Emerging Treatments for Disorders of Consciousness in Paediatric Age
Abstract
1. Introduction
2. Neurorehabilitation
3. Pharmacologic and Regenerative Therapies
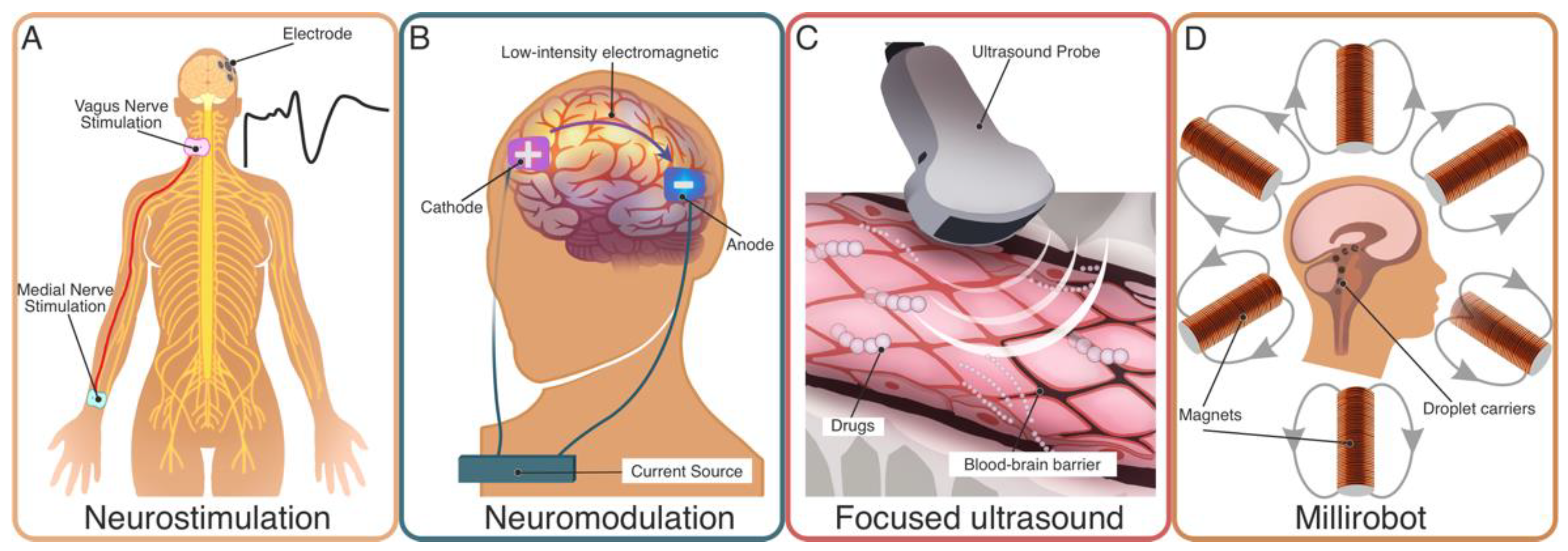
4. Medical Technologies for Treatment
4.1. Neurostimulation
4.2. Neuromodulation
4.3. (Targeted) Drug Delivery
5. Challenges and Opportunities in Low- and Middle-Income Countries
6. The Way Forward
6.1. Standardisation and/or Protocol Adaptation
6.2. Precision
6.3. Investments
6.4. Comparability, Open-Sourcing, and Data Enrichment
6.5. Ethics
6.6. Strategic Investments in Low- and Middle-Income Countries (LMICSs)
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNCS | Coma/Near Coma Scale |
| CNS | Central Nervous System |
| CPE | Continuous Professional Education |
| CRS-R | Coma Recovery Scale—Revised |
| DoC | Disorder of Consciousness |
| HIC | High-Income Countries |
| LMIC | Low- and Middle-Income Countries |
| LOCFAS | Levels of Cognitive Functioning Assessment Scale |
| MCS | Minimally Conscious State |
| NCCPC-PV | Non-Communicating Children’s Pain Checklist–Postoperative version |
| NGO | Non-governmental organization |
| PALOC | Post-Acute Level of Consciousness scale |
| TBI | Traumatic Brain Injury |
| tES | Transcranial Electrical Stimulation |
| UWS | Unresponsive Wakefulness Syndrome, here used as synonym of Vegetative State |
| VS | Vegetative State, here used as synonym of Unresponsive Wakefulness Syndrome |
References
- Plunkett, A.; Parslow, R.C. Is It Taking Longer to Die in Paediatric Intensive Care in England and Wales? Arch. Dis. Child. 2016, 101, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Pisa, F.E.; Biasutti, E.; Drigo, D.; Barbone, F. The Prevalence of Vegetative and Minimally Conscious States: A Systematic Review and Methodological Appraisal. J. Head Trauma Rehabil. 2014, 29, E23–E30. [Google Scholar] [CrossRef] [PubMed]
- The Multi-Society Task Force on PVS Medical Aspects of the Persistent Vegetative State. N. Engl. J. Med. 1994, 330, 1499–1508. [CrossRef] [PubMed]
- Leonardi, M.; Sattin, D.; Raggi, A. An Italian Population Study on 600 Persons in Vegetative State and Minimally Conscious State. Brain Inj. 2013, 27, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Estraneo, A.; Moretta, P.; Loreto, V.; Lanzillo, B.; Santoro, L.; Trojano, L. Late Recovery after Traumatic, Anoxic, or Hemorrhagic Long-Lasting Vegetative State. Neurology 2010, 75, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Houston, A.L.; Wilson, N.S.; Morrall, M.C.; Lodh, R.; Oddy, J.R. Interventions to Improve Outcomes in Children and Young People with Unresponsive Wakefulness Syndrome Following Acquired Brain Injury: A Systematic Review. Eur. J. Paediatr. Neurol. 2020, 25, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Edlow, B.L.; Claassen, J.; Schiff, N.D.; Greer, D.M. Recovery from Disorders of Consciousness: Mechanisms, Prognosis and Emerging Therapies. Nat. Rev. Neurol. 2021, 17, 135–156. [Google Scholar] [CrossRef]
- Wong, C.P.; Forsyth, R.J.; Kelly, T.P.; Eyre, J.A. Incidence, Aetiology, and Outcome of Non-Traumatic Coma: A Population Based Study. Arch. Dis. Child. 2001, 84, 193–199. [Google Scholar] [CrossRef]
- Wade, D.T. How Many Patients in a Prolonged Disorder of Consciousness Might Need a Best Interests Meeting about Starting or Continuing Gastrostomy Feeding? Clin. Rehabil. 2018, 32, 1551–1564. [Google Scholar] [CrossRef]
