The Shape of the Olfactory Bulb Predicts Olfactory Function
Abstract
:1. Introduction
2. Methods
2.1. MRI Acquisition
2.2. Olfactory Testing
2.3. Evaluation of the Volume of the OB
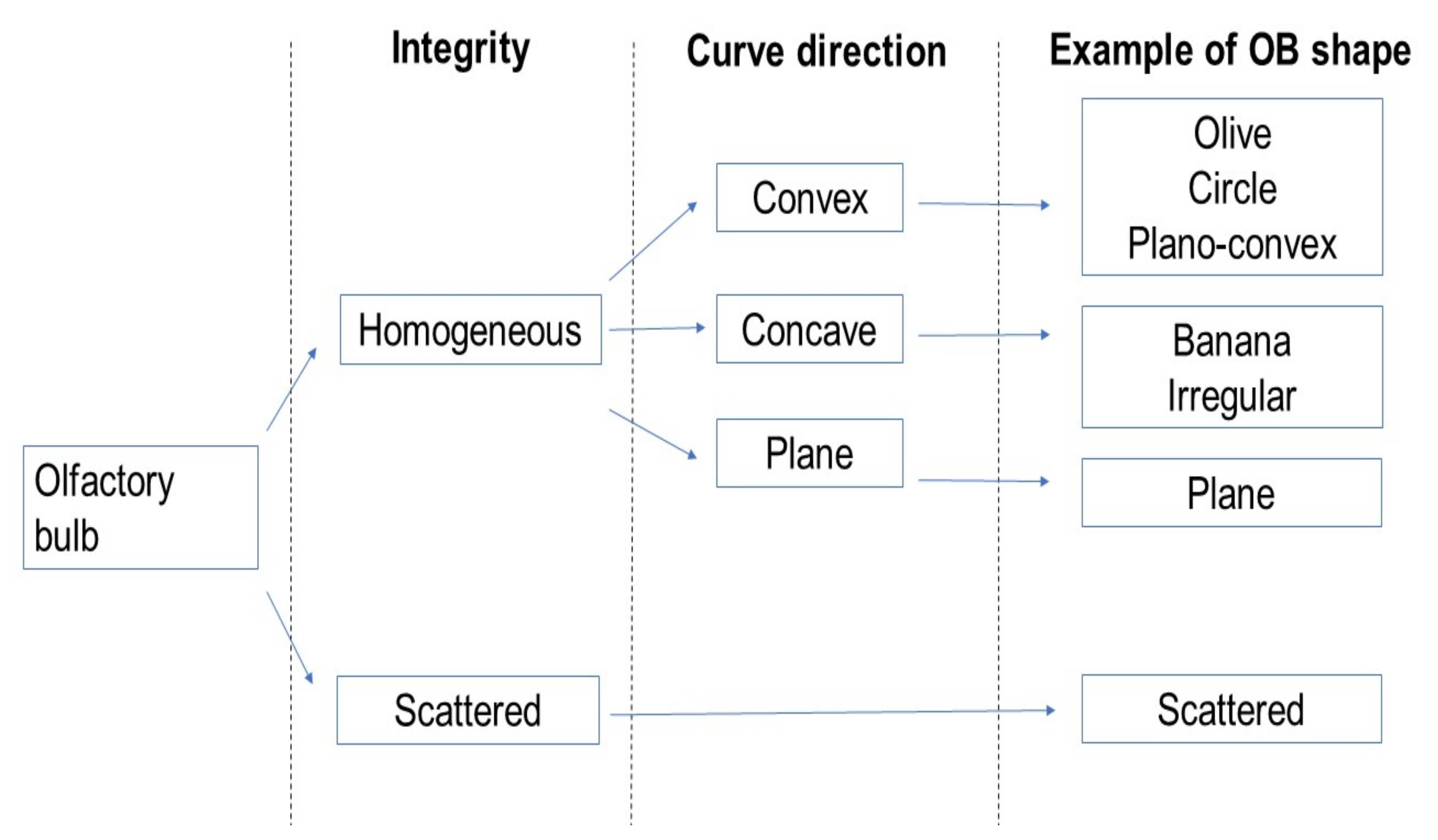
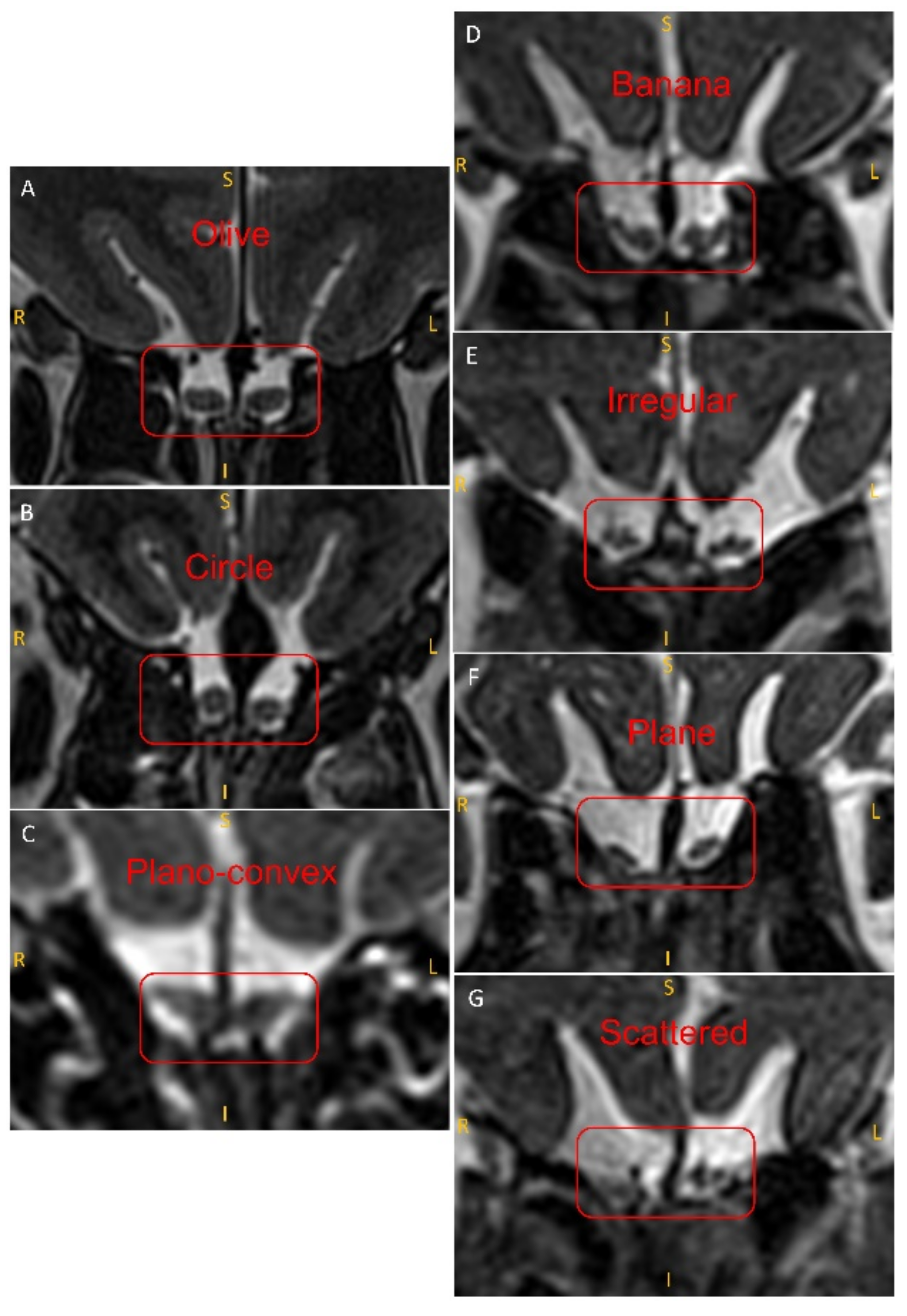
2.4. Evaluation of the Shape of the OB
2.5. Statistical Analysis
3. Results
3.1. Participant Demographics
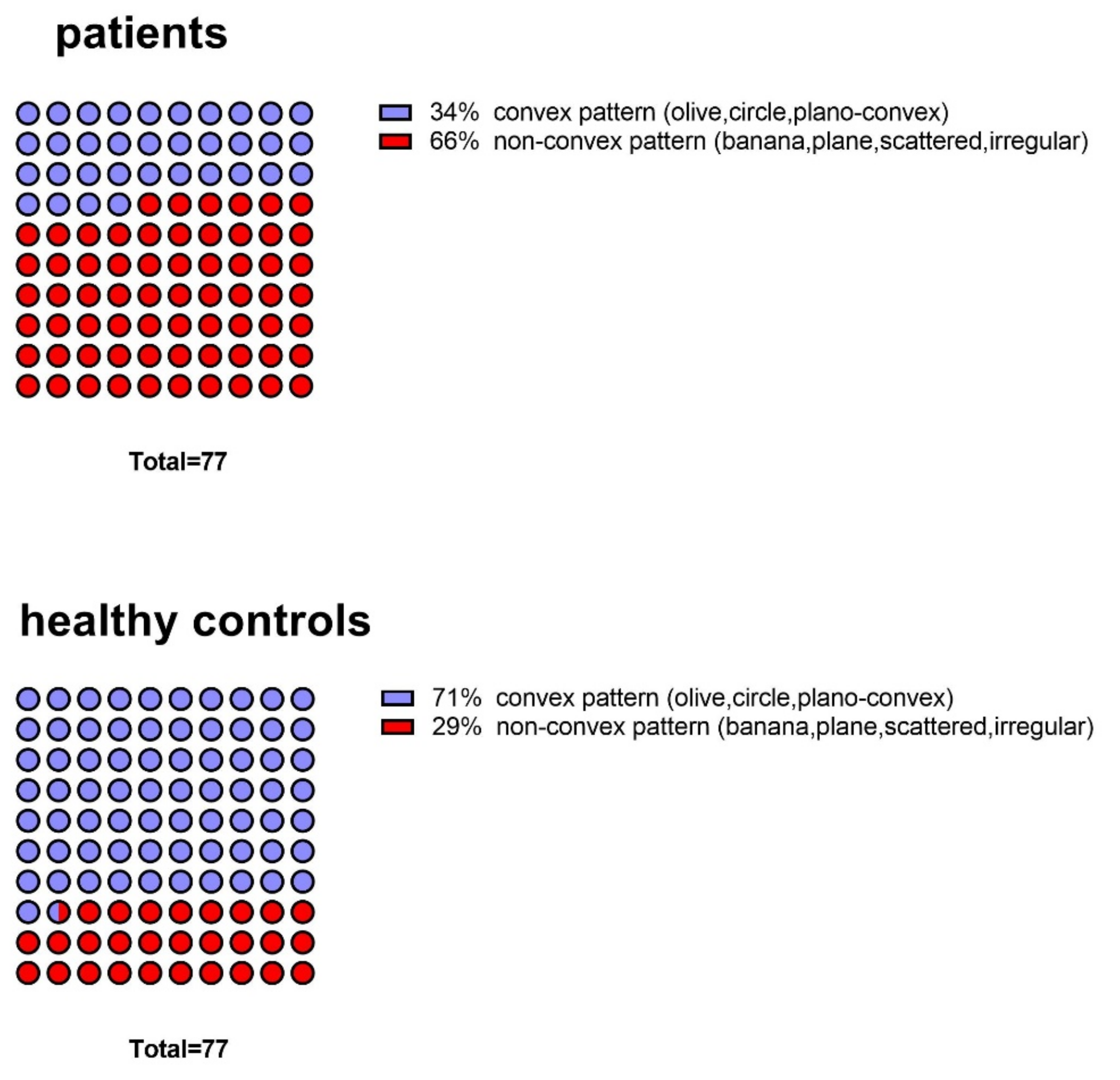
3.2. Comparison of Patients and Controls in Terms of OB Shapes
3.3. Correlation of OB Shapes with the Olfactory Function
3.4. OB Shape and OB Volume
3.5. Correlation of OB Shape with Age
3.6. OB Shapes Associated with the Causes of Olfactory Loss in Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andres, K.H. Anatomy and Ultkastkuctuke of the Olfactory Bulb in Fish, Amphibia, Reptiles, Birds and Mammals. In Ciba Foundation Symposium—Taste and Smell in Vertebrates; Novartis Foundation Symposia: Chichester, UK, 1970; pp. 177–196. [Google Scholar]
- Liem, K.F.; Bemis, W.E.; Walker, W.F.; Grande, L. Functional Anatomy of the Vertebrates: An Evolutionary Perspective; Cengage Learning: Boston, MA, USA, 2001. [Google Scholar]
- Curtis, M.A.; Kam, M.; Nannmark, U.; Anderson, M.F.; Axell, M.Z.; Wikkelso, C.; Holtas, S.; van Roon-Mom, W.M.; Bjork-Eriksson, T.; Nordborg, C.; et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007, 315, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Huart, C.; Rombaux, P.; Hummel, T. Plasticity of the human olfactory system: The olfactory bulb. Molecules 2013, 18, 11586–11600. [Google Scholar] [CrossRef] [Green Version]
- Axel, R. The molecular logic of smell. Sci. Am. 1995, 273, 154–159. [Google Scholar] [CrossRef]
- Zou, D.-J.; Chesler, A.; Firestein, S. How the olfactory bulb got its glomeruli: A just so story? Nat. Rev. Neurosci. 2009, 10, 611–618. [Google Scholar] [CrossRef]
