EEG Correlates of Active Stopping and Preparation for Stopping in Chronic Tic Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anticipated Response Stop Signal Task (Slater-Hammel Task)
2.3. EEG Recording and Pre-Processing
2.4. EEG Source Modeling
2.5. EEG Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic, Clinical, and Behavioral Data
3.2. EEG: Quality Control
3.3. EEG: Outright Stopping (Stop Trials)
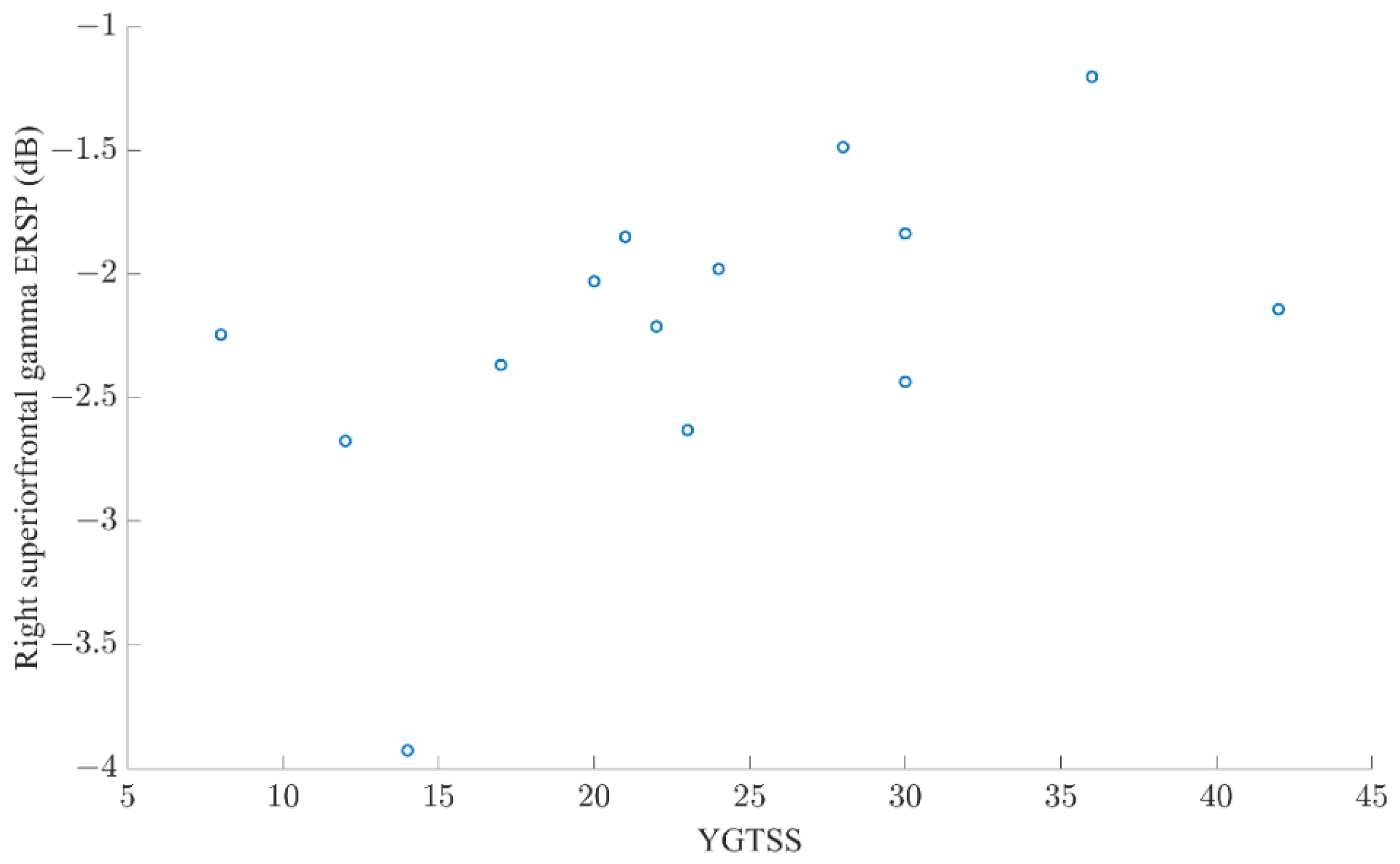
3.4. EEG: Preparation for Possibly Stopping (All Trials)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knight, T.; Steeves, T.; Day, L.; Lowerison, M.; Jette, N.; Pringsheim, T. Prevalence of tic disorders: A systematic review and meta-analysis. Pediatr. Neurol. 2012, 47, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Seri, S.; Cavanna, A.E. The effects of Gilles de la Tourette syndrome and other chronic tic disorders on quality of life across the lifespan: A systematic review. Eur. Child Adolesc. Psychiatry 2016, 25, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, M.-Y.C.; Shahed, J.; Jankovic, J. Malignant Tourette syndrome. Mov. Disord. 2007, 22, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Zhang, H.; Vitale, A.; Lahnin, F.; Lynch, K.; Bondi, C.; Kim, Y.; Peterson, B.S. Course of tic severity in Tourette syndrome: The first two decades. Pediatrics 1998, 102, 14–19. [Google Scholar] [CrossRef]
- Banaschewski, T.; Woerner, W.; Rothenberger, A. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: Developmental aspects in children and adolescents. Dev. Med. Child Neurol. 2003, 45, 700–703. [Google Scholar] [CrossRef]
- Jackson, G.M.; Draper, A.; Dyke, K.; Pépés, S.; Jackson, S.R. Inhibition, Disinhibition, and the Control of Action in Tourette Syndrome. Trends Cogn. Sci. 2015, 19, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Greene, D.J.; Robichaux-Viehoever, A.; Bihun, E.C.; Koller, J.M.; Acevedo, H.; Schlaggar, B.L.; Black, K.J. Tic Suppression in Children with Recent-Onset Tics Predicts 1-Year Tic Outcome. J. Child Neurol. 2019, 34, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Morand-Beaulieu, S.; Grot, S.; Lavoie, J.; Leclerc, J.B.; Luck, D.; Lavoie, M.E. The puzzling question of inhibitory control in Tourette syndrome: A meta-analysis. Neurosci. Biobehav. Rev. 2017, 80, 240–262. [Google Scholar] [CrossRef]
