Direct and Indirect Effects of Blood Levels of Omega-3 and Omega-6 Fatty Acids on Reading and Writing (Dis)Abilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures and Procedure
2.2.1. Blood Measurements of Fatty Acids
2.2.2. Neuropsychological Tests
- (a)
- Cognitive measures. The Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) [53] and the Raven’s Coloured Progressive Matrices Test (CPM) [54,55] were adopted to assess inclusion criteria related to normal intelligence. verbal comprehension, perceptual reasoning, working memory, and processing speed indexes were calculated in addition to full scale IQ (FS-IQ) for the WISC-IV. The WISC-IV was part of the clinical assessment for children diagnosed with DD, whereas the CPM test was used for TDchildren.
- (b)
- Reading and writing tests.
- (c)
- Neuropsychological tests: auditory.
- (d)
- Neuropsychological tests: visual.
2.3. Data Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Correlations between PUFA Levels and Reading and Writing Measures
3.3. Correlations between Reading and Writing Measures and Visual/Auditory Neuropsychological Tasks
3.4. Correlations between PUFA Levels and Auditory/Visual Neuropsychological Functions
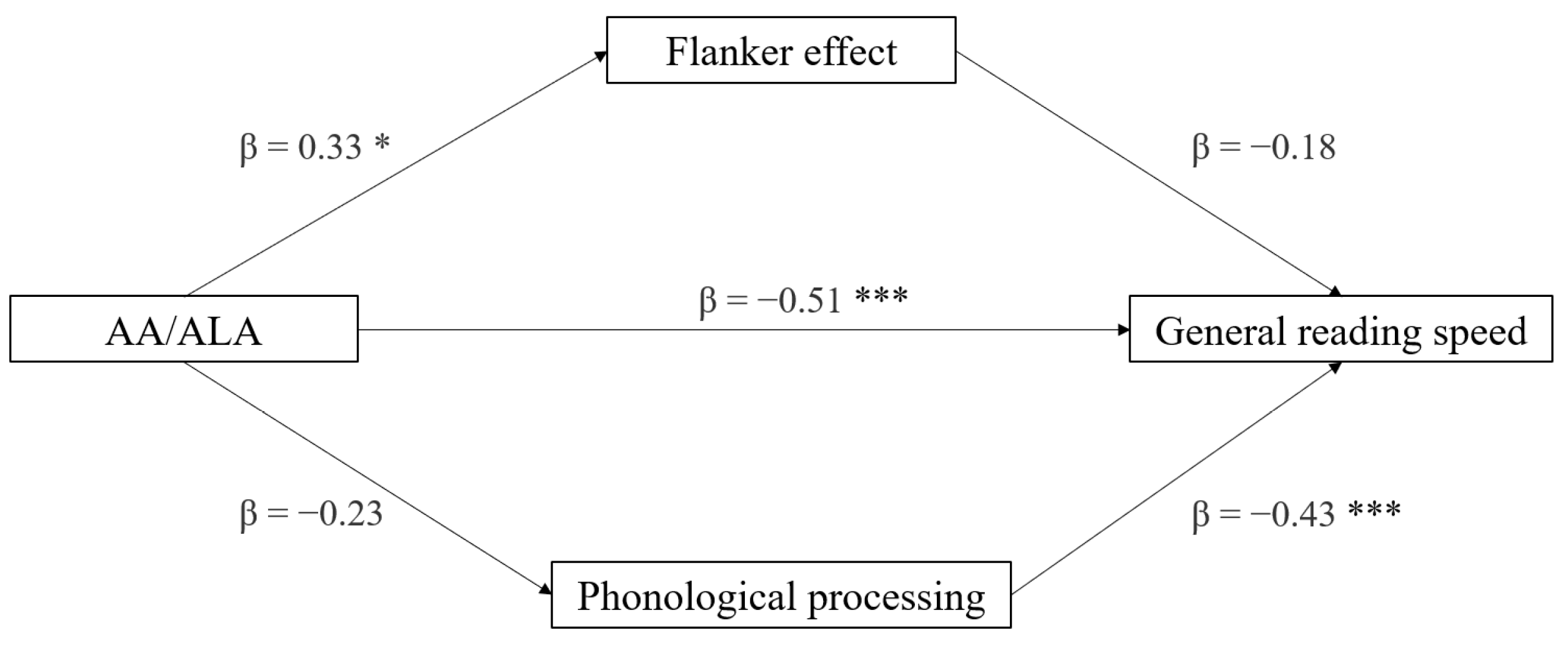
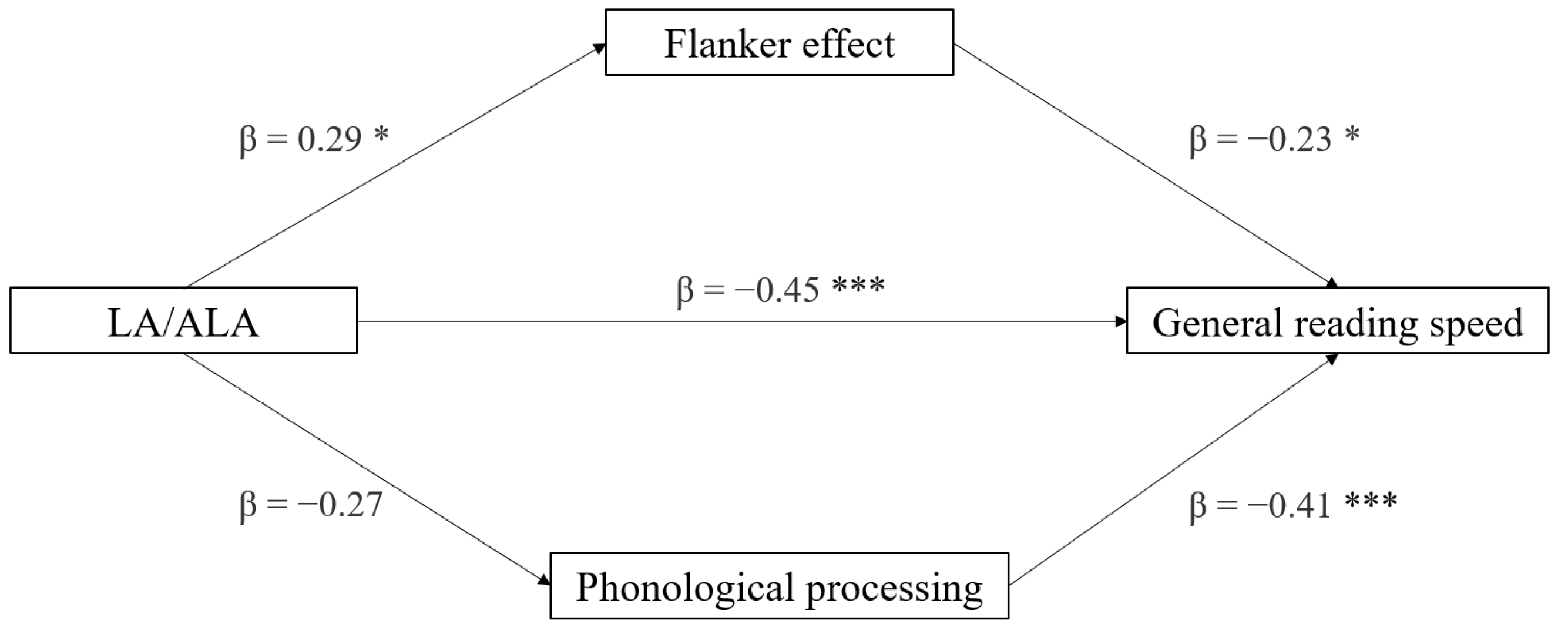
3.5. Mediation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyon, G.R.; Shaywitz, S.E.; Shaywitz, B.A. A definition of dyslexia. Ann. Dyslexia 2003, 53, 1–14. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems—10th Revision 2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Pennington, B.F. From single to multiple deficit models of developmental disorders. Cognition 2006, 101, 385–413. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.M.; Peterson, R.L.; Pennington, B.F. The multiple deficit model: Progress, problems, and prospects. Sci. Stud. Read. 2020, 24, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Annu. Rev. Clin. Psychol. 2015, 11, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F.; White, S.; Frith, U. Weighing the evidence between competing theories of dyslexia. Dev. Sci. 2006, 9, 265–269. [Google Scholar] [CrossRef]
- Facoetti, A.; Trussardi, A.N.; Ruffino, M.; Lorusso, M.L.; Cattaneo, C.; Galli, R.; Zorzi, M. Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. J. Cogn. NeuroSci. 2010, 22, 1011–1025. [Google Scholar] [CrossRef]
- Franceschini, S.; Gori, S.; Ruffino, M.; Viola, S.; Molteni, M.; Facoetti, A. Action video games make dyslexic children read better. Curr. Biol. 2013, 23, 462–466. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.; Walsh, V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997, 20, 147–152. [Google Scholar] [CrossRef]
- Valdois, S.; Reilhac, C.; Ginestet, E.; Line Bosse, M. Varieties of Cognitive Profiles in Poor Readers: Evidence for a VAS-Impaired Subtype. J. Learn. Disabil. 2020, 54, 221–233. [Google Scholar] [CrossRef]
- Facoetti, A.; Paganoni, P.; Lorusso, M.L. The spatial distribution of visual attention in developmental dyslexia. Exp. Brain Res. 2000, 132, 531–538. [Google Scholar] [CrossRef]