- Yousem, D.M.; Geckle, R.J.; Bilker, W.B.; Doty, R.L. Olfactory bulb and tract and temporal lobe volumes: Normative data across decades. Ann. N. Y. Acad. Sci. 1998, 855, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Whitcroft, K.L.; Hummel, T. Clinical Diagnosis and Current Management Strategies for Olfactory Dysfunction: A Review. JAMA Otolaryngol.-Head Neck Surg. 2019, 145, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Buschhüter, D.; Smitka, M.; Puschmann, S.; Gerber, J.C.; Witt, M.; Abolmaali, N.D.; Hummel, T. Correlation between olfactory bulb volume and olfactory function. Neuroimage 2008, 42, 498–502. [Google Scholar] [CrossRef]
- Gudziol, V.; Buschhüter, D.; Abolmaali, N.; Gerber, J.; Rombaux, P.; Hummel, T. Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis—A longitudinal study. Brain A J. Neurol. 2009, 132, 3096–3101. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhinology 2016, 56, 1–30. [Google Scholar] [CrossRef]
- Welge-Luessen, A.; Leopold, D.; Miwa, T. Smell and taste disorders—Diagnostic and clinical work-up. In Management of Smell and Taste Disorders—A Practical Guide for Clinicians; Thieme: Stuttgart, Germany, 2013; pp. 49–57. [Google Scholar]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odor Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Rombaux, P.; Potier, H.; Markessis, E.; Duprez, T.; Hummel, T. Olfactory bulb volume and depth of olfactory sulcus in patients with idiopathic olfactory loss. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 1551–1556. [Google Scholar] [CrossRef]
- Mazal, P.P.; Haehner, A.; Hummel, T. Relation of the volume of the olfactory bulb to psychophysical measures of olfactory function. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1–7. [Google Scholar] [CrossRef]