- Jung, J.; Jackson, S.R.; Parkinson, A.; Jackson, G.M. Cognitive control over motor output in Tourette syndrome. Neurosci. Biobehav. Rev. 2013, 37, 1016–1025. [Google Scholar] [CrossRef]
- Verbruggen, F.; Aron, A.R.; Band, G.P.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H.; et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 2019, 8, 8. [Google Scholar] [CrossRef]
- Aron, A.R. From Reactive to Proactive and Selective Control: Developing a Richer Model for Stopping Inappropriate Responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef] [Green Version]
- Leunissen, I.; Zandbelt, B.B.; Potocanac, Z.; Swinnen, S.P.; Coxon, J.P. Reliable estimation of inhibitory efficiency: To anticipate, choose, or simply react? Eur. J. Neurosci. 2017, 45, 1512–1523. [Google Scholar] [CrossRef] [Green Version]
- Gavazzi, G.; Giovannelli, F.; Currò, T.; Mascalchi, M.; Viggiano, M.P. Contiguity of proactive and reactive inhibitory brain areas: A cognitive model based on ALE meta-analyses. Brain Imaging Behav. 2021, 15, 2199–2214. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Pekar, J.; Mostofsky, S.H. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 2008, 46, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Swann, N.C.; Cai, W.; Conner, C.R.; Pieters, T.A.; Claffey, M.P.; George, J.S.; Aron, A.R.; Tandon, N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. NeuroImage 2012, 59, 2860–2870. [Google Scholar] [CrossRef] [Green Version]
- Chikazoe, J.; Jimura, K.; Hirose, S.; Yamashita, K.-I.; Miyashita, Y.; Konishi, S. Preparation to Inhibit a Response Complements Response Inhibition during Performance of a Stop-Signal Task. J. Neurosci. 2009, 29, 15870–15877. [Google Scholar] [CrossRef]
- Fonken, Y.; Rieger, J.; Tzvi, E.; Crone, N.E.; Chang, E.; Parvizi, J.; Knight, R.T.; Krämer, U.M. Frontal and motor cortex contributions to response inhibition: Evidence from electrocorticography. J. Neurophysiol. 2016, 115, 2224–2236. [Google Scholar] [CrossRef]
- Wagner, J.; Wessel, J.; Ghahremani, A.; Aron, A.R. Establishing a Right Frontal Beta Signature for Stopping Action in Scalp EEG: Implications for Testing Inhibitory Control in Other Task Contexts. J. Cogn. Neurosci. 2018, 30, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Wessel, J.R. β-Bursts Reveal the Trial-to-Trial Dynamics of Movement Initiation and Cancellation. J. Neurosci. 2020, 40, 411–423. [Google Scholar] [CrossRef]
