two hormones.
As discussed previously, cortisol functions by binding to GRs and MRs. Genetic variation in GR has been postulated to play a role in the physiological response to endogenous cortisol. Over 3000 single nucleotide polymorphisms (SNPs) in the GR gene have been documented. Most studies show that BclI and N363S gene variants are associated with clinical measures of increased glucocorticoid sensitivity, while the ER22/23EK and GR-9β are associated with decreased glucocorticoid sensitivity []. The ER22/23EK polymorphism links with improved survival and lower levels of the inflammatory marker C-reactive protein (CRP) []. BclI has consistently been shown to be associated with a higher susceptibility to major depression []. It is currently unclear if and how these genotypes impact PD. Lastly, polymorphisms in the catechol-O-methyl-transferase (COMT) gene have also been associated with glucocorticoid responsivity and cortisol levels. In particular, individuals with the Met/Met COMT homozygote polymorphisms are more sensitive to stressful events and have higher cortisol responses []. Interestingly in people with PD, Met/Met COMT homozygote polymorphism associates with lower IQ score and greater motor severity of disease [].
Genetic variations in the KL gene may influence systemic klotho levels or its function. KL variant rs9315202 downregulates klotho mRNA expression and associates with advanced epigenetic age and elevated aging markers such as CRP [,]. There is also a well-studied protective variant, termed KL-VS, that contains two SNPs, rs95536314 and rs9527025, in complete linkage disequilibrium. Carrying one copy of KL-VS increases klotho levels [,], while carrying two copies, decreases it []. KL-VS heterozygosity is associated with longer lifespan [], slowed epigenetic age [], and higher cognitive function [,] in most but not all populations. In a population at risk for dementia, KL-VS allele attenuates Aβ burden and associates with reduced risk of conversion to mild cognitive impairment or AD []. In PD, KL-VS heterozygotes have higher CSF klotho levels; however, the haplotype itself is associated with shorter interval between onset of PD and progression to MCI and worse motor phenotype []. Therefore, it remains to be clarified how genetic variations in KL gene may affect function of the klotho protein and affect individuals with PD.
It is unknown whether there are direct correlations between cortisol or klotho and the genes linked to PD. However, as both hormones are involved in mechanisms that become aberrant in PD, there may be undiscovered connections. For example, both cortisol and klotho influence mitochondrial function in opposite ways and may interact with genetic variations in genes linked to mitochondrial dysfunction in PD (i.e., Parkin, PINK1, DJ-1, LRRK2).
4.4. Physical Exercise
Just as cortisol and klotho are sensitive to aging and psychological stress, they are also responsive (in the opposite direction) to external stimuli that promote healthy aging, such as physical activity. The past decade has produced much evidence to support that physical exercise is potentially neuroprotective in PD.
Physical exercise can improve dysregulated cortisol levels in healthy individuals and those with major depressive disorder []. High intensity exercise (>90% heart rate reserve) decreases fluctuations in salivary cortisol []. Another study showed that exercising intensely (70% heart rate reserve) suppresses the subsequent cortisol response to a psychosocial stressor []. Smyth et al. have shown that high intensity treadmill exercise in individuals with PD reduces cortisol secretion during the post-awakening period after nocturnal sleep []. Yoga and dance movement therapy also lead to decreased cortisol levels [,].
Contrarily, klotho levels are amplified by treadmill exercise in both young and aged mice [,]. In humans, higher levels of klotho are associated with superior lower extremity strength and functioning [,] Klotho levels also tend to be higher in exercise-trained individuals compared to their untrained counter-partners []. In healthy adults, various exercise programs (endurance, resistance, high intensity interval training) boost plasma klotho levels either acutely or after a 12–16-week training period [,,,,]. Recent study also finds that yoga, consisting of deep breathing exercises, meditation, and postures, upregulates expression of the KL gene [].
5. Candidate Mechanisms of Cortisol and Klotho Interactions in PD
Together cortisol and klotho represent powerful yet complementary mechanisms by which life stress can be internalized and aging can be regulated. We go on to highlight 4 candidate mechanisms where klotho and cortisol may be competing in the life course of PD.
5.1. Mitochondrial Dysfunction and Oxidative Stress
Mitochondrial dysfunction (altered morphology, turnover and transport) plays a fundamental role in the pathogenesis of PD by chronic production of reactive oxygen species and induction of α-synuclein misfolding, promoting neurodegeneration in the substantia nigra []. The causes of mitochondrial dysfunction are complex and multipart, including damage to mitochondrial DNA, environmental neurotoxins, and mutations of the PINK1, DJ-1, and Parkin genes linked to PD [,]. In addition, mitochondrial function is intimately interlinked with other cell processes including iron, copper, and glutathione metabolism []. Dysfunction in any one process impacts the others, leading to a disruptive vicious cycle driving neuronal cell death and pathology. Mitochondrial dysfunction also occurs as a consequence of aging [] and chronic stress [,] with changes linked to increased inflammatory responses. This is because, in addition to their other roles, mitochondria are now considered central hubs in regulating innate immunity and inflammatory responses [].
The hormone cortisol is intricately coupled with mitochondrial function. Once the HPA axis is activated, it is synthesized within the mitochondria of the zona fasciculata of the adrenal cortex and has potent reciprocal effects on mitochondrial function throughout the body []. In this way, mitochondria are both mediators and targets of the main stress axis, with cortisol as a liaison for whole body mitochondria-to-mitochondria communication to regulate energy metabolism. Aging and stress-associated increase in cortisol can reduce the activity of specific mitochondrial electron transport chain complexes and increase mitochondrial oxidative stress []. In mouse models of PD, psychological stress diminishes up to 50% of mitochondrial respiration and glycolysis and links to cell death and exacerbation of motor symptoms [].
Age-related declines in klotho can drive dysfunctional mitochondrial bioenergetics in skeletal muscle and kidney whilst systemic delivery of exogenous klotho rejuvenates and enhances function [,]. Mitochondria not only regulate the normal ROS level, but excessive ROS can also directly damage mitochondria and lead to apoptosis and cell death. Klotho induces the expression of the manganese superoxide dismutase (MnSOD) protein, a mitochondrial antioxidant enzyme that detoxifies superoxides, and thus reduces ROS []. A study in human stem cells shows that klotho attenuates cellular damage and cell apoptosis induced by oxidative stress by protecting mitochondrial structure []. Klotho is also known to play a significant role in brain metabolism as an antioxidant [,]. Reduction at these levels result in the inability of astrocytes to rapidly modify their metabolic activity to support adjacent neurons, making them more vulnerable to neurodegeneration [].
5.2. Neuroinflammation
Several lines of evidence from humans and animal models support the involvement of inflammation in the onset and progression of PD. While inflammation may be a consequence of neuronal loss in PD, the chronic inflammatory response may also contribute to the progression of PD. Neuroinflammation stems from crosstalk between neurons, microglia, astroglia and endothelial cells [], which are susceptible to α-synuclein aggregates and mitochondrial dysfunction. Under disease conditions, the homeostatic functions of the microglia and astroglia are disrupted, leading to reduced secretion of neurotrophic factors, increased secretion of proinflammatory cytokines (interleukin (IL)-6, IL1β, Tumor Necrosis Factor α (TNFα), interferon (IFN)-γ, etc.)) and chemokines (CCL2, CXCL1, etc.) and increased receptor expression for proinflammatory markers and major histocompatibility complex (MHC-I) in microglial cells []. Additionally, peripheral immune cells (such as CD4+ T cells) are recruited to the brain parenchyma, further augmenting the proinflammatory environment.
Cortisol plays a significant and beneficial role in regulation of inflammation, but old age and chronic stress are associated with dysregulation of the HPA axis and cortisol secretion []. Cortisol and pro-inflammatory cytokines interact on multiple levels. Under normal conditions, cortisol inhibits the immune system cells that produce peripheral cytokines. It also inhibits transcription and action of many of the pro-inflammatory cytokines including IL-1β, IL-6, and TNFα [,,]. In a reciprocal relationship, cytokines can also influence glucocorticoid secretion, availability, and signaling. IL-1β and IL-6 can activate the HPA axis directly, while IL-1 and TNFα can impair cortisol signaling by interfering with GR phosphorylation [,]. In settings of chronic stress, excessive cortisol secretion leads to compensatory down-regulation or resistance of the GR and its anti-inflammatory actions, resulting instead in a pro-inflammatory milieu facilitating a wide range of disease risks including neurotoxicity [,]. In this scenario, increased levels of cortisol are associated with an increase in pro-inflammatory cytokines such as IL-6 []. Therefore, cortisol can have dual effects—it can limit inflammation under normal conditions but promote inflammation under conditions of chronic stress.