- Hummel, T.; Urbig, A.; Huart, C.; Duprez, T.; Rombaux, P. Volume of olfactory bulb and depth of olfactory sulcus in 378 consecutive patients with olfactory loss. J. Neurol. 2015, 262, 1046–1051. [Google Scholar] [CrossRef]
- Schneider, J.F.; Floemer, F. Maturation of the olfactory bulbs: MR imaging findings. AJNR Am. J. Neuroradiol. 2009, 30, 1149–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmeister, H.P.; Bitter, T.; Heiler, P.M.; Irintchev, A.; Fröber, R.; Dietzel, M.; Baltzer, P.A.; Schad, L.R.; Reichenbach, J.R.; Gudziol, H.; et al. Imaging of lamination patterns of the adult human olfactory bulb and tract: In vitro comparison of standard- and high-resolution 3T MRI, and MR microscopy at 9.4 T. Neuroimage 2012, 60, 1662–1670. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Jones-Gotman, M.; Evans, A.C.; Meyer, E. Functional localization and lateralization of human olfactory cortex. Nature 1992, 360, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, A.; Kunkel, F.; Larsson, M.; Hummel, T. Consequences of undetected olfactory loss for human chemosensory communication and well-being. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2020, 375, 20190265. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, J.; Hummel, T. Clinical Usefulness of Self-Rated Olfactory Performance-A Data Science-Based Assessment of 6000 Patients. Chem. Senses 2019, 44, 357–364. [Google Scholar] [CrossRef]
- Yao, L.; Yi, X.; Pinto, J.M.; Yuan, X.; Guo, Y.; Liu, Y.; Wei, Y. Olfactory cortex and Olfactory bulb volume alterations in patients with post-infectious Olfactory loss. Brain Imaging Behav. 2018, 12, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Negoias, S.; Pietsch, K.; Hummel, T. Changes in olfactory bulb volume following lateralized olfactory training. Brain Imaging Behav. 2017, 11, 998–1005. [Google Scholar] [CrossRef]