- Molteni, E.; Colombo, K.; Pastore, V.; Galbiati, S.; Recla, M.; Locatelli, F.; Galbiati, S.; Fedeli, C.; Strazzer, S. Joint Neuropsychological Assessment through Coma/Near Coma and Level of Cognitive Functioning Assessment Scales Reduces Negative Findings in Pediatric Disorders of Consciousness. Brain Sci. 2020, 10, 162. [Google Scholar] [CrossRef]
- Kelly, D.F.; Becker, D.P. Advances in Management of Neurosurgical Trauma: Usa and Canada. World J. Surg. 2001, 25, 1179. [Google Scholar] [CrossRef]
- Colantonio, A.; Croxford, R.; Farooq, S.; Laporte, A.; Coyte, P.C. Trends in Hospitalization Associated with Trau-matic Brain Injury in a Publicly Insured Population, 1992–2002. J. Trauma-Inj. Infect. Crit. Care 2009, 66, 179–183. [Google Scholar] [CrossRef]
- Andelic, N.; Anke, A.; Skandsen, T.; Sigurdardottir, S.; Sandhaug, M.; Ader, T.; Roe, C. Incidence of Hospital-Admitted Severe Traumatic Brain Injury and in-Hospital Fatality in Norway: A National Cohort Study. Neuroepidemiology 2012, 38, 259–267. [Google Scholar] [CrossRef]
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The Impact of Traumatic Brain Injuries: A Global Perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef]
- Odebode, T.O.; Abubakar, A.M. Childhood Head Injury: Causes, Outcome, and Outcome Predictors-A Nigerian Perspective. Pediatric Surg. Int. 2004, 20, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.F. Virus and Immune-Mediated Encephalitides: Epidemiology, Diagnosis, Treatment, and Prevention. Pediatric Neurol. 2015, 53, 3–12. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; El-lenbogen, R.G.; et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Eilander, H.J.; van de Wiel, M.; Wijers, M.; van Heugten, C.M.; Buljevac, D.; Lavrijsen, J.C.M.; Hoenderdaal, P.L.; de Letter-van der Heide, L.; Wijnen, V.J.M.; Scheirs, J.G.M.; et al. The Reliability and Validity of the PALOC-s: A Post-Acute Level of Consciousness Scale for Assessment of Young Patients with Prolonged Disturbed Consciousness after Brain Injury. Neuropsychol. Rehabil. 2009, 19, 1–27. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement Characteristics and Diagnostic Utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Magee, W.L.; Ghetti, C.M.; Moyer, A. Feasibility of the Music Therapy Assessment Tool for Awareness in Disorders of Consciousness (MATADOC) for Use with Pediatric Populations. Front. Psychol. 2015, 6, 698. [Google Scholar] [CrossRef] [PubMed]
- Duszyk, A.; Dovgialo, M.; Pietrzak, M.; Zieleniewska, M.; Durka, P. Event-Related Potentials in the Odd-Ball Paradigm and Behavioral Scales for the Assessment of Children and Adolescents with Disorders of Consciousness: A Proof of Concept Study. Clin. Neuropsychol. 2019, 33, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Mouthon, A.L.; van Hedel, H.J.A.; Meyer-Heim, A.; Kurth, S.; Ringli, M.; Pugin, F.; Huber, R. High-Density Electro-encephalographic Recordings during Sleep in Children with Disorders of Consciousness. Neuroimage-Clin. 2016, 11, 468–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicholas, C.R.; McLaren, D.G.; Gawrysiak, M.J.; Rogers, B.P.; Dougherty, J.H.; Nash, M.R. Functional Neuroimaging of Personally-Relevant Stimuli in a Paediatric Case of Impaired Awareness. Brain Inj. 2014, 28, 1135–1138. [Google Scholar] [CrossRef]
- Slomine, B.S.; Suskauer, S.J.; Nicholson, R.; Giacino, J.T.; Slomine, B.S.; Suskauer, S.J.; Nicholson, R.; Giacino, J.T. Preliminary Validation of the Coma Recovery Scale for Pediatrics in Typically Developing Young Children. Brain Inj. 2019, 33, 1640–1645. [Google Scholar] [CrossRef]