Recent studies suggest that klotho could also play a role in mediating the interface between the brain and immune system in the choroid plexus. Selectively reducing klotho within the choroid plexus of mice triggers inflammation and enhances activation of innate immune cells []. As a separate pathway, klotho suppresses activation of macrophages by enhancing FGF23 signaling []. Klotho also decreases activation of NF-κB and influences expression of the pro-inflammatory cytokines, IFNγ and TNFα [,], the latter being a ‘master regulator’ of production of pro-inflammatory cytokines. To counter inflammation, klotho increases production of IL-10, which is responsible for inhibiting the expression of pro-inflammatory cytokines such as TNFα [].
A key event in the neuroinflammatory processes is the activation of inflammasomes, multiprotein complexes that mediate pro-inflammatory cytokine secretion and maturation. The inflammasome component NLRP3 is strongly linked to neuroinflammation. Klotho overexpression inhibits the NLRP3/caspase signaling pathways and enhances cognition in animal models of neurodegenerative disease []. In contrast, high cortisol levels activate NLRP1 and NLRP3 inflammasomes and promote neuroinflammation and neuronal injury [,].
5.3. Insulin Resistance
Insulin and insulin-like growth factor 1 (IGF-1) signaling represent an evolutionary conserved pathway of longevity. Oxidative stress is implicated in the onset and progression of insulin resistance and type 2 diabetes [], which is a prominent feature of normal aging and a preclinical indicator in many neurodegenerative disorders []. Peripheral insulin resistance is related to impairment of central insulin signaling and reported to be an early etiological factor in development of PD [,]. It is also associated with more rapid progression of PD disease and related cognitive impairment and dementia []. Functional brain imaging in PD further shows hypometabolism in the inferior parietal cortex and the caudate nucleus, which correlate with cognitive deficits and motor symptoms, respectively []. In PD, insulin resistance is proposed to lead to a state of bioenergetic failure and hypometabolism in the brain that may promote neurotoxicity [].
Elevated cortisol is a major causal candidate for the development of insulin resistance with aging. It is well-known that while insulin exerts anabolic actions, cortisol exerts catabolic actions and the two hormones counteract each other in many metabolic functions, from glucose utilization to lipid storage []. On the other hand, klotho deficiency decreases insulin production and increases insulin sensitivity [,]. While the mechanisms of this are not entirely clear, it is known that klotho suppresses the downstream signaling pathway of the IGF-1 reception and insulin receptor substrate without directly binding to these receptors. Insulin also increases shedding of klotho, thereby increasing circulating klotho [].
5.4. Vitamin D Metabolism
Another route by which cortisol and klotho may contribute to the multifactorial toxic cycle implicated in PD is via their actions on the neuroprotective hormone vitamin D. Vitamin D is a fat-soluble hormone that can pass the blood–brain barrier, supporting its significance in the central nervous system. Vitamin D insufficiency is associated with an increased risk of several CNS diseases including PD [,]. Vitamin D is reported to regulate more than 200 genes, influencing a variety of cellular processes such as neurotransmission neuroprotection, and downregulation of inflammation and oxidative stress []. It stimulates expression of many neurotrophic factors including neurotrophin 3 (NT-3), BDNF, glial cell-derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), and neuroprotective cytokine IL-34 [].
The vitamin D receptor is found throughout the human brain but crucially, is abundant in the substantia nigra pars compacta, the primary target of neurodegeneration in PD []. Additionally, 1α-hydroxylase—the enzyme that converts vitamin D to its active form,1,25(OH)2D3—is highly expressed in the substantia nigra, suggesting that vitamin D may be directly or indirectly related to the pathogenesis of PD via loss of protection for vulnerable dopaminergic neurons in this brain region []. In the past two decades, a high prevalence of vitamin D deficiency has been noted in individuals with PD []. Vitamin D concentrations also negatively correlate with PD risk and disease severity []. A small but significant association between vitamin D status at baseline and disease motor severity at 36 months has been reported []. Higher vitamin D concentrations link to better cognitive function and mood in individuals with PD [,]. Unfortunately, a (somewhat limited) trial of Vitamin D supplementation did not appear to improve PD symptoms [].
Vitamin D is now suggested to be a biomarker of healthy aging with a strong association between low levels and higher all-cause mortality with large and significant effect sizes in multiple studies []. Cortisol has an antagonist relationship with vitamin D. Higher cortisol levels correlate with lower vitamin D levels []. Several studies also suggest that vitamin D may regulate the HPA axis. In hippocampal cell cultures, vitamin D suppresses glucocorticoid-induced transcription and cytotoxicity []. In the CNS, the most intense staining for vitamin D receptor and activating enzyme is described to be in the hypothalamus, including in the paraventricular nucleus (PVN) containing the corticotrophin releasing hormone (CRH)-positive neurons []. These neurons also stain positive for vitamin D 24-hydroxylase, and therefore are likely vitamin D responsive [].
The anti-aging protein klotho plays a key role in regulating vitamin D metabolism. Membrane bound klotho is a cofactor for FGF23. Together, they form the receptor complex instrumental in Vitamin D production []. The biological functions of Vitamin D and klotho are highly intertwined because vitamin D induces the expression of klotho, and klotho keeps vitamin D levels in check. Klotho inhibits 1α-hydroxylase to decrease the active form of vitamin D—1-25(OH)2 D3, and increases activity of 24-hydroxylase, which converts both vitamin D and 1-25(OH)2 D3 into 24-hydroxylated products targeted for excretion. Lastly, low vitamin D levels are associated with depression and chronic stress, both conditions also linked to decreased klotho and elevated cortisol [,,].
6. Cortisol and Klotho Associations with PD Symptomatology
6.1. Mood and Cognition
Initial studies revealed that cortisol and klotho may influence non-motor symptoms of PD. Cortisol levels have most commonly been correlated with neuropsychiatric symptoms. A large number of people with Major Depressive Disorder (MDD) show abnormalities in the HPA axis functioning, with coinciding increased plasma levels of cortisol []. MDD patients also show neurochemical changes in CRH in the PVN, a structure now known to contain inclusions of α-synuclein, hallmark of PD pathology []. In individuals with PD, cortisol has been shown to correlate with the severity of depression [] and with prevalence of anxiety and anhedonia []. In PD patients with impulse control disorders, increased cortisol is associated with more risk-taking behavior [].
There is a growing body of evidence that increased cortisol is associated with late-life cognitive decline in normal aging in people with pre-clinical or clinical AD [,,]. In non-demented patients, high cortisol correlates with decreased total brain volume, particularly in grey matter, and poorer cognitive function [,]. Elevated cortisol has also been linked to hippocampal atrophy, correlating with memory dysfunction []. Studies are needed to evaluate the link between cortisol and cognition in PD.
While klotho levels have not been related to psychiatric symptoms in PD itself, low klotho levels have been associated with depression []. Interestingly, a small exploratory study also found that CSF klotho levels are increased by electroconvulsive treatment for depression []. Lastly, KL gene polymorphisms can influence responsiveness to selective serotonin reuptake inhibitors (SSRIs) in people with depression [].
Klotho has been shown to confer cognitive resilience in healthy aging and neurodegenerative disease. In animal models, klotho overexpression increases long-term potentiation and enhances spatial learning and memory []. In normal aging individuals, carrying the KL genetic variant (KL-VS), resulting in higher system klotho protein levels, links to enhanced cognition and enhanced functional brain connectivity [,]. Higher klotho levels also associate with enhanced volume of dorsolateral prefrontal cortex, an area that drives executive function []. Clinical studies studying klotho in relation to cognition in PD are lacking but initial studies reveal that acute elevation of klotho by peripheral delivery is sufficient to restore cognition in transgenic mouse models of PD [].
6.2. Circadian Rhythm
In recent years, it has become increasingly apparent that the circadian rhythm influences PD, with patients experiencing diurnal fluctuations in motor and non-motor symptoms, despite stable pharmacokinetics of dopaminergic medications []. While mechanisms behind this remain unclear, it is known that neurodegeneration affects the central structures responsible for sleep and wakefulness, which may in turn affect input to the hypothalamic SCN, housing the molecular clock of the circadian system. This molecular clock consists of core clock genes, and disruption in their function in the pathogenesis of PD has recently gained attention []. In people with PD, degeneration of the dopamine containing cells in the retina may further affect input needed for alignment of dark/light cycles []. Lastly, dopaminergic therapy has a bidirectional relationship with circadian rhythm—responsiveness of motor symptoms to medication declines later in the day and medication leads to uncoupling of circadian and sleep regulation [,,].