- Maresh, A.; Gil, D.R.; Whitman, M.C.; Greer, C.A. Principles of glomerular organization in the human olfactory bulb–implications for odor processing. PLoS ONE 2008, 3, e2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogland, P.V.; van den Berg, R.; Huisman, E. Misrouted olfactory fibres and ectopic olfactory glomeruli in normal humans and in Parkinson and Alzheimer patients. Neuropathol. Appl. Neurobiol. 2003, 29, 303–311. [Google Scholar] [CrossRef]
- Zapiec, B.; Dieriks, B.V.; Tan, S.; Faull, R.L.M.; Mombaerts, P.; Curtis, M.A. A ventral glomerular deficit in Parkinson’s disease revealed by whole olfactory bulb reconstruction. Brain 2017, 140, 2722–2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altundag, A.; Salihoglu, M.; Tekeli, H.; Saglam, M.; Cayonu, M.; Hummel, T. Lateralized differences in olfactory function and olfactory bulb volume relate to nasal septum deviation. J. Craniofac. Surg. 2014, 25, 359–362. [Google Scholar] [CrossRef]
- Ennis, M.; Holy, T.E. Anatomy and neurobiology of the main and accessory olfactory bulbs. In Handbook of Olfaction and Gustation; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 157–182. [Google Scholar]
- Haehner, A.; Hummel, T.; Reichmann, H. Olfactory loss in Parkinson’s disease. Parkinsons Dis. 2011, 2011, 450939. [Google Scholar] [CrossRef] [Green Version]
- Croy, I.; Hummel, T. Olfaction as a marker for depression. J. Neurol. 2017, 264, 631–638. [Google Scholar] [CrossRef]
- Hummel, T.; Haehner, A.; Hummel, C.; Croy, I.; Iannilli, E. Lateralized differences in olfactory bulb volume relate to lateralized differences in olfactory function. Neuroscience 2013, 237, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Breton-Provencher, V.; Bakhshetyan, K.; Hardy, D.; Bammann, R.R.; Cavarretta, F.; Snapyan, M.; Côté, D.; Migliore, M.; Saghatelyan, A. Principal cell activity induces spine relocation of adult-born interneurons in the olfactory bulb. Nat. Commun. 2016, 7, 12659. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Yu, B.; Komiyama, T. Plasticity in olfactory bulb circuits. Curr. Opin. Neurobiol. 2020, 64, 17–23. [Google Scholar] [CrossRef]