- De Tanti, A.; Scarponi, F.; Bertoni, M.; Gasperini, G.; Lanzillo, B.; Molteni, F.; Posteraro, F.; Vitale, D.F.; Zanpolini, M. Management of Intrathecal Baclofen Therapy for Severe Acquired Brain Injury: Consensus and Recommendations for Good Clinical Practice. Neurol. Sci. 2017, 38, 1429–1435. [Google Scholar] [CrossRef]
- Enslin, J.M.N.; Rohlwink, U.K.; Figaji, A. Management of Spasticity After Traumatic Brain Injury in Children. Front. Neurol. 2020, 11, 126. [Google Scholar] [CrossRef]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness. Neurology 2018, 99, 1699–1709. [Google Scholar] [CrossRef]
- Fink, E.L.; Beers, S.R.; Houtrow, A.J.; Richichi, R.; Burns, C.; Doughty, L.; Ortiz-Aguayo, R.; Madurski, C.A.; Valenta, C.; Chrisman, M.; et al. Early Protocolized Versus Usual Care Rehabilitation for Pediatric Neurocritical Care Patients: A Randomized Controlled Trial. Pediatric Crit. Care Med. 2019, 20, 540. [Google Scholar] [CrossRef] [PubMed]
- Luft, A.R. Rehabilitation and Plasticity. Front. Neurol. Neurosci. 2013, 32, 88–94. [Google Scholar] [CrossRef]
- Eifert, B.; Maurer-Karattup, P.; Schorl, M. Integration of Intensive Care Treatment and Neurorehabilitation in Patients With Disorders of Consciousness: A Program Description and Case Report. Arch. Phys. Med. Rehabil. 2013, 94, 1924–1933. [Google Scholar] [CrossRef]
- Xu, G.; Sheng, Q.; Xin, Q.; Song, Y.; Zhang, G.; Yuan, L.; Zhao, P.; Liang, J. EEG Assessment in a 2-Year-Old Child with Prolonged Disorders of Consciousness: 3 Years’ Follow-Up. Comput. Intell. Neurosci. 2020, 2020. [Google Scholar] [CrossRef]
- Torrisi, M.; Piccolo, A.; de Luca, R.; Berenati, M.; Olivo, A.; Maresca, G.; Naro, A.; Calabrò, R.S. Are You There? The Growing Need to Get the Right Diagnosis in Disorder of Consciousness. J. Neurosci. Nurs. 2018, 50, 107–110. [Google Scholar] [CrossRef]
- Liscio, M.; Adduci, A.; Galbiati, S.; Poggi, G.; Sacchi, D.; Strazzer, S.; Castelli, E.; Flannery, J. Cognitive-Behavioural Stimulation Protocol for Severely Brain-Damaged Patients in the Post-Acute Stage in Developmental Age. Disabil. Rehabil. 2008, 30, 275–285. [Google Scholar] [CrossRef]
- Fadiga, L.; Fogassi, L.; Pavesi, G.; Rizzolatti, G. Motor Facilitation during Action Observation: A Magnetic Stimulation Study. J. Neurophysiol. 1995, 73, 2608–2611. [Google Scholar] [CrossRef]
- Seel, R.T.; Douglas, J.; Dennison, A.C.; Heaner, S.; Farris, K.; Rogers, C. Specialized Early Treatment for Persons with Disorders of Consciousness: Program Components and Outcomes. Arch. Phys. Med. Rehabil. 2013, 94, 1908–1923. [Google Scholar] [CrossRef]
- Nacoti, M.; Fazzi, F.; Biroli, F.; Zangari, R.; Barbui, T.; Kochanek, P.M. Addressing Key Clinical Care and Clinical Research Needs in Severe Pediatric Traumatic Brain Injury: Perspectives From a Focused International Conference. Front. Pediatrics 2021, 8, 814. [Google Scholar]
- Molteni, E.; Ranzini, M.B.M.; Beretta, E.; Modat, M.; Strazzer, S. Individualized Prognostic Prediction of the Long-Term Functional Trajectory in Pediatric Acquired Brain Injury. J. Pers. Med. 2021, 11, 675. [Google Scholar] [CrossRef]
- Hediger, K.; Boek, F.; Sachers, J.; Blankenburg, U.; Antonius-Kluger, E.; Rist, B.; Schaudek, M.; Staudt, M.; Kluger, G. Dog-Assisted Therapy in Neurorehabilitation of Children with Severe Neurological Impairment: An Explorative Study. Neuropediatrics 2020, 51, 267–274. [Google Scholar] [CrossRef]