The circadian pattern of cortisol secretion provides a key signal from the SCN to peripheral clock genes. PD-associated changes in HPA axis function leads to a flatter circadian profile for cortisol and signaling to peripheral clock genes is compromised with resultant circadian disorder [,]. It is interesting to note that chronic kidney disease is associated with dysregulation of the SCN [], and kidney disease is a risk factor for PD.
Unlike the hormone cortisol, there is not much written about klotho and circadian function. An early report in healthy humans found that serum klotho showed a circadian rhythm with falling levels in the evening, a marked nadir at midnight and levels rising again by the morning []. No such circadian variation in klotho has been found in human CSF [] or in the serum of healthy rats []. However, a relationship between klotho and sleep appears more robust with subjective sleep quality being positively associated with klotho in sedentary middle-aged adults []. Lower levels of klotho are also reported in people with obstructive sleep apnea and associated with overnight markers of hypoxemia []. Klotho levels are also decreased with excessive sleep duration [], which is known to increase the risk of inflammatory diseases.
6.3. Motor Symptoms
When looking at healthy aging populations, cortisol negatively correlates with grip strength [] while lower levels of klotho associate with decreased grip strength and knee strength [,]. In people newly diagnosed with PD, higher cortisol levels correlate with greater motor burden of disease, measured using the-Unified Parkinson’s Disease Rating Scale (UPDRS) part III []. Thus far, only one study reports that lower CSF levels of klotho link to increased UPDRS III scores and higher Hoehn and Yahr disease stage []. When mice deficient in klotho were initially described, a parkinsonian phenotype was noted, with development of hypokinesis and decreased stride length along with midbrain dopaminergic neuronal loss at 5 weeks of age []. Later studies demonstrated that treatment with klotho, either via intracerebroventricular injection in toxin mouse model of PD or administered intraperitoneally in α-synuclein transgenic mouse model of PD, ameliorates motor deficits []. More studies are needed in humans to confirm that changes in cortisol and klotho affect PD motor symptoms.
7. Proposed Model
Evidently there exists a complex interplay between aging and chronic stress in the pathogenesis of PD through mechanisms involving mitochondrial dysfunction and oxidative stress, neuroinflammation, insulin resistance, and vitamin D. It is difficult to extract precise cause and effect in the vicious circle resulting in neurodegeneration; however, there is a case that the hormones cortisol and klotho can contribute to the disease process in a yin-yang manner. Moreover, given their broad effects, these hormones may be key players in both idiopathic and familial PD. Our model proposed in Figure 1 suggests that in PD, there is accelerated aging and increased stress, leading to decrease in circulating klotho and increase in cortisol. As the balanced dualism of these hormones becomes dysregulated, there is resultant pro-inflammatory environment and mitochondrial dysfunction facilitating a wide range of pathways leading to neurotoxicity. Vitamin D metabolism may also be shifted along with increase in insulin resistance, further facilitating disease processes. Interestingly, both cortisol and klotho levels may be amenable to change, with physical activity increasing klotho and decreasing cortisol, and psychological stress/depression decreasing klotho and increasing cortisol.
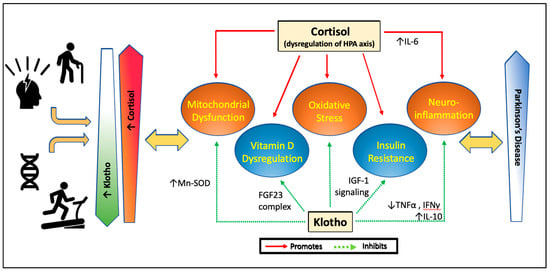
Figure 1.
Proposed model for effects of cortisol and klotho on pathways linked to Parkinson’s disease. With stress and aging, cortisol levels increase due to dysregulation of the HPA axis and klotho levels decrease. Exercise decreases cortisol levels and increases klotho levels. Certain genetic variations may further dampen cortisol sensitivity and cellular response to stress or increase klotho levels or change its function. Chronic elevation of cortisol with aging or stress leads to increase in mitochondrial dysfunction, oxidative stress and activation of inflammatory cytokines, while promoting insulin resistance and correlating with lower vitamin D levels. Klotho normally protects mitochondrial structure and function, decreases oxidative stress, decreases inflammatory states, suppresses the downstream signaling pathway that leads to insulin resistance, and downregulates vitamin D metabolizing enzymes to control active vitamin D levels. This dysregulation of cortisol and reduction in klotho with aging and stress, important risk factors for PD, are hypothesized to affect the course of PD. Changes in cortisol and klotho in disease conditions may affects signs, symptoms, or progression of PD.
8. Conclusions
In summary, we have highlighted the multifactorial actions of cortisol and klotho in the pathogenesis of PD. Downstream effects of cortisol and klotho may influence non-motor and motor symptoms of PD. Given that PD is a heterogenous disorder with multiple pathways involved in neurodegeneration, identifying strategies with a broad neuroprotective potential, e.g., via exercise-induced modifications of cortisol and klotho secretion, offers the potential of increasing the brain’s overall resilience. Future studies on how these hormones of aging and stress play a role in PD will lend evidence to whether these can be potential biomarkers or novel targets for interventional strategies.
Author Contributions
Conceptualization: N.S.L. and D.M.C.; Writing—Original Draft Preparation: N.S.L. and A.C; Writing—Review and Editing, N.S.L., A.C. and D.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number U01NS113851. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was also supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Foffani, G.; Dehay, B.; Bezard, E.; Obeso, J.A. Motor and non-motor circuit disturbances in early Parkinson disease: Which happens first? Nat. Rev. Neurosci. 2022, 23, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Sauerbier, A. Parkinson disease. Unravelling the nonmotor mysteries of Parkinson disease. Nat. Rev. Neurol. 2016, 12, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.C.; Gao, X.; Baker, J.M.; Stephen, C.; Kim, I.Y.; Valeri, L.; Schwarzschild, M.A.; Ascherio, A. Non-motor features of Parkinson’s disease in a nested case-control study of US men. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1288–1295. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 509. [Google Scholar] [CrossRef]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Pang, S.Y.; Ho, P.W.; Liu, H.F.; Leung, C.T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl Neurodegener 2019, 8, 23. [Google Scholar] [CrossRef]
- Marinus, J.; Zhu, K.; Marras, C.; Aarsland, D.; van Hilten, J.J. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018, 17, 559–568. [Google Scholar] [CrossRef]
- Hindle, J.V. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 2010, 39, 156–161. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; Juster, R.P.; Raymond, C.; Marin, M.F. The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Front. Neuroendocrinol. 2018, 49, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Rackova, L.; Mach, M.; Brnoliakova, Z. An update in toxicology of ageing. Environ. Toxicol. Pharmacol. 2021, 84, 103611. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Dubal, D.B.; Zhu, L.; Sanchez, P.E.; Worden, K.; Broestl, L.; Johnson, E.; Ho, K.; Yu, G.Q.; Kim, D.; Betourne, A.; et al. Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. J. Neurosci. 2015, 35, 2358–2371. [Google Scholar] [CrossRef]