- Hasegawa, S.; Yamagishi, M.; Nakano, Y. Microscopic studies of human olfactory epithelia following traumatic anosmia. Arch. Otorhinolaryngol. 1986, 243, 112–116. [Google Scholar] [CrossRef]
- Naessen, R. An enquiry on the morphological characteristics and possible changes with age in the olfactory region of man. Acta Oto-Laryngol. 1971, 71, 49–62. [Google Scholar] [CrossRef]
- Mombaerts, P.; Wang, F.; Dulac, C.; Chao, S.K.; Nemes, A.; Mendelsohn, M.; Edmondson, J.; Axel, R. Visualizing an olfactory sensory map. Cell 1996, 87, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Ekdale, E.G. Form and function of the mammalian inner ear. J. Anat. 2016, 228, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Verkicharla, P.K.; Mathur, A.; Mallen, E.A.; Pope, J.M.; Atchison, D.A. Eye shape and retinal shape, and their relation to peripheral refraction. Ophthalmic. Physiol. Opt. 2012, 32, 184–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousem, D.M.; Geckle, R.J.; Bilker, W.B.; Kroger, H.; Doty, R.L. Posttraumatic smell loss: Relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad. Radiol. 1999, 6, 264–272. [Google Scholar] [CrossRef]
- Rombaux, P.; Mouraux, A.; Bertrand, B.; Nicolas, G.; Duprez, T.; Hummel, T. Retronasal and orthonasal olfactory function in relation to olfactory bulb volume in patients with posttraumatic loss of smell. Laryngoscope 2006, 116, 901–905. [Google Scholar] [CrossRef] [Green Version]
- Meisami, E.; Mikhail, L.; Baim, D.; Bhatnagar, K.P. Human olfactory bulb: Aging of glomeruli and mitral cells and a search for the accessory olfactory bulb. Ann. N. Y. Acad. Sci. 1998, 855, 708–715. [Google Scholar] [CrossRef]