- Facoetti, A.; Zorzi, M.; Cestnick, L.; Lorusso, M.L.; Molteni, M.; Paganoni, P.; Umiltà, C.; Mascetti, G.G. The relationship between visuo-spatial attention and nonword reading in developmental dyslexia. Cogn. Neuropsychol. 2006, 23, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Matelli, M. Two different streams form the dorsal visual system: Anatomy and functions. Exp. Brain Res. 2003, 153, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Stein, J. The current status of the magnocellular theory of developmental dyslexia. Neuropsychologia 2019, 130, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Peters, J.L.; Parsons, C.; Crewther, D.P.; Crewther, S.G. Efficiency in Magnocellular Processing: A Common Deficit in Neurodevelopmental Disorders. Front. Hum. Neurosci. 2020, 14, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschle, N.M.; Stering, P.L.; Meissner, S.N.; Gaab, N. Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cereb. Cortex 2014, 24, 2489–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, G.; Lettvin, J.Y. Dyslexia and reading as examples of alternative visual strategies. In Brain and Reading; Von Euler, C., Lundberg, I., Lennerstrand, G., Eds.; International School for Advanced Studies (ISAS): Trieste, Italy, 1989; pp. 331–343. [Google Scholar]
- Spinelli, D.; De Luca, M.; Judica, A.; Zoccolotti, P. Crowding effects on word identification in developmental dyslexia. Cortex 2002, 38, 179–200. [Google Scholar] [CrossRef]
- Vidyasagar, T.R. Neural underpinnings of dyslexia as a disorder of visuo-spatial attention. Clin. Exp. Optom. 2004, 87, 4–10. [Google Scholar] [CrossRef]
- Sperling, A.J.; Lu, Z.L.; Manis, F.R.; Seidenberg, M.S. Deficits in perceptual noise exclusion in developmental dyslexia. Nat. Neurosci. 2005, 8, 862–863. [Google Scholar] [CrossRef]
- Goswami, U. A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci. 2011, 15, 3–10. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur. J. Pediatr. 2010, 169, 149–164. [Google Scholar] [CrossRef]
- Tan, M.L.; Ho, J.J.; Teh, K.H. Polyunsaturated fatty acids (PUFAs) for children with specific learning disorders. Cochrane Database Syst. Rev. 2016, 9, CD009398. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.V.; Hibbeln, J.R. Omega-3 and treatment implications in Attention Deficit Hyperactivity Disorder (ADHD) and associated behavioral symptoms. Lipid Technol. 2014, 26, 7–10. [Google Scholar] [CrossRef]
- Cyhlarova, E.; Bell, J.G.; Dick, J.R.; MacKinlay, E.E.; Stein, J.F.; Richardson, A.J. Membrane fatty acids, reading and spelling in dyslexic and non-dyslexic adults. Eur. Neuropsychopharmacol. 2007, 17, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Laasonen, M.; Erkkilä, A.T.; Isotalo, E.; Pulkkinen, J.J.; Haapanen, M.L.; Virsu, V. Serum lipid fatty acids, phonological processing, and reading in children with oral clefts. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zelcer, M.; Goldman, R.D. Omega-3 and dyslexia: Uncertain connection. Can. Fam. Physician 2015, 61, 768–770. [Google Scholar] [PubMed]