- Lahey, S.; Beaulieu, C.; Sandbach, K.; Colaiezzi, A.; Balkan, S. The Role of the Psychologist With Disorders of Con-sciousness in Inpatient Pediatric Neurorehabilitation: A Case Series. Rehabil. Psychol. 2017, 62, 238–248. [Google Scholar] [CrossRef]
- Van Stan, J.H.; Whyte, J.; Duffy, J.R.; Barkmeier-Kraemer, J.M.; Doyle, P.B.; Gherson, S.; Kelchner, L.; Muise, J.; Petty, B.; Roy, N.; et al. Rehabilitation Treatment Specification System: Methodology to Identify and Describe Unique Targets and Ingredients. Arch. Phys. Med. Rehabil. 2021, 102, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.; Dijkers, M.P.; Whyte, J.; Turkstra, L.S.; Zanca, J.M.; Packel, A.; van Stan, J.H.; Ferraro, M.; Chen, C. A Theory-Driven System for the Specification of Rehabilitation Treatments. Arch. Phys. Med. Rehabil. 2019, 100, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Pagani, M.; Giovannetti, A.M.; Raggi, A.; Sattin, D. Burnout in Healthcare Professionals Working with Patients with Disorders of Consciousness. Work 2013, 45, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Whyte, J.; Bagiella, E.; Kalmar, K.; Childs, N.; Khademi, A.; Eifert, B.; Long, D.; Katz, D.I.; Cho, S.; et al. Placebo-Controlled Trial of Amantadine for Severe Traumatic Brain Injury. N. Engl. J. Med. 2012, 366, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Suskauer, S.J.; Trovato, M.K. Update on Pharmaceutical Intervention for Disorders of Consciousness and Agitation After Traumatic Brain Injury in Children. PMR 2013, 5, 142–147. [Google Scholar] [CrossRef]
- Thibaut, A.; Schiff, N.; Giacino, J.; Laureys, S.; Gosseries, O. Therapeutic Interventions in Patients with Prolonged Disorders of Consciousness. Lancet Neurol. 2019, 18, 600–614. [Google Scholar] [CrossRef]
- Edlow, B.L.; Sanz, L.R.D.; Polizzotto, L.; Pouratian, N.; Rolston, J.D.; Snider, S.B.; Thibaut, A.; Stevens, R.D.; Gosseries, O.; Akbari, Y.; et al. Therapies to Restore Consciousness in Patients with Severe Brain Injuries: A Gap Analysis and Future Directions. Neurocritical Care 2021, 35, 68–85. [Google Scholar] [CrossRef]
- Schiff, N.D. Recovery of Consciousness after Brain Injury: A Mesocircuit Hypothesis. Trends Neurosci. 2010, 33, 1–9. [Google Scholar] [CrossRef]
- Fridman, E.A.; Beattie, B.J.; Broft, A.; Laureys, S.; Schif, N.D. Role of Anterior Forebrain Mesocircuit Dysfunction in the Severely Injured Brain. Proc. Natl. Acad. Sci. USA 2014, 111, 6473–6478. [Google Scholar] [CrossRef]
- Danner, O.K.; Kern, Q.; Matthews, R.; Sola, R., Jr.; Butler, C.; Udobi, K. Selective Serotonin Reuptake Inhibitor Therapy Reduces Time to Emergence and Arousal from TBI-Induced Prolonged Coma: A Pilot Study. J. Clin. Med. Res. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Lant, N.D.; Gonzalez-Lara, L.E.; Owen, A.M.; Fernández-Espejo, D. Relationship between the Anterior Forebrain Mesocircuit and the Default Mode Network in the Structural Bases of Disorders of Consciousness. NeuroImage Clin. 2016, 10, 27–35. [Google Scholar] [CrossRef]
- Baumann, C.R.; Bassetti, C.L. Hypocretins (Orexins) and Sleep-Wake Disorders. Lancet Neurol. 2005, 4, 673–682. [Google Scholar] [CrossRef]
- Valente, M.; Placidi, F.; Oliveira, A.J.; Bigagli, A.; Morghen, I.; Proietti, R.; Gigli, G.L. Sleep Organization Pattern as a Prognostic Marker at the Subacute Stage of Post-Traumatic Coma. Clin. Neurophysiol. 2002, 113, 1798–1805. [Google Scholar] [CrossRef]
- Paparrigopoulos, T.; Melissaki, A.; Tsekou, H.; Efthymiou, A.; Kribeni, G.; Baziotis, N.; Geronikola, X. Melatonin Secretion after Head Injury: A Pilot Study. Brain Inj. 2006, 20, 873–878. [Google Scholar] [CrossRef]