- Bhatnagar, K.P.; Kennedy, R.C.; Baron, G.; Greenberg, R.A. Number of mitral cells and the bulb volume in the aging human olfactory bulb: A quantitative morphological study. Anat. Rec. 1987, 218, 73–87. [Google Scholar] [CrossRef]
- Richard, M.B.; Taylor, S.R.; Greer, C.A. Age-induced disruption of selective olfactory bulb synaptic circuits. Proc. Natl. Acad. Sci. USA 2010, 107, 15613–15618. [Google Scholar] [CrossRef] [Green Version]
- Mirich, J.M.; Williams, N.C.; Berlau, D.J.; Brunjes, P.C. Comparative study of aging in the mouse olfactory bulb. J. Comp. Neurol. 2002, 454, 361–372. [Google Scholar] [CrossRef]
- Hummel, T.; Oleszkiewicz, A. Age-related changes of chemosensory function. In The Senses: A Comprehensive Reference; Fritzsch, B., Ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]


| Characteristic | Before Matching | After Matching | |||
|---|---|---|---|---|---|
| Patients | Patients | Healthy Control | Statistics | p | |
| Age (mean [SD]) | 57 [12.8] | 51 [15.2] | 51 [15.5] | t = 0.07 | NS |
| Gender (number (%)) | χ2 = 0 | NS | |||
| Male (n (%)) | 86 (45) | 39 (49) | 39 (49) | ||
| Female (n (%)) | 106 (55) | 38 (51) | 38 (51) | ||
| Total (n) | 192 | 77 | 77 | ||
| Smell function (mean [SD]) | |||||
| TDI | 18.1 [7.6] | 18.6 [8.5] | 33.5 [4.2] | t = 13.7 | p < 0.001 |
| T | 2.7 [2.7] | 3.2 [3.1] | 7.4 [2.9] | t = 8.6 | p < 0.001 |
| D | 8.2 [3.0] | 8.4 [3.0] | 12.5 [2.1] | t = 10.2 | p < 0.001 |
| I | 7.4 [3.5] | 7.4 [4.1] | 13.6 [1.5] | t = 12.3 | p < 0.001 |
| Characteristic | Convex Pattern | Non-Convex Pattern | Statistic | p |
|---|---|---|---|---|
| Age (mean [SD]) | 51 [15.2] | 51 [15.5] | t = 0.106 | NS |
| Gender, n (%) | χ2 = 0.922 | NS | ||
| Male (n (%)) | 44 (54) | 34 (47) | ||
| Female (n (%)) | 37 (46) | 39 (53) | ||
| Total (n) | 81 | 73 | ||
| Smell function (median) | ||||