- Dubal, D.B.; Yokoyama, J.S.; Zhu, L.; Broestl, L.; Worden, K.; Wang, D.; Sturm, V.E.; Kim, D.; Klein, E.; Yu, G.Q.; et al. Life extension factor klotho enhances cognition. Cell Rep 2014, 7, 1065–1076. [Google Scholar] [CrossRef]
- Dedovic, K.; Duchesne, A.; Andrews, J.; Engert, V.; Pruessner, J.C. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage 2009, 47, 864–871. [Google Scholar] [CrossRef]
- Hermans, E.J.; Henckens, M.J.; Joels, M.; Fernandez, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab 2010, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Viho, E.M.G.; Buurstede, J.C.; Mahfouz, A.; Koorneef, L.L.; van Weert, L.; Houtman, R.; Hunt, H.J.; Kroon, J.; Meijer, O.C. Corticosteroid Action in the Brain: The Potential of Selective Receptor Modulation. Neuroendocrinology 2019, 109, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Fietta, P.; Fietta, P.; Delsante, G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin. Neurosci. 2009, 63, 613–622. [Google Scholar] [CrossRef]
- Liston, C.; Gan, W.B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 16074–16079. [Google Scholar] [CrossRef]
- Ratman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.M.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.S.; Meng, F.; Thompson, R.C. Glucocorticoid treatment of astrocytes results in temporally dynamic transcriptome regulation and astrocyte-enriched mRNA changes in vitro. Physiol. Genom. 2012, 44, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.D.; Rubin, T.G.; Hunter, R.G.; McEwen, B.S. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol. Psychiatry 2014, 19, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Joels, M. Corticosteroids and the brain. J. Endocrinol. 2018, 238, R121–R130. [Google Scholar] [CrossRef]
- Van Wamelen, D.J.; Wan, Y.M.; Ray Chaudhuri, K.; Jenner, P. Stress and cortisol in Parkinson’s disease. Int. Rev. Neurobiol. 2020, 152, 131–156. [Google Scholar]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Arking, D.E.; Atzmon, G.; Arking, A.; Barzilai, N.; Dietz, H.C. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke and longevity. Circ. Res. 2005, 96, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Arking, D.E.; Becker, D.M.; Yanek, L.R.; Fallin, D.; Judge, D.P.; Moy, T.F.; Becker, L.C.; Dietz, H.C. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am. J. Hum. Genet. 2003, 72, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011, 59, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Katz, R.; Kritchevsky, S.; Ix, J.; Shlipak, M.; Gutierrez, O.M.; Newman, A.; Hoofnagle, A.; Fried, L.; Semba, R.D.; et al. Association between Soluble Klotho and Change in Kidney Function: The Health Aging and Body Composition Study. J. Am. Soc. Nephrol. 2017, 28, 1859–1866. [Google Scholar] [CrossRef]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef]
- Semba, R.D.; Moghekar, A.R.; Hu, J.; Sun, K.; Turner, R.; Ferrucci, L.; O’Brien, R. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci. Lett. 2014, 558, 37–40. [Google Scholar] [CrossRef]
- Zimmermann, M.; Kohler, L.; Kovarova, M.; Lerche, S.; Schulte, C.; Wurster, I.; Machetanz, G.; Deuschle, C.; Hauser, A.K.; Gasser, T.; et al. The longevity gene Klotho and its cerebrospinal fluid protein profiles as a modifier for Parkinson’s disease. Eur. J. Neurol. 2021, 28, 1557–1565. [Google Scholar] [CrossRef]
- Clinton, S.M.; Glover, M.E.; Maltare, A.; Laszczyk, A.M.; Mehi, S.J.; Simmons, R.K.; King, G.D. Expression of klotho mRNA and protein in rat brain parenchyma from early postnatal development into adulthood. Brain Res. 2013, 1527, 1–14. [Google Scholar] [CrossRef]
- German, D.C.; Khobahy, I.; Pastor, J.; Kuro, O.M.; Liu, X. Nuclear localization of Klotho in brain: An anti-aging protein. Neurobiol. Aging 2012, 33, 1483.e25–1483.e30. [Google Scholar] [CrossRef][Green Version]
- Brobey, R.K.; German, D.; Sonsalla, P.K.; Gurnani, P.; Pastor, J.; Hsieh, C.C.; Papaconstantinou, J.; Foster, P.P.; Kuro-o, M.; Rosenblatt, K.P. Klotho Protects Dopaminergic Neuron Oxidant-Induced Degeneration by Modulating ASK1 and p38 MAPK Signaling Pathways. PLoS ONE 2015, 10, e0139914. [Google Scholar] [CrossRef]
- Li, S.A.; Watanabe, M.; Yamada, H.; Nagai, A.; Kinuta, M.; Takei, K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct. Funct. 2004, 29, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Utsugi, T.; Ohno, T.; Ohyama, Y.; Uchiyama, T.; Saito, Y.; Matsumura, Y.; Aizawa, H.; Itoh, H.; Kurabayashi, M.; Kawazu, S.; et al. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism 2000, 49, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J.; et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef]
- Chang, Q.; Hoefs, S.; van der Kemp, A.W.; Topala, C.N.; Bindels, R.J.; Hoenderop, J.G. The beta-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science 2005, 310, 490–493. [Google Scholar] [CrossRef]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Erben, R.G. alpha-Klotho’s effects on mineral homeostasis are fibroblast growth factor-23 dependent. Curr. Opin. Nephrol. Hypertens. 2018, 27, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Hum, J.M.; O’Bryan, L.; Smith, R.C.; White, K.E. Novel functions of circulating Klotho. Bone 2017, 100, 36–40. [Google Scholar] [CrossRef]
- Kuro, O.M. Molecular Mechanisms Underlying Accelerated Aging by Defects in the FGF23-Klotho System. Int. J. Nephrol. 2018, 2018, 9679841. [Google Scholar] [CrossRef]
- Razzaque, M.S. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef]
- Huang, C.L.; Moe, O.W. Klotho: A novel regulator of calcium and phosphorus homeostasis. Pflügers Arch.-Eur. J. Physiol. 2011, 462, 185–193. [Google Scholar] [CrossRef]
- Tsujikawa, H.; Kurotaki, Y.; Fujimori, T.; Fukuda, K.; Nabeshima, Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003, 17, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; de Groot, M.H.; Owen, B.M.; Lee, S.; Gautron, L.; Lawrence, H.L.; Ding, X.; Elmquist, J.K.; Takahashi, J.S.; Mangelsdorf, D.J.; et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013, 19, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, L.; Sheng, C.; Cheng, Z.; Cui, L.; Li, M.; Zhao, Y.; Shi, T.; Yau, T.O.; Li, F.; et al. Increased Serum Levels of Cortisol and Inflammatory Cytokines in People with Depression. J. Nerv. Ment. Dis. 2019, 207, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Fiksdal, A.; Hanlin, L.; Kuras, Y.; Gianferante, D.; Chen, X.; Thoma, M.V.; Rohleder, N. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology 2019, 102, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.M.; Prolo, P.; Mastorakos, G.; Vela-Bueno, A.; Kales, A.; Chrousos, G.P. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. J. Clin. Endocrinol. Metab. 2001, 86, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Tsigos, C.; Bixler, E.O.; Stratakis, C.A.; Zachman, K.; Kales, A.; Vela-Bueno, A.; Chrousos, G.P. Chronic insomnia and activity of the stress system: A preliminary study. J Psychosom Res 1998, 45, 21–31. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Costa, C.M.; Oliveira, G.L.; Fonseca, A.C.S.; Lana, R.C.; Polese, J.C.; Pernambuco, A.P. Levels of cortisol and neurotrophic factor brain-derived in Parkinson’s disease. Neurosci. Lett. 2019, 708, 134359. [Google Scholar] [CrossRef]
- Barbosa, I.G.; Rocha, N.P.; Alpak, G.; Vieira, E.L.M.; Huguet, R.B.; Rocha, F.L.; de Oliveira Diniz, B.S.; Teixeira, A.L. Klotho dysfunction: A pathway linking the aging process to bipolar disorder? J. Psychiatr. Res. 2017, 95, 80–83. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Arenander, J.; Broestl, L.; Garay, B.I.; Wang, D.; Dubal, D.B. Longevity factor klotho and chronic psychological stress. Transl. Psychiatry 2015, 5, e585. [Google Scholar] [CrossRef]