- Yehuda, S. Omega-6/omega-3 ratio and brain-related functions. World Rev. Nutr. Diet. 2003, 92, 37–56. [Google Scholar]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164. [Google Scholar]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Di Nicolantonio, J.J.; O’Keefe, J.H. The importance of marine omega-3s for brain development and the prevention and treatment of behavior, mood, and other brain disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative and neurological disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Niyonsenga, T.; Duff, J. Omega-3 and omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS ONE 2016, 11, e0156432. [Google Scholar] [CrossRef]
- LaChance, L.; McKenzie, K.; Taylor, V.H.; Vigod, S.N. Omega-6 to omega-3 fatty acid ratio in patients with ADHD: A meta-analysis. J. Can. Acad. Child Adolesc. Psychiatry 2016, 25, 87. [Google Scholar]
- Sheppard, K.W.; Cheatham, C.L. Omega-6 to omega-3 fatty acid ratio and higher-order cognitive functions in 7-to 9-y-olds: A cross-sectional study. Am. J. Clin. Nutr. 2013, 98, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.J.; Calvin, C.M.; Clisby, C.; Schoenheimer, D.R.; Montgomery, P.; Hall, J.A.; Stein, J.F. Fatty acid deficiency signs predict the severity of reading and related difficulties in dyslexic children. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2000, 63, 69–74. [Google Scholar] [CrossRef]
- Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol. Psychol. 2012, 89, 183–190. [Google Scholar] [CrossRef]
- Jiang, L.; Shi, Y.; Wang, L.; Yang, Z. The influence of orally administered docosahexaenoic acid on cognitive ability in aged mice. J. Nutr. Biochem. 2009, 20, 735–741. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Fontani, G.; Corradeschi, F.; Felici, A.; Alfatti, F.; Migliorini, S.; Lodi, L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur. J. Clin. Investig. 2005, 35, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Fontani, G.; Lodi, L.; Migliorini, S.; Corradeschi, F. Effect of omega-3 and policosanol supplementation on attention and reactivity in athletes. J. Am. Coll. Nutr. 2009, 28 (Suppl. 4), 473S–481S. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, N.; Kaysar, N.; Zaruk-Adasha, Y.; Pelled, D.; Brichon, G.; Zwingelstein, G.; Bodennec, J. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: Effect of dietary n − 3 fatty acids containing phospholipids. Am. J. Clin. Nutr. 2008, 87, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Emery, S.; Häberling, I.; Berger, G.; Walitza, S.; Schmeck, K.; Albert, T.; Drechsler, R. Omega-3 and its domain-specific effects on cognitive test performance in youths: A meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Saksida, A.; Iannuzzi, S.; Bogliotti, C.; Chaix, Y.; Démonet, J.F.; Bricout, L.; Ramus, F. Phonological skills, visual attention span, and visual stress in developmental dyslexia. Dev. Psychol. 2016, 52, 1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, C.; Aleci, C. To Be Or Not To Be? What Makes a Child Dyslexic: An Overview on Risk Factors and Correlated Clinical Aspects. Arch. Curr. Res. Int. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Snowling, M.J. Early identification and interventions for dyslexia: A contemporary view. J. Res. Spec. Educ. Needs 2013, 13, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Johnston, R.; Pitchford, N.J.; Roach, N.W.; Ledgeway, T. Why is the processing of global motion impaired in adults with developmental dyslexia? Brain Cogn. 2016, 108, 20–31. [Google Scholar] [CrossRef]
- Brizzolara, D.; Chilosi, A.; Cipriani, P.; Di Filippo, G.; Gasperini, F.; Mazzotti, S.; Zoccolotti, P. Do phonologic and rapid automatized naming deficits differentially affect dyslexic children with and without a history of language delay? A study of Italian dyslexic children. Cogn. Behav. Neurol. 2006, 19, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Marangoni, F.; Colombo, C.; Galli, C. A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: Applicability to nutritional and epidemiological studies. Anal. Biochem. 2004, 326, 267–272. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; Psychological Corp.: San Antonio, TX, USA, 2003. [Google Scholar]
- Raven, J.; Raven, J.C.; Court, J.H. Section 2: Coloured Progressive Matrices. Introducing the parallel version of the test. In Manual for the Raven’s Progressive Matrices and Vocabulary Scales; 1998 Edition; Oxford Psychologist Press: Oxford, UK, 1998. [Google Scholar]
- Raven, J.C.; Court, J.H.; Raven, J. Section 2: Coloured Progressive Matrices. In Manual for the Raven’s Progressive Matrices and Vocabulary Scales; 1990 Edition with US Norms; Oxford Psychologist Press: Oxford, UK, 1990. [Google Scholar]
- Sartori, G.; Job, R. DDE-2: Batteria per la Valutazione della Dislessia e della Disortografia Evolutiva-2 (Assessment Battery for Developmental Reading and Spelling Disorders); Organizzazioni Speciali: Florence, Italy, 2007. [Google Scholar]
- Cantiani, C.; Lorusso, M.L.; Valnegri, C.; Molteni, M. Perception of non-verbal auditory stimuli in Italian dyslexic children. Dev. Neuropsychol. 2009, 35, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY-II, 2nd ed.; Harcourt Assessment: San Antonio, TX, USA, 2007. [Google Scholar]
- De Luca, M.; Di Filippo, G.; Judica, A.; Spinelli, D.; Zoccolotti, P. Test di Denominazione Rapida e Ricerca Visiva di Colori, Figure e Numeri (Rapid Automatized Naming and Visual Search Test-Color, Figures, Digits); IRCCS Fondazione Santa Lucia: Rome, Italy, 2005. [Google Scholar]
- De Sonneville, L.M.J. Amsterdam Neuropsychological Tasks: A computer-aided assessment program. Cogn. Ergon. Clin. Assess. Comput.-Assist. Learn. Comput. Psychol. 1999, 6, 204–217. [Google Scholar]
- Benassi, M.; Giovagnoli, S.; Pansell, T.; Mandolesi, L.; Bolzani, R.; Magri, S.; Hellgren, K. Developmental trajectories of global motion and global form perception from 4 years to adulthood. J. Exp. Child Psychol. 2021, 207, 105092. [Google Scholar] [CrossRef] [PubMed]