- Chang, A.; Ide, J.S.; Li, H.-H.; Chen, C.-C.; Li, C.-S.R. Proactive Control: Neural Oscillatory Correlates of Conflict Anticipation and Response Slowing. Eneuro 2017, 4, 4. [Google Scholar] [CrossRef]
- Cooper, P.S.; Wong, A.S.; McKewen, M.; Michie, P.T.; Karayanidis, F. Frontoparietal theta oscillations during proactive control are associated with goal-updating and reduced behavioral variability. Biol. Psychol. 2017, 129, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, L.; Yu, S.; Blair, C.; Gunter, B.C.; Wang, Z.; Marsh, R.; Peterson, B.S. An fMRI Study of Frontostriatal Circuits During the Inhibition of Eye Blinking in Persons with Tourette Syndrome. Am. J. Psychiatry 2010, 167, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, B.S.; Skudlarski, P.; Anderson, A.W.; Zhang, H.; Gatenby, J.C.; Lacadie, C.M.; Leckman, J.F.; Gore, J.C. A Functional Magnetic Resonance Imaging Study of Tic Suppression in Tourette Syndrome. Arch. Gen. Psychiatry 1998, 55, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Salm, S.M.; van der Meer, J.N.; Cath, D.C.; Groot, P.F.; van der Werf, Y.D.; Brouwers, E.; de Wit, S.J.; Coppens, J.C.; Nederveen, A.J.; van Rootselaar, A.-F.; et al. Distinctive tics suppression network in Gilles de la Tourette syndrome distinguished from suppression of natural urges using multimodal imaging. NeuroImage Clin. 2018, 20, 783–792. [Google Scholar] [CrossRef]
- Marsh, R.; Zhu, H.; Wang, Z.; Skudlarski, P.; Peterson, B.S. A developmental fMRI study of self-regulatory control in Tourette’s syndrome. Am. J. Psychiatry. 2007, 164, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Ganos, C.; Kühn, S.; Kahl, U.; Schunke, O.; Feldheim, J.; Gerloff, C.; Roessner, V.; Bäumer, T.; Thomalla, G.; Haggard, P.; et al. Action inhibition in Tourette syndrome. Mov. Disord. 2014, 29, 1532–1538. [Google Scholar] [CrossRef]
- Thomalla, G.; Jonas, M.; Bäumer, T.; Siebner, H.R.; Biermann-Ruben, K.; Ganos, C.; Orth, M.; Hummel, F.C.; Gerloff, C.; Müller-Vahl, K.; et al. Costs of control: Decreased motor cortex engagement during a Go/NoGo task in Tourette’s syndrome. Brain 2014, 137, 122–136. [Google Scholar] [CrossRef] [Green Version]
- Baym, C.L.; Corbett, B.A.; Wright, S.B.; Bunge, S.A. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain 2008, 131, 165–179. [Google Scholar] [CrossRef] [Green Version]