- Emami Aleagha, M.S.; Siroos, B.; Ahmadi, M.; Balood, M.; Palangi, A.; Haghighi, A.N.; Harirchian, M.H. Decreased concentration of Klotho in the cerebrospinal fluid of patients with relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2015, 281, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Teocchi, M.A.; Ferreira, A.E.; da Luz de Oliveira, E.P.; Tedeschi, H.; D’Souza-Li, L. Hippocampal gene expression dysregulation of Klotho, nuclear factor kappa B and tumor necrosis factor in temporal lobe epilepsy patients. J. Neuroinflammation 2013, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Hineno, T.; Kakimoto, Y.; Hiratani, K. Increase of plasma adrenocorticotrophin and cortisol in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated dogs. Brain Res. 1993, 612, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Jadavji, N.M.; Colwell, K.L.; Katrina Perehudoff, S.; Metz, G.A. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2008, 27, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Veldhuis, J.D.; Deuschle, M.; Standhardt, H.; Heuser, I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 1997, 18, 285–289. [Google Scholar] [CrossRef]
- Skogar, O.; Fall, P.A.; Hallgren, G.; Lokk, J.; Bringer, B.; Carlsson, M.; Lennartsson, U.; Sandbjork, H.; Tornhage, C.J. Diurnal salivary cortisol concentrations in Parkinson’s disease: Increased total secretion and morning cortisol concentrations. Int. J. Gen. Med. 2011, 4, 561–569. [Google Scholar] [CrossRef][Green Version]
- Van den Heuvel, L.L.; du Plessis, S.; Stalder, T.; Acker, D.; Kirschbaum, C.; Carr, J.; Seedat, S. Hair glucocorticoid levels in Parkinson’s disease. Psychoneuroendocrinology 2020, 117, 104704. [Google Scholar] [CrossRef]
- Belloy, M.E.; Napolioni, V.; Han, S.S.; Le Guen, Y.; Greicius, M.D.; Alzheimer’s Disease Neuroimaging Initiative. Association of Klotho-VS Heterozygosity with Risk of Alzheimer Disease in Individuals Who Carry APOE4. JAMA Neurol. 2020, 77, 849–862. [Google Scholar] [CrossRef]
- Kosakai, A.; Ito, D.; Nihei, Y.; Yamashita, S.; Okada, Y.; Takahashi, K.; Suzuki, N. Degeneration of mesencephalic dopaminergic neurons in klotho mouse related to vitamin D exposure. Brain Res. 2011, 1382, 109–117. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Eftekhari, S.M.; Jamali-Raeufy, N.; Haghani, S.; Zeinali, H.; Roghani, M. The anti-aging protein klotho alleviates injury of nigrostriatal dopaminergic pathway in 6-hydroxydopamine rat model of Parkinson’s disease: Involvement of PKA/CaMKII/CREB signaling. Exp. Gerontol. 2017, 100, 70–76. [Google Scholar] [CrossRef]
- Leon, J.; Moreno, A.J.; Garay, B.I.; Chalkley, R.J.; Burlingame, A.L.; Wang, D.; Dubal, D.B. Peripheral Elevation of a Klotho Fragment Enhances Brain Function and Resilience in Young, Aging and alpha-Synuclein Transgenic Mice. Cell Rep 2017, 20, 1360–1371. [Google Scholar] [CrossRef]
- Kakar, R.S.; Pastor, J.V.; Moe, O.W.; Ambrosio, F.; Castaldi, D.; Sanders, L.H. Peripheral Klotho and Parkinson’s Disease. Mov. Disord. 2021, 36, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Grillo, P.; Basilicata, M.; Schirinzi, T. Alpha-Klotho in Parkinson’s disease: A perspective on experimental evidence and potential clinical implications. Neural. Regen. Res. 2022, 17, 2687–2688. [Google Scholar]
- Muller, T.; Muhlack, S. Acute levodopa intake and associated cortisol decrease in patients with Parkinson disease. Clin. Neuropharmacol. 2007, 30, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Welnic, J.; Muhlack, S. Acute levodopa administration reduces cortisol release in patients with Parkinson’s disease. J. Neural. Transm. 2007, 114, 347–350. [Google Scholar] [CrossRef]
- Tanner, C.M.; Goldman, S.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1996, 14, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Collier, T.J.; Kanaan, N.M.; Kordower, J.H. Ageing as a primary risk factor for Parkinson’s disease: Evidence from studies of non-human primates. Nat. Rev. Neurosci. 2011, 12, 359–366. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Moffat, S.D.; An, Y.; Resnick, S.M.; Diamond, M.P.; Ferrucci, L. Longitudinal Change in Cortisol Levels Across the Adult Life Span. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 394–400. [Google Scholar] [CrossRef]
- Iob, E.; Steptoe, A. Cardiovascular Disease and Hair Cortisol: A Novel Biomarker of Chronic Stress. Curr. Cardiol. Rep. 2019, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G. Chronic Stress and Diabetes Mellitus: Interwoven Pathologies. Curr. Diabetes Rev. 2020, 16, 546–556. [Google Scholar] [PubMed]
- Al-Rawaf, H.A.; Alghadir, A.H.; Gabr, S.A. Circulating MicroRNA Expression, Vitamin D, and Hypercortisolism as Predictors of Osteoporosis in Elderly Postmenopausal Women. Dis. Markers 2021, 2021, 3719919. [Google Scholar] [CrossRef]
- Lara, V.P.; Caramelli, P.; Teixeira, A.L.; Barbosa, M.T.; Carmona, K.C.; Carvalho, M.G.; Fernandes, A.P.; Gomes, K.B. High cortisol levels are associated with cognitive impairment no-dementia (CIND) and dementia. Clin. Chim. Acta 2013, 423, 18–22. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Imura, A.; Urakawa, I.; Shimada, T.; Murakami, J.; Aono, Y.; Hasegawa, H.; Yamashita, T.; Nakatani, K.; Saito, Y.; et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 2010, 398, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and mortality risk in older community-dwelling adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: The InCHIANTI study. Eur. J. Appl. Physiol. 2012, 112, 1215–1220. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Sieurin, J.; Andel, R.; Tillander, A.; Valdes, E.G.; Pedersen, N.L.; Wirdefeldt, K. Occupational stress and risk for Parkinson’s disease: A nationwide cohort study. Mov. Disord. 2018, 33, 1456–1464. [Google Scholar] [CrossRef]
- Vlajinac, H.; Sipetic, S.; Marinkovic, J.; Ratkov, I.; Maksimovic, J.; Dzoljic, E.; Kostic, V. The stressful life events and Parkinson’s disease: A case-control study. Stress Health 2013, 29, 50–55. [Google Scholar] [CrossRef]
- White, D.L.; Kunik, M.E.; Yu, H.; Lin, H.L.; Richardson, P.A.; Moore, S.; Sarwar, A.I.; Marsh, L.; Jorge, R.E. Post-Traumatic Stress Disorder is Associated with further Increased Parkinson’s Disease Risk in Veterans with Traumatic Brain Injury. Ann. Neurol. 2020, 88, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Farkas, D.K.; Gradus, J.L.; Lash, T.L.; Sorensen, H.T. Adjustment disorder and risk of Parkinson’s disease. Eur. J. Neurol. 2016, 23, 751–756. [Google Scholar] [CrossRef]
- Marin, M.F.; Lord, C.; Andrews, J.; Juster, R.P.; Sindi, S.; Arsenault-Lapierre, G.; Fiocco, A.J.; Lupien, S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem. 2011, 96, 583–595. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar] [CrossRef] [PubMed]
- Nollet, M.; Wisden, W.; Franks, N.P. Sleep deprivation and stress: A reciprocal relationship. Interface Focus 2020, 10, 20190092. [Google Scholar] [CrossRef]
- Abel, T.; Havekes, R.; Saletin, J.M.; Walker, M.P. Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol. 2013, 23, R774–R788. [Google Scholar] [CrossRef] [PubMed]
- Metz, G.A. Stress as a modulator of motor system function and pathology. Rev. Neurosci. 2007, 18, 209–222. [Google Scholar] [CrossRef]
- Van der Heide, A.; Meinders, M.J.; Speckens, A.E.M.; Peerbolte, T.F.; Bloem, B.R.; Helmich, R.C. Stress and Mindfulness in Parkinson’s Disease: Clinical Effects and Potential Underlying Mechanisms. Mov. Disord. 2021, 36, 64–70. [Google Scholar] [CrossRef]