- Menghini, D.; Finzi, A.; Benassi, M.; Bolzani, R.; Facoetti, A.; Giovagnoli, S.; Ruffino, S.; Vicari, S. Different underlying neurocognitive deficits in developmental dyslexia: A comparative study. Neuropsychologia 2010, 48, 863–872. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Gallucci, M. jAMM: Model Building. Available online: https://jamovi-amm.github.io/model_building.html (accessed on 30 November 2021).
- Wagner, R.K.; Torgesen, J.K.; Laughon, P.; Simmons, K.; Rashotte, C.A. Development of young readers’ phonological processing abilities. J. Educ. Psychol. 1993, 85, 83. [Google Scholar] [CrossRef]
- Wolf, M.; Bowers, P.G. The double-deficit hypothesis for the developmental dyslexias. J. Educ. Psychol. 1999, 91, 415. [Google Scholar] [CrossRef]
- Hari, R.; Renvall, H.; Tanskanen, T. Left minineglect in dyslexic adults. Brain 2001, 124, 1373–1380. [Google Scholar] [CrossRef] [Green Version]
- Sireteanu, R.; Goertz, R.; Bachert, I.; Wandert, T. Children with developmental dyslexia show a left visual “minineglect”. Vis. Res. 2005, 45, 3075–3082. [Google Scholar] [CrossRef] [Green Version]
- Geiger, G.; Lettvin, J.Y.; Zegarra-Moran, O. Task-determined strategies of visual process. Cogn. Brain Res. 1992, 1, 39–52. [Google Scholar] [CrossRef]
- Lorusso, M.L.; Facoetti, A.; Pesenti, S.; Cattaneo, C.; Molteni, M.; Geiger, G. Wider recognition in peripheral vision common to different subtypes of dyslexia. Vis. Res. 2004, 44, 2413–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoni, S.; Franceschini, S.; Ronconi, L.; Gori, S.; Facoetti, A. Is excessive visual crowding causally linked to developmental dyslexia? Neuropsychologia 2019, 130, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Lee, M.H.; Lee, S.; Cho, E.J. Neuroprotective effect of alpha-linolenic acid against Aβ-mediated inflammatory responses in C6 glial cell. J. Agric. Food Chem. 2018, 66, 4853–4861. [Google Scholar] [CrossRef]
- Julvez, J.; Gignac, F.; Fernández-Barrés, S.; Romaguera, D.; Sala-Vila, A.; Ranzani, O.T.; Sunyer, J. Walnuts, Long-Chain Polyunsaturated Fatty Acids, and Adolescent Brain Development: Protocol for the Walnuts Smart Snack Dietary Intervention Trial. Front. Pediatr. 2021, 9, 593847. [Google Scholar] [CrossRef]
- Kim, H.; Lee, E.; Kim, Y.; Ha, E.H.; Chang, N. Association between maternal intake of n-6 to n-3 fatty acid ratio during pregnancy and infant neurodevelopment at 6 months of age: Results of the MOCEH cohort study. Nutr. J. 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, J.Y.; Armand, M.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.-A.; Heude, B. The association between linoleic acid levels in colostrum and child cognition at 2 and 3 y in the EDEN cohort. Pediatr. Res. 2015, 77, 829–835. [Google Scholar] [CrossRef]
- Bernard, J.Y.; Armand, M.; Peyere, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.-A.; Heude, B.; EDEN Mother-Child Cohort Study Group. Breastfeeding, polyunsaturated fatty acid levels in colostrum and child intelligence quotient at age 5–6 years. J. Pediatr. 2017, 183, 43–50.e3. [Google Scholar] [CrossRef]