- Chiaretti, A.; Genovese, O.; Riccardi, R.; di Rocco, C.; di Giuda, D.; Mariotti, P.; Pulitanò, S.; Piastra, M.; Polidori, G.; Colafati, G.S.; et al. Intraventricular Nerve Growth Factor Infusion: A Possible Treatment for Neurological Deficits Following Hypoxic-Ischemic Brain Injury in Infants. Neurol. Res. 2005, 27, 741–746. [Google Scholar] [CrossRef]
- Bornstein, N.; Poon, W.S. Accelerated Recovery from Acute Brain Injuries: Clinical Efficacy of Neurotrophic Treatment in Stroke and Traumatic Brain Injuries. Drugs Today 2012, 48, 43–61. [Google Scholar]
- Liem, N.T.; Chinh, V.D.; Phuong, D.T.M.; van Doan, N.; Forsyth, N.R.; Heke, M.; Thi, P.A.N.; Nguyen, X.H. Outcomes of Bone Marrow-Derived Mononuclear Cell Transplantation for Patients in Persistent Vegetative State After Drowning: Report of Five Cases. Front. Pediatrics 2020, 8, 564. [Google Scholar] [CrossRef]
- Jozwiak, S.; Habich, A.; Kotulska, K.; Sarnowska, A.; Kropiwnicki, T.; Janowski, M.; Jurkiewicz, E.; Lukomska, B.; Kmiec, T.; Walecki, J.; et al. Intracerebroventricular Transplantation of Cord Blood-Derived Neural Progenitors in a Child With Severe Global Brain Ischemic Injury. Cell Med. 2010, 1, 71–80. [Google Scholar] [CrossRef]
- Maski, K.; Trotti, L.M.; Kotagal, S.; Auger, R.R.; Rowley, J.A.; Hashmi, S.D.; Watson, N.F. Treatment of Central Disorders of Hypersomnolence: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2021, 17, 1881–1893. [Google Scholar] [CrossRef]
- Maski, K.; Trotti, L.M.; Kotagal, S.; Robert Auger, R.; Swick, T.J.; Rowley, J.A.; Hashmi, S.D.; Watson, N.F. Treatment of Central Disorders of Hypersomnolence: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and Grade Assessment. J. Clin. Sleep Med. 2021, 17, 1895–1945. [Google Scholar] [CrossRef]
- Mayer, G.; Happe, S.; Evers, S.; Hermann, W.; Jansen, S.; Kallweit, U.; Muntean, M.-L.; Pöhlau, D.; Riemann, D.; Saletu, M.; et al. Insomnia in Neurological Diseases. Neurol. Res. Pract. 2021, 3, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, S.; Cairns, V. Providing Evidence of Efficacy for a New Drug. Stat. Med. 1998, 17, 1813–1823. [Google Scholar] [CrossRef]
- Huster, W.J.; Enas, G.G. A Framework Establishing Clear Decision Criteria for the Assessment of Drug Efficacy. Stat. Med. 1998, 17, 1829–1838. [Google Scholar] [CrossRef]
- Rabinstein, A.A. Erratum: Rabinstein AA. Lifelong Learning in Neurology (CONTINUUM Lifelong Learning in Neurology (2018) 24:6 (1708-1731) DOI:10.1212/CON.0000000000000666). CONTINUUM Lifelong Learn. Neurol. 2019, 25, 435–445. [Google Scholar]
- Ji, X.L.; Ma, L.; Zhou, W.H.; Xiong, M. Narrative Review of Stem Cell Therapy for Ischemic Brain Injury. Transl. Pediatrics 2021, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Vissel, B. Inflammation-Sleep Interface in Brain Disease: TNF, Insulin, Orexin. J. Neuroinflammation 2014, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Schiff, N.D. Coma and Consciousness: Paradigms (Re)Framed by Neuroimaging. NeuroImage 2012, 61, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.A.; Hanes, S.D. Pharmacokinetic Alterations after Severe Head Injury. Clin. Pharmacokinet. 1998, 35, 209–221. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, J.S.; Vig, P.J. Current Strategies for Therapeutic Drug Delivery after Traumatic CNS Injury. Ther. Deliv. 2019, 10, 251–263. [Google Scholar] [CrossRef]
- Pozzi, M.; Galbiati, S.; Locatelli, F.; Carnovale, C.; Radice, S.; Strazzer, S.; Clementi, E. Drug Use in Pediatric Patients Admitted to Rehabilitation For Severe Acquired Brain Injury: Analysis of the Associations with Rehabilitation Outcomes. Pediatric Drugs 2021, 23, 75–86. [Google Scholar] [CrossRef]