- Wolf, E.J.; Morrison, F.G.; Sullivan, D.R.; Logue, M.W.; Guetta, R.E.; Stone, A.; Schichman, S.A.; McGlinchey, R.E.; Milberg, W.P.; Miller, M.W. The goddess who spins the thread of life: Klotho, psychiatric stress, and accelerated aging. Brain Behav. Immun. 2019, 80, 193–203. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, W.N.; Fan, H.; Liu, L.E.; Zhan, J.Q.; Li, Y.H.; Chen, C.N.; Jiang, S.Z.; Xiong, J.W.; Yu, Z.M.; et al. Life extension factor klotho regulates behavioral responses to stress via modulation of GluN2B function in the nucleus accumbens. Neuropsychopharmacology 2022, 47, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Koper, J.W.; van Rossum, E.F.; van den Akker, E.L. Glucocorticoid receptor polymorphisms and haplotypes and their expression in health and disease. Steroids 2014, 92, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, E.F.; Feelders, R.A.; van den Beld, A.W.; Uitterlinden, A.G.; Janssen, J.A.; Ester, W.; Brinkmann, A.O.; Grobbee, D.E.; de Jong, F.H.; Pols, H.A.; et al. Association of the ER22/23EK polymorphism in the glucocorticoid receptor gene with survival and C-reactive protein levels in elderly men. Am. J. Med. 2004, 117, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, E.F.; Binder, E.B.; Majer, M.; Koper, J.W.; Ising, M.; Modell, S.; Salyakina, D.; Lamberts, S.W.; Holsboer, F. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol. Psychiatry 2006, 59, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R.; Enoch, M.A.; Sorocco, K.H.; Vincent, A.S.; Acheson, A.; Cohoon, A.J.; Hodgkinson, C.A.; Goldman, D. Joint Impact of Early Life Adversity and COMT Val158Met (rs4680) Genotypes on the Adult Cortisol Response to Psychological Stress. Psychosom. Med. 2017, 79, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zou, Y.B.; Xiao, J.; Pan, C.D.; Jiang, S.D.; Zheng, Z.J.; Yan, Z.R.; Tang, K.Y.; Tan, L.M.; Tang, M.S. COMT Val158Met polymorphism and Parkinson’s disease risk: A pooled analysis in different populations. Neurol. Res. 2019, 41, 319–325. [Google Scholar] [CrossRef]
- Wolf, E.J.; Chen, C.D.; Zhao, X.; Zhou, Z.; Morrison, F.G.; Daskalakis, N.P.; Stone, A.; Schichman, S.; Grenier, J.G.; Fein-Schaffer, D.; et al. Klotho, PTSD and advanced epigenetic age in cortical tissue. Neuropsychopharmacology 2021, 46, 721–730. [Google Scholar] [CrossRef]
- Yokoyama, J.S.; Sturm, V.E.; Bonham, L.W.; Klein, E.; Arfanakis, K.; Yu, L.; Coppola, G.; Kramer, J.H.; Bennett, D.A.; Miller, B.L.; et al. Variation in longevity gene KLOTHO is associated with greater cortical volumes. Ann. Clin. Transl. Neurol. 2015, 2, 215–230. [Google Scholar] [CrossRef]
- Beserra, A.H.N.; Kameda, P.; Deslandes, A.C.; Schuch, F.B.; Laks, J.; Moraes, H.S. Can physical exercise modulate cortisol level in subjects with depression? A systematic review and meta-analysis. Trends Psychiatry Psychother. 2018, 40, 360–368. [Google Scholar] [CrossRef]
- Gottschall, J.S.; Davis, J.J.; Hastings, B.; Porter, H.J. Exercise Time and Intensity: How Much Is Too Much? Int. J. Sports Physiol. Perform. 2020, 15, 808–815. [Google Scholar] [CrossRef]
- Caplin, A.; Chen, F.S.; Beauchamp, M.R.; Puterman, E. The effects of exercise intensity on the cortisol response to a subsequent acute psychosocial stressor. Psychoneuroendocrinology 2021, 131, 105336. [Google Scholar] [CrossRef] [PubMed]
- Smyth, N.; Skender, E.; David, F.J.; Munoz, M.J.; Fantuzzi, G.; Clow, A.; Goldman, J.G.; Corcos, D.M. Endurance exercise reduces cortisol in Parkinson’s disease with mild cognitive impairment. Mov. Disord. 2019, 34, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017, 86, 152–168. [Google Scholar] [CrossRef]
- Ho, R.T.H.; Fong, T.C.T.; Chan, W.C.; Kwan, J.S.K.; Chiu, P.K.C.; Yau, J.C.Y.; Lam, L.C.W. Psychophysiological Effects of Dance Movement Therapy and Physical Exercise on Older Adults with Mild Dementia: A Randomized Controlled Trial. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2020, 75, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Luan, J.; Hu, F.; Zhao, Y.; Lv, B.; Wang, W.; Xia, M.; Zhao, X.; Lao, K. Aerobic exercise-stimulated Klotho upregulation extends life span by attenuating the excess production of reactive oxygen species in the brain and kidney. Exp. Ther. Med. 2018, 16, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zheng, L.; Huang, H.; Feng, Y.; Shi, R. α-Klotho Expression in Mouse Tissues Following Acute Exhaustive Exercise. Front. Physiol. 2019, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Simonsick, E.; Turner, R.; Miljkovic, I.; Harris, T.; Schwartz, A.V.; Asao, K.; Kritchevsky, S.; et al. Low Plasma Klotho Concentrations and Decline of Knee Strength in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 103–108. [Google Scholar] [CrossRef]
- Crasto, C.L.; Semba, R.D.; Sun, K.; Cappola, A.R.; Bandinelli, S.; Ferrucci, L. Relationship of low-circulating “anti-aging” klotho hormone with disability in activities of daily living among older community-dwelling adults. Rejuvenation Res. 2012, 15, 295–301. [Google Scholar] [CrossRef]
- Saghiv, M.S.; Sira, D.B.; Goldhammer, E.; Sagiv, M. The effects of aerobic and anaerobic exercises on circulating soluble-Klotho and IGF-I in young and elderly adults and in CAD patients. J. Circ. Biomark. 2017, 6, 1849454417733388. [Google Scholar] [CrossRef]
- Matsubara, T.; Miyaki, A.; Akazawa, N.; Choi, Y.; Ra, S.G.; Tanahashi, K.; Kumagai, H.; Oikawa, S.; Maeda, S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H348–H355. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la, O.A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; de Haro, T.; Gutierrez, A.; Ruiz, J.R.; Castillo, M.J. Exercise training increases the S-Klotho plasma levels in sedentary middle-aged adults: A randomised controlled trial. The FIT-AGEING study. J. Sports. Sci. 2019, 37, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; de-la-O, A.; Jurado-Fasoli, L.; Gutierrez, A.; Ruiz, J.R.; Castillo, M.J. Association of physical activity and fitness with S-Klotho plasma levels in middle-aged sedentary adults: The FIT-AGEING study. Maturitas 2019, 123, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Santos-Dias, A.; MacKenzie, B.; Oliveira-Junior, M.C.; Moyses, R.M.; Consolim-Colombo, F.M.; Vieira, R.P. Longevity protein klotho is induced by a single bout of exercise. Br. J. Sports Med. 2017, 51, 549–550. [Google Scholar] [CrossRef]
- Tan, S.J.; Chu, M.M.; Toussaint, N.D.; Cai, M.M.; Hewitson, T.D.; Holt, S.G. High-intensity physical exercise increases serum α-klotho levels in healthy volunteers. J. Circ. Biomark. 2018, 7, 1849454418794582. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, U.; Kumar, M.; Rana, D.; Dada, R. Yoga improves mitochondrial health and reduces severity of autoimmune inflammatory arthritis: A randomized controlled trial. Mitochondrion 2021, 58, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Liddell, J.R.; White, A.R. Nexus between mitochondrial function, iron, copper and glutathione in Parkinson’s disease. Neurochem. Int. 2018, 117, 126–138. [Google Scholar] [CrossRef]
- Currais, A. Ageing and inflammation—A central role for mitochondria in brain health and disease. Ageing Res. Rev. 2015, 21, 30–42. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Olivenza, R.; Moro, M.A.; Lizasoain, I.; Lorenzo, P.; Rodrigo, J.; Leza, J.C. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 2001, 24, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Caruncho, H.J.; Kalynchuk, L.E. Severe life stress, mitochondrial dysfunction, and depressive behavior: A pathophysiological and therapeutic perspective. Mitochondrion 2021, 56, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Manda, G.; Bosca, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Grigoruta, M.; Martinez-Martinez, A.; Dagda, R.Y.; Dagda, R.K. Psychological Stress Phenocopies Brain Mitochondrial Dysfunction and Motor Deficits as Observed in a Parkinsonian Rat Model. Mol. Neurobiol. 2020, 57, 1781–1798. [Google Scholar] [CrossRef]
- Sahu, A.; Mamiya, H.; Shinde, S.N.; Cheikhi, A.; Winter, L.L.; Vo, N.V.; Stolz, D.; Roginskaya, V.; Tang, W.Y.; St Croix, C.; et al. Age-related declines in alpha-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 2018, 9, 4859. [Google Scholar] [CrossRef]