- Niccolai, V.; Van Dijk, H.; Franzkowiak, S.; Finis, J.; Südmeyer, M.; Jonas, M.; Thomalla, G.; Siebner, H.R.; Müller-Vahl, K.; Münchau, A.; et al. Increased beta rhythm as an indicator of inhibitory mechanisms in tourette syndrome. Mov. Disord. 2016, 31, 384–392. [Google Scholar] [CrossRef]
- Zapparoli, L.; Macerollo, A.; Joyce, E.M.; Martino, D.; Kilner, J.M. Voluntary tic suppression and the normalization of motor cortical beta power in Gilles de la Tourette syndrome: An EEG study. Eur. J. Neurosci. 2019, 50, 3944–3957. [Google Scholar] [CrossRef]
- Franzkowiak, S.; Pollok, B.; Biermann-Ruben, K.; Südmeyer, M.; Paszek, J.; Jonas, M.; Thomalla, G.; Bäumer, T.; Orth, M.; Münchau, A.; et al. Altered pattern of motor cortical activation-inhibition during voluntary movements in Tourette syndrome. Mov. Disord. 2010, 25, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Serrien, D.J.; Orth, M.; Evans, A.H.; Lees, A.J.; Brown, P. Motor inhibition in patients with Gilles de la Tourette syndrome: Functional activation patterns as revealed by EEG coherence. Brain 2005, 128, 116–125. [Google Scholar] [CrossRef]
- Hong, H.J.; Sohn, H.; Cha, M.; Kim, S.; Oh, J.; Chu, M.K.; Namkoong, K.; Jeong, J. Increased Frontomotor Oscillations During Tic Suppression in Children with Tourette Syndrome. J. Child Neurol. 2013, 28, 615–624. [Google Scholar] [CrossRef]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial Testing of a Clinician-Rated Scale of Tic Severity. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef]
- Woods, D.W.; Piacentini, J.; Himle, M.B.; Chang, S. Premonitory Urge for Tics Scale (PUTS): Initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. J. Dev. Behav. Pediatr. 2005, 26, 397–403. [Google Scholar] [CrossRef]
- Scahill, L.; Riddle, M.A.; McSWiggin-Hardin, M.; Ort, S.I.; King, R.A.; Goodman, W.K.; Cicchetti, D.; Leckman, J.F. Children’s Yale-Brown Obsessive Compulsive Scale: Reliability and Validity. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 844–852. [Google Scholar] [CrossRef]
- DuPaul, G.J.; Power, T.J.; Anastopoulos, A.D.; Reid, R. ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation; Guilford Press: New York, NY, USA, 1998. [Google Scholar]