- Binvignat, O.; Olloquequi, J. Excitotoxicity as a Target Against Neurodegenerative Processes. Curr. Pharm. Des. 2020, 26, 1251–1262. [Google Scholar] [CrossRef]
- Garavaglia, L.; Molteni, E.; Beretta, E.; Vassena, E.; Strazzer, S.; Pittaccio, S. Pilot Study of the Cortical Correlates and Clinical Effects of Passive Ankle Mobilisation in Children with Upper Motorneuron Lesions. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar]
- Wu, X.; Zhang, C.; Feng, J.; Mao, Q.; Gao, G.; Jiang, J. Right Median Nerve Electrical Stimulation for Acute Traumatic Coma (the Asia Coma Electrical Stimulation Trial): Study Protocol for a Randomised Controlled Trial. Trials 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Corazzol, M.; Lio, G.; Lefevre, A.; Deiana, G.; Tell, L.; André-Obadia, N.; Bourdillon, P.; Guenot, M.; Desmurget, M.; Luauté, J.; et al. Restoring Consciousness with Vagus Nerve Stimulation. Curr. Biol. 2017, 27, R994–R996. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.J.; Reiser, M.; Padberg, F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during FMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Bolognini, N.; Pascual-Leone, A.; Fregni, F. Using Non-Invasive Brain Stimulation to Augment Motor Training-Induced Plasticity. J. NeuroEngineering Rehabil. 2009, 6, 1–13. [Google Scholar] [CrossRef]
- Carrière, M.; Mortaheb, S.; Raimondo, F.; Annen, J.; Barra, A.; Binda Fossati, M.C.; Chatelle, C.; Hermann, B.; Martens, G.; di Perri, C.; et al. Neurophysiological Correlates of a Single Session of Prefrontal Tdcs in Patients with Pro-longed Disorders of Consciousness: A Pilot Double-Blind Randomized Controlled Study. Brain Sci. 2020, 10, 469. [Google Scholar] [CrossRef]
- Thibaut, A.; Bruno, M.A.; Ledoux, D.; Demertzi, A.; Laureys, S. TDCS in Patients with Disorders of Consciousness: Sham-Controlled Randomized Double-Blind Study. Neurology 2014, 82, 1112–1128. [Google Scholar] [CrossRef]
- Angelakis, E.; Liouta, E.; Andreadis, N.; Korfias, S.; Ktonas, P.; Stranjalis, G.; Sakas, D.E. Transcranial Direct Current Stimulation Effects in Disorders of Consciousness. Arch. Phys. Med. Rehabil. 2014, 95, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of Deep Brain Stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.; Huckhagel, T.; Gulberti, A.; Pötter-Nerger, M.; Vettorazzi, E.; Hidding, U.; Choe, C.U.; Zittel, S.; Braaß, H.; Ludewig, P.; et al. Towards Unambiguous Reporting of Complications Related to Deep Brain Stimulation Surgery: A Retrospective Single-Center Analysis and Systematic Review of the Literature. PLoS ONE 2018, 13, e0198529. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.G.; Lo, W.D. Noninvasive Brain Stimulation: The Potential for Use in the Rehabilitation of Pediatric Acquired Brain Injury. Arch. Phys. Med. Rehabil. 2015, 96, S129–S137. [Google Scholar] [CrossRef]
- Stoykov, M.E.; Madhavan, S. Motor Priming in Neurorehabilitation. J. Neurol. Phys. Ther. 2015, 39, 33. [Google Scholar] [CrossRef]
- Lazzari, R.D.; Politti, F.; Santos, C.A.; Lopesdumont, A.J.; Rezende, F.L.; Collangegrecco, L.A.; Braunferreira, L.A.; Oliveira, C.S. Effect of a Single Session of Transcranial Direct-Current Stimulation Combined with Virtual Reality Training on the Balance of Children with Cerebral Palsy: A Randomized, Controlled, Double-Blind Trial. J. Phys. Ther. Sci. 2015, 27, 763–768. [Google Scholar] [CrossRef]