- Miao, J.; Huang, J.; Luo, C.; Ye, H.; Ling, X.; Wu, Q.; Shen, W.; Zhou, L. Klotho retards renal fibrosis through targeting mitochondrial dysfunction and cellular senescence in renal tubular cells. Physiol. Rep. 2021, 9, e14696. [Google Scholar] [CrossRef]
- Lim, S.W.; Jin, L.; Luo, K.; Jin, J.; Shin, Y.J.; Hong, S.Y.; Yang, C.W. Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 2017, 8, e2972. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Fu, C.; Wu, X.; Peng, Y.; Lin, X.; Wang, Y. Recombinant Klotho Protects Human Periodontal Ligament Stem Cells by Regulating Mitochondrial Function and the Antioxidant System during H2O2-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 9261565. [Google Scholar] [CrossRef]
- Emami Aleagha, M.S.; Harirchian, M.H.; Lavasani, S.; Javan, M.; Allameh, A. Differential Expression of Klotho in the Brain and Spinal Cord is Associated with Total Antioxidant Capacity in Mice with Experimental Autoimmune Encephalomyelitis. J. Mol. Neurosci. 2018, 64, 543–550. [Google Scholar] [CrossRef]
- Typiak, M.; Piwkowska, A. Antiinflammatory Actions of Klotho: Implications for Therapy of Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 956. [Google Scholar] [CrossRef] [PubMed]
- Mazucanti, C.H.; Kawamoto, E.M.; Mattson, M.P.; Scavone, C.; Camandola, S. Activity-dependent neuronal Klotho enhances astrocytic aerobic glycolysis. J. Cereb. Blood Flow Metab. 2019, 39, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Vieira, E.M. Stress, Inflammation and Aging: An Association Beyond Chance. Am. J. Geriatr. Psychiatry 2018, 26, 964–965. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol. Metab. 1999, 10, 359–368. [Google Scholar] [CrossRef]
- Swolin-Eide, D.; Ohlsson, C. Effects of cortisol on the expression of interleukin-6 and interleukin-1 beta in human osteoblast-like cells. J. Endocrinol. 1998, 156, 107–114. [Google Scholar] [CrossRef]
- Mastorakos, G.; Chrousos, G.P.; Weber, J.S. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J. Clin. Endocrinol. Metab. 1993, 77, 1690–1694. [Google Scholar]
- Turnbull, A.V.; Rivier, C. Regulation of the HPA axis by cytokines. Brain Behav. Immun. 1995, 9, 253–275. [Google Scholar] [CrossRef]
- Chen, X.; Gianferante, D.; Hanlin, L.; Fiksdal, A.; Breines, J.G.; Thoma, M.V.; Rohleder, N. HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology 2017, 78, 168–176. [Google Scholar] [CrossRef]
- Zhu, L.; Stein, L.R.; Kim, D.; Ho, K.; Yu, G.Q.; Zhan, L.; Larsson, T.E.; Mucke, L. Klotho controls the brain-immune system interface in the choroid plexus. Proc. Natl. Acad. Sci. USA 2018, 115, E11388–E11396. [Google Scholar] [CrossRef] [PubMed]
- Urabe, A.; Doi, S.; Nakashima, A.; Ike, T.; Morii, K.; Sasaki, K.; Doi, T.; Arihiro, K.; Masaki, T. Klotho deficiency intensifies hypoxia-induced expression of IFN-α/βthrough upregulation of RIG-I in kidneys. PLoS ONE 2021, 16, e0258856. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Zhai, Y.; Ao, L.; Cleveland, J.C.; Liu, H., Jr.; Fullerton, D.A.; Meng, X. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget 2017, 8, 15663–15676. [Google Scholar] [CrossRef]
- Mytych, J.; Romerowicz-Misielak, M.; Koziorowski, M. Klotho protects human monocytes from LPS-induced immune impairment associated with immunosenescent-like phenotype. Mol. Cell. Endocrinol. 2018, 470, 1–13. [Google Scholar] [CrossRef]
- Xiang, T.; Luo, X.; Ye, L.; Huang, H.; Wu, Y. Klotho alleviates NLRP3 inflammasome-mediated neuroinflammation in a temporal lobe epilepsy rat model by activating the Nrf2 signaling pathway. Epilepsy Behav. 2022, 128, 108509. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Xu, T.; Yin, Y.; Huang, R.; Wang, Y.; Zhang, J.; Huang, D.; Li, W. Chronic dexamethasone treatment results in hippocampal neurons injury due to activate NLRP1 inflammasome in vitro. Int. Immunopharmacol. 2017, 49, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhao, Y.; Yang, T.; Song, M.; Wang, C.; Yao, Y.; Fan, H. Glucocorticoid-Driven NLRP3 Inflammasome Activation in Hippocampal Microglia Mediates Chronic Stress-Induced Depressive-Like Behaviors. Front. Mol. Neurosci. 2019, 12, 210. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Camandola, S.; Mattson, M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef]
- Liu, W.; Tang, J. Association between diabetes mellitus and risk of Parkinson’s disease: A prisma-compliant meta-analysis. Brain Behav. 2021, 11, e02082. [Google Scholar] [CrossRef]
- Deischinger, C.; Dervic, E.; Kaleta, M.; Klimek, P.; Kautzky-Willer, A. Diabetes Mellitus is Associated with a Higher Relative Risk for Parkinson’s Disease in Women than in Men. J. Parkinson’s Dis. 2021, 11, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Plastino, M.; Cristiano, D.; Colica, C.; Ermio, C.; De Bartolo, M.; Mungari, P.; Fonte, G.; Consoli, D.; Consoli, A.; et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J. Neurol. Sci. 2012, 315, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, F.; Ballarini, T.; Neumann, J.; Schroeter, M.L. FDG-PET hypometabolism is more sensitive than MRI atrophy in Parkinson’s disease: A whole-brain multimodal imaging meta-analysis. Neuroimage Clin. 2019, 21, 101594. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.T.D.; Le, T.T.; Vo, V.G. Role of Insulin Resistance in the Alzheimer’s Disease Progression. Neurochem. Res. 2020, 45, 1481–1491. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Alexander, N.; Bornstein, S.R.; Gao, W.; Miller, R.; Stark, S.; Bosch, J.A.; Fischer, J.E. Cortisol in hair and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2573–2580. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.; Chae, D.W.; Lee, K.B.; Sung, S.A.; Yoo, T.H.; Han, S.H.; Ahn, C.; Oh, K.H. Serum klotho is inversely associated with metabolic syndrome in chronic kidney disease: Results from the KNOW-CKD study. BMC Nephrol. 2019, 20, 119. [Google Scholar] [CrossRef]
- Chen, C.D.; Podvin, S.; Gillespie, E.; Leeman, S.E.; Abraham, C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA 2007, 104, 19796–19801. [Google Scholar] [CrossRef]
- Tuohimaa, P.; Keisala, T.; Minasyan, A.; Cachat, J.; Kalueff, A. Vitamin D, nervous system and aging. Psychoneuroendocrinology 2009, 34, S278–S286. [Google Scholar] [CrossRef]
- Knekt, P.; Kilkkinen, A.; Rissanen, H.; Marniemi, J.; Saaksjarvi, K.; Heliovaara, M. Serum vitamin D and the risk of Parkinson disease. Arch. Neurol. 2010, 67, 808–811. [Google Scholar] [CrossRef]
- Lv, L.; Tan, X.; Peng, X.; Bai, R.; Xiao, Q.; Zou, T.; Tan, J.; Zhang, H.; Wang, C. The relationships of vitamin D, vitamin D receptor gene polymorphisms, and vitamin D supplementation with Parkinson’s disease. Transl. Neurodegener. 2020, 9, 34. [Google Scholar] [CrossRef]
- DeLuca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pelekanos, M.; Liu, P.Y.; Burne, T.H.; McGrath, J.J.; Eyles, D.W. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience 2013, 236, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Evatt, M.L.; DeLong, M.R.; Kumari, M.; Auinger, P.; McDermott, M.P.; Tangpricha, V.; Parkinson Study Group DATATOP Investigators. High prevalence of hypovitaminosis D status in patients with early Parkinson disease. Arch. Neurol. 2011, 68, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, I.; Aspray, T.; Lawson, R.; Coleman, S.; Duncan, G.; Khoo, T.K.; Schoenmakers, I.; Rochester, L.; Burn, D.; Yarnall, A. The Role of Vitamin D in Disease Progression in Early Parkinson’s Disease. J. Parkinson’s Dis. 2017, 7, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Newmark, H.L.; Newmark, J. Vitamin D and Parkinson’s disease—A hypothesis. Mov. Disord. 2007, 22, 461–468. [Google Scholar] [CrossRef]