| TDI | 32.8 | 21.5 | U = 1703.500 | p < 0.001 |
| T | 6.5 | 2.3 | U = 1644.500 | p < 0.001 |
| D | 12 | 10 | U = 1967.500 | p < 0.001 |
| I | 13 | 9 | U = 1869.000 | p < 0.001 |
| Causes, OB Shape | Olive, no. (%) | Circle, no. (%) | Plano-Convex, no. (%) | Banana, no. (%) | Irregular, no. (%) | Plane, no. (%) | Scattered, no. (%) | Total |
|---|---|---|---|---|---|---|---|---|
| Sinonasal | 4 (19.0%) | 3 (14.3%) | 2 (9.5%) | 6 (28.6%) | 3 (14.3%) | 1 (4.8%) | 2 (4.8%) | 21 |
| idiopathic | 6 (10.5%) | 5 (8.8%) | 1 (1.8%) | 18 (31.6%) | 9 (15.8%) | 4 (7.0%) | 14 (24.6%) | 57 |
| Parkinson’s disease | 1 (9.1%) | 4 (36.4%) | 0 (0.0%) | 3 (27.3%) | 3 (27.3%) | 0 (0.0%) | 0 (0.0%) | 11 |
| PIOD | 3 (5.2%) | 9 (15.5%) | 7 (12.1%) | 15 (25.9%) | 9 (15.5%) | 11 (19.0%) | 7 (12.1%) | 58 |
| PTOD | 9 (20.0%) | 0 (0.0%) | 4 (8.9%) | 11 (24.4%) | 12 (26.7%) | 3 (6.7%) | 4 (8.9%) | 45 |
| Total, no. | 53 | 36 | 23 | 21 | 19 | 26 | 14 | 192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Joshi, A.; Zang, Y.; Assunção, F.; Fernandes, H.M.; Hummel, T. The Shape of the Olfactory Bulb Predicts Olfactory Function. Brain Sci. 2022, 12, 128. https://doi.org/10.3390/brainsci12020128
Yan X, Joshi A, Zang Y, Assunção F, Fernandes HM, Hummel T. The Shape of the Olfactory Bulb Predicts Olfactory Function. Brain Sciences. 2022; 12(2):128. https://doi.org/10.3390/brainsci12020128
Chicago/Turabian StyleYan, Xiaoguang, Akshita Joshi, Yunpeng Zang, Francisca Assunção, Henrique M. Fernandes, and Thomas Hummel. 2022. "The Shape of the Olfactory Bulb Predicts Olfactory Function" Brain Sciences 12, no. 2: 128. https://doi.org/10.3390/brainsci12020128
APA StyleYan, X., Joshi, A., Zang, Y., Assunção, F., Fernandes, H. M., & Hummel, T. (2022). The Shape of the Olfactory Bulb Predicts Olfactory Function. Brain Sciences, 12(2), 128. https://doi.org/10.3390/brainsci12020128