- Rivera-Urbina, G.N.; Nitsche, M.A.; Vicario, C.M.; Molero-Chamizo, A. Applications of Transcranial Direct Current Stimulation in Children and Pediatrics. Rev. Neurosci. 2017, 28, 173–184. [Google Scholar] [CrossRef]
- Krishna, V.; Sammartino, F.; Rezai, A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology Advances in Diagnosis and Treatment. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-Responsive Biohybrid Neutrobots for Active Target Delivery. Sci. Robot. 2020, 6, 9519eaaz. [Google Scholar] [CrossRef]
- Mair, L.O.; Adam, G.; Chowdhury, S.; Davis, A.; Arifin, D.R.; Vassoler, F.M.; Engelhard, H.H.; Li, J.; Tang, X.; Weinberg, I.N.; et al. Soft Capsule Magnetic Millirobots for Region-Specific Drug Delivery in the Central Nervous System. Front. Robot. AI 2021, 8, 226. [Google Scholar] [CrossRef]
- Hermann, B.; Raimondo, F.; Hirsch, L.; Huang, Y.; Denis-Valente, M.; Pérez, P.; Engemann, D.; Faugeras, F.; Weiss, N.; Demeret, S.; et al. Combined Behavioral and Electrophysiological Evidence for a Direct Cortical Effect of Pre-frontal TDCS on Disorders of Consciousness. bioRxiv 2019. [Google Scholar] [CrossRef]
- Cavinato, M.; Genna, C.; Formaggio, E.; Gregorio, C.; Storti, S.F.; Manganotti, P.; Casanova, E.; Piperno, R.; Piccione, F. Behavioural and Electrophysiological Effects of TDCS to Prefrontal Cortex in Patients with Disorders of Consciousness. Clin. Neurophysiol. 2019, 130, 231–238. [Google Scholar] [CrossRef]
- Martens, G.; Lejeune, N.; O’Brien, A.T.; Fregni, F.; Martial, C.; Wannez, S.; Laureys, S.; Thibaut, A. Randomized Controlled Trial of Home-Based 4-Week TDCS in Chronic Minimally Conscious State. Brain Stimul. 2018, 11, 982–990. [Google Scholar] [CrossRef]
- Wu, M.; Yu, Y.; Luo, L.; Wu, Y.; Gao, J.; Ye, X.; Luo, B. Efficiency of Repetitive Transcranial Direct Current Stimulation of the Dorsolateral Prefrontal Cortex in Disorders of Consciousness: A Randomized Sham-Controlled Study. Neural Plast. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Saleem, G.T.; Ewen, J.B.; Crasta, J.E.; Slomine, B.S.; Cantarero, G.L.; Suskauer, S.J. Single-Arm, Open-Label, Dose Es-calation Phase I Study to Evaluate the Safety and Feasibility of Transcranial Direct Current Stimulation with Elec-troencephalography Biomarkers in Paediatric Disorders of Consciousness: A Study Protocol. BMJ Open 2019, 9, e029967. [Google Scholar] [CrossRef] [PubMed]
- Ekici, B. Transcranial Direct Current Stimulation-Induced Seizure: Analysis of a Case. Clin. EEG Neurosci. 2015, 46, 169. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Hallett, M.; Rothwell, J.C. Repetitive Transcranial Magnetic Stimulation or Transcranial Direct Current Stimulation? Brain Stimul. 2009, 2, 169. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Lima, M.C.; Ferreira, M.J.L.; Wagner, T.; Rigonatti, S.P.; Castro, A.W.; Souza, D.R.; Riberto, M.; Freedman, S.D.; et al. A Sham-Controlled, Phase II Trial of Transcranial Direct Current Stimulation for the Treatment of Central Pain in Traumatic Spinal Cord Injury. Pain 2006, 122, 197–209. [Google Scholar] [CrossRef]
- Breau, L.M.; Finley, G.A.; McGrath, P.J.; Camfield, C.S. Validation of the Non-Communicating Children’s Pain Checklist-Postoperative Version. Anesthesiology 2002, 96, 528–535. [Google Scholar] [CrossRef]
- Non-Communicating Children’s Pain Checklist–Revised (NCCPC-R). Available online: Https://Oxfordmedicine.Com/Fileasset/Files/Paediatric%20Pain/Med_9780199642656_file_003.Pdf (accessed on 30 January 2022).