- Peterson, A.L.; Murchison, C.; Zabetian, C.; Leverenz, J.B.; Watson, G.S.; Montine, T.; Carney, N.; Bowman, G.L.; Edwards, K.; Quinn, J.F. Memory, mood and vitamin D in persons with Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 547–555. [Google Scholar] [CrossRef]
- Hiller, A.L.; Murchison, C.F.; Lobb, B.M.; O’Connor, S.; O’Connor, M.; Quinn, J.F. A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson’s disease: Does age matter? PLoS ONE 2018, 13, e0203637. [Google Scholar] [CrossRef]
- Caristia, S.; Filigheddu, N.; Barone-Adesi, F.; Sarro, A.; Testa, T.; Magnani, C.; Aimaretti, G.; Faggiano, F.; Marzullo, P. Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients 2019, 11, 2384. [Google Scholar] [CrossRef]
- Guarnotta, V.; Di Gaudio, F.; Giordano, C. Vitamin D Deficiency in Cushing’s Disease: Before and after Its Supplementation. Nutrients 2022, 14, 973. [Google Scholar] [CrossRef]
- Obradovic, D.; Gronemeyer, H.; Lutz, B.; Rein, T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J. Neurochem. 2006, 96, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Schuurman, K.G.; van Strien, M.E.; Melief, J.; Hendrickx, D.; Hol, E.M.; van Eden, C.; Luchetti, S.; Huitinga, I. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue. J. Neuropathol. Exp. Neurol. 2013, 72, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Dermaku-Sopjani, M.; Kurti, F.; Xuan, N.T.; Sopjani, M. Klotho-Dependent Role of 1,25(OH)2D3 in the Brain. Neurosignals 2021, 29, 14–23. [Google Scholar] [PubMed]
- Hoogendijk, W.J.; Lips, P.; Dik, M.G.; Deeg, D.J.; Beekman, A.T.; Penninx, B.W. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch. Gen. Psychiatry 2008, 65, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.J.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Pruessner, J.; Sousa, N.; Almeida, O.F.; Van Dam, A.M.; Rajkowska, G.; Swaab, D.F.; Czeh, B. Neuropathology of stress. Acta Neuropathol. 2014, 127, 109–135. [Google Scholar] [CrossRef]
- Seifried, C.; Boehncke, S.; Heinzmann, J.; Baudrexel, S.; Weise, L.; Gasser, T.; Eggert, K.; Fogel, W.; Baas, H.; Badenhoop, K.; et al. Diurnal variation of hypothalamic function and chronic subthalamic nucleus stimulation in Parkinson’s disease. Neuroendocrinology 2013, 97, 283–290. [Google Scholar] [CrossRef]
- Djamshidian, A.; O’Sullivan, S.S.; Papadopoulos, A.; Bassett, P.; Shaw, K.; Averbeck, B.B.; Lees, A. Salivary cortisol levels in Parkinson’s disease and its correlation to risk behaviour. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1107–1111. [Google Scholar] [CrossRef]
- Sang, Y.M.; Wang, L.J.; Mao, H.X.; Lou, X.Y.; Zhu, Y.J. The association of short-term memory and cognitive impairment with ghrelin, leptin and cortisol levels in non-diabetic and diabetic elderly individuals. Acta Diabetol. 2018, 55, 531–539. [Google Scholar] [CrossRef]
- Ouanes, S.; Castelao, E.; Gebreab, S.; von Gunten, A.; Preisig, M.; Popp, J. Life events, salivary cortisol and cognitive performance in nondemented subjects: A population-based study. Neurobiol. Aging 2017, 51, 1–8. [Google Scholar] [CrossRef]
- Geerlings, M.I.; Sigurdsson, S.; Eiriksdottir, G.; Garcia, M.E.; Harris, T.B.; Gudnason, V.; Launer, L.J. Salivary cortisol, brain volumes and cognition in community-dwelling elderly without dementia. Neurology 2015, 85, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Conner, S.C.; Himali, J.J.; Maillard, P.; DeCarli, C.S.; Beiser, A.S.; Vasan, R.S.; Seshadri, S. Circulating cortisol and cognitive and structural brain measures: The Framingham Heart Study. Neurology 2018, 91, e1961–e1970. [Google Scholar] [CrossRef] [PubMed]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul Med. 2014, 87, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, C.; Sartorius, A.; Aksay, S.S.; Bumb, J.M.; Janke, C.; Thiel, M.; Haffner, D.; Leifheit-Nestler, M.; Kranaster, L. Electroconvulsive therapy enhances the anti-ageing hormone Klotho in the cerebrospinal fluid of geriatric patients with major depression. Eur. Neuropsychopharmacol. 2018, 28, 428–435. [Google Scholar] [CrossRef]
- Paroni, G.; Seripa, D.; Fontana, A.; D’Onofrio, G.; Gravina, C.; Urbano, M.; Addante, F.; Lozupone, M.; Copetti, M.; Pilotto, A.; et al. Klotho Gene and Selective Serotonin Reuptake Inhibitors: Response to Treatment in Late-Life Major Depressive Disorder. Mol. Neurobiol. 2017, 54, 1340–1351. [Google Scholar] [CrossRef]
- Yokoyama, J.S.; Marx, G.; Brown, J.A.; Bonham, L.W.; Wang, D.; Coppola, G.; Seeley, W.W.; Rosen, H.J.; Miller, B.L.; Kramer, J.H.; et al. Systemic klotho is associated with KLOTHO variation and predicts intrinsic cortical connectivity in healthy human aging. Brain Imaging Behav. 2017, 11, 391–400. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, F.; Hu, L.F.; Liu, C.F. A New Perspective for Parkinson’s Disease: Circadian Rhythm. Neurosci. Bull. 2017, 33, 62–72. [Google Scholar] [CrossRef]
- Shkodina, A.D.; Tan, S.C.; Hasan, M.M.; Abdelgawad, M.; Chopra, H.; Bilal, M.; Boiko, D.I.; Tarianyk, K.A.; Alexiou, A. Roles of clock genes in the pathogenesis of Parkinson’s disease. Ageing Res. Rev. 2022, 74, 101554. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. 2004, 108, 17–39. [Google Scholar] [CrossRef]
- Bonuccelli, U.; Del Dotto, P.; Lucetti, C.; Petrozzi, L.; Bernardini, S.; Gambaccini, G.; Rossi, G.; Piccini, P. Diurnal motor variations to repeated doses of levodopa in Parkinson’s disease. Clin. Neuropharmacol. 2000, 23, 28–33. [Google Scholar] [CrossRef]
- Nyholm, D.; Lennernas, H.; Johansson, A.; Estrada, M.; Aquilonius, S.M. Circadian rhythmicity in levodopa pharmacokinetics in patients with Parkinson disease. Clin. Neuropharmacol. 2010, 33, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Bolitho, S.J.; Naismith, S.L.; Rajaratnam, S.M.; Grunstein, R.R.; Hodges, J.R.; Terpening, Z.; Rogers, N.; Lewis, S.J. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014, 15, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Egstrand, S.; Olgaard, K.; Lewin, E. Circadian rhythms of mineral metabolism in chronic kidney disease-mineral bone disorder. Curr. Opin. Nephrol. Hypertens 2020, 29, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Insogna, K.L.; Zhang, J.H.; Ellis, B.; Nieman, S.; Simpson, C.; Olear, E.; Gundberg, C.M. Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: Circadian variance, effects of treatment and relationship to parathyroid status. J. Clin. Endocrinol. Metab. 2010, 95, E352–E357. [Google Scholar] [CrossRef]
- Nordholm, A.; Egstrand, S.; Gravesen, E.; Mace, M.L.; Morevati, M.; Olgaard, K.; Lewin, E. Circadian rhythm of activin A and related parameters of mineral metabolism in normal and uremic rats. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1079–1094. [Google Scholar] [CrossRef]
- Mochon-Benguigui, S.; Carneiro-Barrera, A.; Castillo, M.J.; Amaro-Gahete, F.J. Is Sleep Associated with the S-Klotho Anti-Aging Protein in Sedentary Middle-Aged Adults? The FIT-AGEING Study. Antioxidants 2020, 9, 738. [Google Scholar] [CrossRef]
- Khurana, S.; Soda, N.; Shiddiky, M.J.A.; Nayak, R.; Bose, S. Current and future strategies for diagnostic and management of obstructive sleep apnea. Expert Rev. Mol. Diagn. 2021, 21, 1287–1301. [Google Scholar] [CrossRef]
- Huang, D.; Wang, S. Association between the Anti-Aging Protein Klotho and Sleep Duration in General Population. Int. J. Gen. Med. 2021, 14, 10023–10030. [Google Scholar] [CrossRef]
- Katsuhara, S.; Yokomoto-Umakoshi, M.; Umakoshi, H.; Matsuda, Y.; Iwahashi, N.; Kaneko, H.; Ogata, M.; Fukumoto, T.; Terada, E.; Sakamoto, R.; et al. Impact of Cortisol on Reduction in Muscle Strength and Mass: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e1477–e1487. [Google Scholar] [CrossRef]
- Haglin, L.; Backman, L. Covariation between plasma phosphate and daytime cortisol in early Parkinson’s disease. Brain Behav. 2016, 6, e00556. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).