- Guthrie, M.D.; Gilbert, D.L.; Huddleston, D.A.; Pedapati, E.V.; Horn, P.S.; Mostofsky, S.H.; Wu, S.W. Online Transcranial Magnetic Stimulation Protocol for Measuring Cortical Physiology Associated with Response Inhibition. J. Vis. Exp. 2018, 132, e56789. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Maiquez, B.M.; Jackson, G.M.; Jackson, S.R. Examining the neural antecedents of tics in Tourette syndrome using electroencephalography. J. Neuropsychol. 2022, in press. [Google Scholar] [CrossRef]
- Richards, J.E.; Sanchez, C.; Phillips-Meek, M.; Xie, W. A database of age-appropriate average MRI templates. NeuroImage 2016, 124, 1254–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; The MIT Press: Cambridge, MS, USA, 2014. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lipszyc, J.; Schachar, R. Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. J. Int. Neuropsychol. Soc. 2010, 16, 1064–1076. [Google Scholar] [CrossRef]
- Zandbelt, B.B.; Bloemendaal, M.; Neggers, S.F.; Kahn, R.S.; Vink, M. Expectations and violations: Delineating the neural network of proactive inhibitory control. Hum. Brain Mapp. 2013, 34, 2015–2024. [Google Scholar] [CrossRef]
- Ishii, R.; Canuet, L.; Ishihara, T.; Aoki, Y.; Ikeda, S.; Hata, M.; Katsimichas, T.; Gunji, A.; Takahashi, H.; Nakahachi, T.; et al. Frontal midline theta rhythm and gamma power changes during focused attention on mental calculation: An MEG beamformer analysis. Front. Hum. Neurosci. 2014, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Rosen, A.; Reiner, M. Right frontal gamma and beta band enhancement while solving a spatial puzzle with insight. Int. J. Psychophysiol. 2017, 122, 50–55. [Google Scholar] [CrossRef]
- Foy, J.G.; Foy, M.R. Dynamic Changes in EEG Power Spectral Densities During NIH-Toolbox Flanker, Dimensional Change Card Sort Test and Episodic Memory Tests in Young Adults. Front. Hum. Neurosci. 2020, 14, 158. [Google Scholar] [CrossRef]
- Loo, S.K.; Miyakoshi, M.; Tung, K.; Lloyd, E.; Salgari, G.; Dillon, A.; Chang, S.; Piacentini, J.; Makeig, S. Neural activation and connectivity during cued eye blinks in Chronic Tic Disorders. NeuroImage Clin. 2019, 24, 101956. [Google Scholar] [CrossRef]
- Hosaka, R.; Nakajima, T.; Aihara, K.; Yamaguchi, Y.; Mushiake, H. The Suppression of Beta Oscillations in the Primate Supplementary Motor Complex Reflects a Volatile State During the Updating of Action Sequences. Cereb. Cortex 2016, 26, 3442–3452. [Google Scholar] [CrossRef] [Green Version]