- Lassek, W.D.; Gaulin, S.J. Linoleic and docosahexaenoic acids in human milk have opposite relationships with cognitive test performance in a sample of 28 countries. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 195–201. [Google Scholar] [CrossRef]
- Han, S.N.; Lichtenstein, A.H.; Ausman, L.M.; Meydani, S.N. Novel soybean oils differing in fatty acid composition alter immune functions of moderately hypercholesterolemic older adults. J. Nutr. 2012, 142, 2182–2187. [Google Scholar] [CrossRef] [Green Version]
- Bourourou, M.; Heurteaux, C.; Blondeau, N. Alpha-linolenic acid given as enteral or parenteral nutritional intervention against sensorimotor and cognitive deficits in a mouse model of ischemic stroke. Neuropharmacology 2016, 108, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Taha, A.Y. Linoleic acid–good or bad for the brain? NPJ Sci. Food 2020, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, D.R.; Liu, H.; Miller, M.; Ramsden, C.; Gao, B.; Feldstein, A.E.; Schuster, S.; McClain, C.J.; Kirpich, I.A. Dietary linoleic acid and its oxidized metabolites exacerbate liver injury caused by ethanol via induction of hepatic proinflammatory response in mice. Am. J. Pathol. 2017, 187, 2232–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egbert, A.H.; Creber, C.; Loren, D.M.; Bohnert, A.M. Executive function and dietary intake in youth: A systematic review of the literature. Appetite 2019, 139, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, S.E.; Escobar, M.D.; Shaywitz, B.A.; Fletcher, J.M.; Makuch, R. Evidence that dyslexia may represent the lower tail of a normal distribution of reading ability. N. Engl. J. Med. 1992, 326, 145–150. [Google Scholar] [CrossRef]
- Cilibrasi, L.; Tsimpli, I. Categorical and Dimensional Diagnoses of Dyslexia: Are They Compatible? Front. Psychol. 2020, 11, 2171. [Google Scholar] [CrossRef]
- Shaywitz, S.E.; Shaywitz, B.A.; Fulbright, R.K.; Skudlarski, P.; Mencl, W.E.; Constable, R.T.; Gore, J.C. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biol. Psychiatry 2003, 54, 25–33. [Google Scholar] [CrossRef]
- Yu, X.; Zuk, J.; Gaab, N. What factors facilitate resilience in developmental dyslexia? Examining protective and compensatory mechanisms across the neurodevelopmental trajectory. Child Dev. Perspect. 2018, 12, 240–246. [Google Scholar] [CrossRef]


| DD Children n = 15 | TD Children n = 15 | Group Comparison | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Independent Samples t-Test: t, p | |
| Age | 10.96 (1.33) | 10.70 (1.56) | 0.494, 0.625 |
| IQ | 103.47 (9.49) | 104.20 (14.99) | −0.160, 0.874 |
| General reading accuracy | −1.80 (1.13) | 0.56 (0.44) | −7.567, <0.001 |
| General reading speed | −3.71 (2.71) | 0.10 (0.65) | −5.287, <0.001 |
| General writing accuracy | −2.20 (2.49) | 0.43 (0.50) | −4.004, <0.001 |
| Mean (SD) | Range | |
|---|---|---|
| Age | 10.83 (1.43) | 8.17–13.58 |
| IQ | 103.83 (12.33) | 85–125 |
| General reading errors | 5.83 (5.28) | 0–21.50 |
| General reading time (s) | 127.46 (89.07) | 55–498.50 |
| General writing errors | 3.03 (3.21) | 0–12.50 |
| Rhythmic pattern discrimination (Pattern total accuracy) | 19.03 (4.98) | 8–24 |