- Hampshire, A.; Sharp, D.J. Contrasting network and modular perspectives on inhibitory control. Trends Cogn. Sci. 2015, 19, 445–452. [Google Scholar] [CrossRef]
- Levy, B.J.; Wagner, A.D. Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Ann. N. Y. Acad. Sci. 2011, 1224, 40–62. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Obeso, I.; Rothwell, J.; Obeso, J.A. A fronto–striato–subthalamic–pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 2015, 16, 719–732. [Google Scholar] [CrossRef]
- Rawji, V.; Modi, S.; Latorre, A.; Rocchi, L.; Hockey, L.; Bhatia, K.; Joyce, E.; Rothwell, J.C.; Jahanshahi, M. Impaired automatic but intact volitional inhibition in primary tic disorders. Brain 2020, 143, 906–919. [Google Scholar] [CrossRef]
- Stenner, M.-P.; Baumgaertel, C.; Heinze, H.-J.; Ganos, C.; Müller-Vahl, K.R. Intact automatic motor inhibition in patients with tourette syndrome. Mov. Disord. 2018, 33, 1800–1804. [Google Scholar] [CrossRef]
- Buckner, R.L.; Di Nicola, L.M. The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef]
- McKiernan, K.A.; D’Angelo, B.R.; Kaufman, J.N.; Binder, J. Interrupting the “stream of consciousness”: An fMRI investigation. NeuroImage 2006, 29, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Heuvel, O.A.V.D.; Cath, D.C.; De Wit, S.J.; Vriend, C.; Veltman, D.J.; van der Werf, Y. Altered Functional Connectivity in Resting State Networks in Tourette’s Disorder. Front. Hum. Neurosci. 2018, 12, 363. [Google Scholar] [CrossRef]
- Church, J.A.; Fair, D.A.; Dosenbach, N.U.F.; Cohen, A.; Miezin, F.M.; Petersen, S.E.; Schlaggar, B.L. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain 2009, 132, 225–238. [Google Scholar] [CrossRef]
- Schmidt, R.; Ruiz, M.H.; Kilavik, B.E.; Lundqvist, M.; Starr, A.P.; Aron, A.R. Beta Oscillations in Working Memory, Executive Control of Movement and Thought, and Sensorimotor Function. J. Neurosci. 2019, 39, 8231–8238. [Google Scholar] [CrossRef] [Green Version]
- Zamorano, F.; Billeke, P.; Hurtado, J.M.; López, V.; Carrasco, X.; Ossandón, T.; Aboitiz, F. Temporal Constraints of Behavioral Inhibition: Relevance of Inter-stimulus Interval in a Go-Nogo Task. PLoS ONE 2014, 9, e87232. [Google Scholar] [CrossRef] [PubMed]
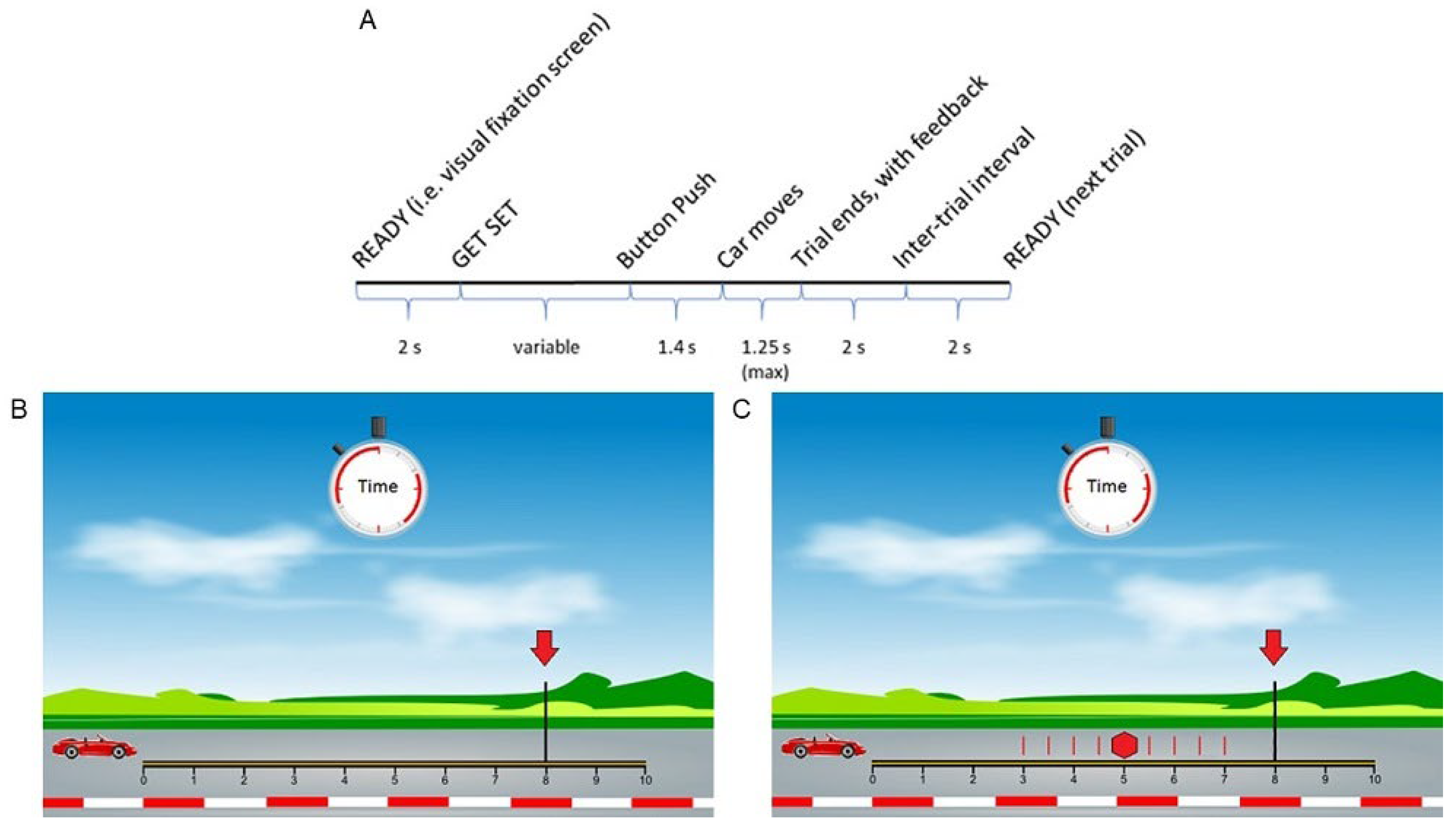