| Phonological processing accuracy | 46.70 (4.22) | 35–52 |
| rapid automatized naming errors | 0.83 (1.51) | 0–6 |
| Rapid automatized naming time (s) | 84.75 (18.24) | 57.87–138 |
| Flanker effect time (s) | 85.93 (93.69) | −189–288 |
| VSA cue effect accuracy | 0.50 (1.65) | −3–4.50 |
| VSA cue effect speed (s) | −3.51 (79.42) | −130.63–294.25 |
| Motion coherence accuracy | 31.07 (4.73) | 23–38 |
| Visual search errors | 0.30 (0.60) | 0–2 |
| Visual search time (s) | 23.01 (7.51) | 13.10–39.68 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. AA/ALA ratio | - | |||||||
| 2. LA/ALA ratio | 0.949 *** | - | ||||||
| 3. General reading time (s) | 0.807 *** | 0.763 *** | - | |||||
| 4. General writing errors | 0.550 ** | 0.522 ** | 0.794 *** | - | ||||
| 5. Phonological processing accuracy | −0.416 * | −0.440 * | −0.670 *** | −0.658 *** | - | |||
| 6. Rapid automatized naming time (s) | 0.117 | 0.065 | 0.277 | 0.396 * | −0.246 | - | ||
| 7. Flanker effect time (s) | 0.540 ** | 0.470 * | 0.569 ** | 0.644 *** | −0.351 | 0.224 | - | |
| 8. VSA cue effect accuracy | 0.287 | 0.316 | 0.242 | 0.426 * | 0.426 * | 0.074 | 0.291 | - |
| Effect | Variable | Estimate | SE | Lower | Upper | β | df | t | p |
|---|---|---|---|---|---|---|---|---|---|
| AA/ALA | General reading speed | −0.044 | 0.010 | −0.063 | −0.024 | −0.656 | 28 | −4.60 | <0.001 |
| LA/ALA | −0.021 | 0.005 | −0.032 | −0.010 | −0.606 | 28 | −4.03 | <0.001 | |
| AA/ALA | General writing accuracy | −0.023 | 0.010 | −0.042 | −0.003 | −0.416 | 28 | −2.42 | 0.022 |
| LA/ALA | −0.010 | 0.005 | −0.020 | −1.11 × 10−4 | −0.364 | 28 | −2.07 | 0.048 |
| Effect | Estimate | SE | Lower | Upper | β | z | p |
|---|---|---|---|---|---|---|---|
| LA/ALA ⇒ VSA cue effect ⇒ General writing accuracy | −0.004 | 0.002 | −0.010 | 5.14 × 10−4 | −0.163 | −1.750 | 0.080 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borasio, F.; Syren, M.-L.; Turolo, S.; Agostoni, C.; Molteni, M.; Antonietti, A.; Lorusso, M.L. Direct and Indirect Effects of Blood Levels of Omega-3 and Omega-6 Fatty Acids on Reading and Writing (Dis)Abilities. Brain Sci. 2022, 12, 169. https://doi.org/10.3390/brainsci12020169
Borasio F, Syren M-L, Turolo S, Agostoni C, Molteni M, Antonietti A, Lorusso ML. Direct and Indirect Effects of Blood Levels of Omega-3 and Omega-6 Fatty Acids on Reading and Writing (Dis)Abilities. Brain Sciences. 2022; 12(2):169. https://doi.org/10.3390/brainsci12020169
Chicago/Turabian StyleBorasio, Francesca, Marie-Louise Syren, Stefano Turolo, Carlo Agostoni, Massimo Molteni, Alessandro Antonietti, and Maria Luisa Lorusso. 2022. "Direct and Indirect Effects of Blood Levels of Omega-3 and Omega-6 Fatty Acids on Reading and Writing (Dis)Abilities" Brain Sciences 12, no. 2: 169. https://doi.org/10.3390/brainsci12020169
APA StyleBorasio, F., Syren, M.-L., Turolo, S., Agostoni, C., Molteni, M., Antonietti, A., & Lorusso, M. L. (2022). Direct and Indirect Effects of Blood Levels of Omega-3 and Omega-6 Fatty Acids on Reading and Writing (Dis)Abilities. Brain Sciences, 12(2), 169. https://doi.org/10.3390/brainsci12020169