| Participant | Diagnosis | Age | Sex | Handed-ness | YGTSS | PUTS | DuPaul ADHD Scale | CY–BOCS | Medication(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TS | 13 | M | L | 30 | 19 | 21 | 0 | citalopram, gabapentin |
| 2 | TS | 14 | F | R | 22 | 11 | 15 | 1 | none |
| 3 | TS | 11 | M | R | 8 | 18 | 5 | 0 | none |
| 4 | TS | 14 | M | R | 21 | 26 | 11 | 0 | none |
| 5 | TS | 11 | M | R | 17 | 19 | 13 | 0 | none |
| 6 | TS | 12 | M | R | 36 | 16 | 18 | 16 | citalopram, clonidine, risperidone |
| 7 | TS | 15 | M | R | 42 | 21 | 40 | 21 | atomoxetine, fluvoxamine |
| 8 | TS | 14 | M | R | 23 | 29 | 21 | 0 | guanfacine, sertraline |
| 9 | TS | 11 | M | R | 20 | 23 | 22 | 0 | none |
| 10 | CMTD | 11 | F | R | 12 | 19 | 4 | 0 | none |
| 11 | TS | 16 | M | R | 14 | 28 | 29 | 22 | desvenlafaxine |
| 12 | TS | 15 | F | R | 30 | 31 | 30 | 22 | none |
| 13 | TS | 16 | M | R | 24 | 15 | 0 | 18 | none |
| 14 | TS | 13 | M | R | 28 | 27 | 13 | 0 | None |
| Chronic Tic Disorders (n = 14) | Healthy Controls (n = 13) | p Value (Diagnosis * Block) | |
|---|---|---|---|
| Probability of Inhibiting | 0.54 ± 0.04 | 0.54 ± 0.06 | 0.54 |
| Stop Signal Reaction Time (ms) | 249.6 + 36.3 | 249.4 + 40.5 | 0.70 |
| Stop Signal Delay (ms) | 548.4 ± 33.9 | 543.8 ± 44.8 | 0.35 |
| Go Reaction Time (ms) | 798.0 ± 25.2 | 793.2 ± 22.6 | 0.15 |
| Go trial success rate * | 0.48 ± 0.15 | 0.53 ± 0.16 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zea Vera, A.; Pedapati, E.V.; Larsh, T.R.; Kohmescher, K.; Miyakoshi, M.; Huddleston, D.A.; Jackson, H.S.; Gilbert, D.L.; Horn, P.S.; Wu, S.W. EEG Correlates of Active Stopping and Preparation for Stopping in Chronic Tic Disorder. Brain Sci. 2022, 12, 151. https://doi.org/10.3390/brainsci12020151
Zea Vera A, Pedapati EV, Larsh TR, Kohmescher K, Miyakoshi M, Huddleston DA, Jackson HS, Gilbert DL, Horn PS, Wu SW. EEG Correlates of Active Stopping and Preparation for Stopping in Chronic Tic Disorder. Brain Sciences. 2022; 12(2):151. https://doi.org/10.3390/brainsci12020151
Chicago/Turabian StyleZea Vera, Alonso, Ernest V. Pedapati, Travis R. Larsh, Kevin Kohmescher, Makoto Miyakoshi, David A. Huddleston, Hannah S. Jackson, Donald L. Gilbert, Paul S. Horn, and Steve W. Wu. 2022. "EEG Correlates of Active Stopping and Preparation for Stopping in Chronic Tic Disorder" Brain Sciences 12, no. 2: 151. https://doi.org/10.3390/brainsci12020151