- Sanchez-Villamañan, M.D.C.; Gonzalez-Vargas, J.; Torricelli, D.; Moreno, J.C.; Pons, J.L. Compliant Lower Limb Ex-oskeletons: A Comprehensive Review on Mechanical Design Principles. J. NeuroEngineering Rehabil. 2019, 16, 55. [Google Scholar] [CrossRef]
- Graf, W.D.; Nagel, S.K.; Epstein, L.G.; Miller, G.; Nass, R.; Larriviere, D. Pediatric Neuroenhancement: Ethical, Legal, Social, and Neurodevelopmental Implications. Neurology 2013, 80, 1251–1260. [Google Scholar] [CrossRef]
- De Silva, M.J.; Roberts, I.; Perel, P.; Edwards, P.; Kenward, M.G.; Fernandes, J.; Shakur, H.; Patel, V. Patient Outcome after Traumatic Brain Injury in High-, Middle- and Low-Income Countries: Analysis of Data on 8927 Patients in 46 Countries. Int. J. Epidemiol. 2009, 38, 452–458. [Google Scholar] [CrossRef]
- Bonow, R.H.; Barber, J.; Temkin, N.R.; Videtta, W.; Rondina, C.; Petroni, G.; Lujan, S.; Alanis, V.; la Fuente, G.; Lavadenz, A.; et al. The Outcome of Severe Traumatic Brain Injury in Latin America. World Neurosurg. 2018, 111, e82–e90. [Google Scholar] [CrossRef]
- Suryanto; Plummer, V.; Boyle, M. EMS Systems in Lower-Middle Income Countries: A Literature Review. Prehospital Disaster Med. 2017, 32, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.; Bikson, M.; Woods, A.J.; Rosen, A.R.; Kessler, S.K. Transcranial Direct Current Stimulation in Pediatric Brain: A Computational Modeling Study. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012. [Google Scholar]
- Kessler, S.K.; Minhas, P.; Woods, A.J.; Rosen, A.; Gorman, C.; Bikson, M. Dosage Considerations for Transcranial Direct Current Stimulation in Children: A Computational Modeling Study. PLoS ONE 2013, 8, e76112. [Google Scholar] [CrossRef] [PubMed]
- Gillick, B.T.; Kirton, A.; Carmel, J.B.; Minhas, P.; Bikson, M. Pediatric Stroke and Transcranial Direct Current Stimu-lation: Methods for Rational Individualized Dose Optimization. Front. Hum. Neurosci. 2014, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Segmiller, F.M.; Epple, A.N.; Freisleder, F.J.; Koutsouleris, N.; Schulte-Körne, G.; Padberg, F. Transcranial Direct Current Stimulation in Children and Adolescents: A Comprehensive Review. J. Neural Transm. 2016, 123, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Moliadze, V.; Schmanke, T.; Andreas, S.; Lyzhko, E.; Freitag, C.M.; Siniatchkin, M. Stimulation Intensities of Tran-scranial Direct Current Stimulation Have to Be Adjusted in Children and Adolescents. Clin. Neurophysiol. 2015, 126, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.; Borrebaeck, C.; Elander, N.; Gasslander, T.; Gawel, D.R.; Gustafsson, M.; Jörnsten, R.; Lee, E.J.; Li, X.; Lilja, S.; et al. Digital Twins to Personalize Medicine. Genome Med. 2019, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.C. Adaptive Clinical Trial Design. Annu. Rev. Med. 2014, 65, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Dulf, D.; Coman, M.A.; Tadevosyan, A.; Chikhladze, N.; Cebanu, S.; Peek-Asa, C. A 3-Country Assessment of Traumatic Brain Injury Practices and Capacity. World Neurosurg. 2020, 146, e517–e526. [Google Scholar] [CrossRef]
- Green, L.B.; Hornyak, J.E.; Hurvitz, E.A. Amantadine in Pediatric Patients with Traumatic Brain Injury: A Retrospective, Case-Controlled Study. Am. J. Phys. Med. Rehabil. 2004, 83, 893–897. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.A.; Vargus-Adams, J.N.; Michaud, L.J.; Bean, J. Effects of Amantadine in Children with Impaired Consciousness Caused by Acquired Brain Injury: A Pilot Study. Am. J. Phys. Med. Rehabil. 2009, 88, 525–532. [Google Scholar] [CrossRef]
- Vargus-Adams, J.N.; McMahon, M.A.; Michaud, L.J.; Bean, J.; Vinks, A.A. Pharmacokinetics of Amantadine in Children With Impaired Consciousness Due to Acquired Brain Injury: Preliminary Findings Using a Sparse-Sampling Technique. PMR 2010, 2, 37–42. [Google Scholar] [CrossRef]
- Zão, A.; Almeida, A.F.; Beça, G.; Nunes, R. From Vegetative State to Participation: Amantadine As a Trigger of the Rehabilitation Program. Acta Med. Port. 2020, 33, 604–609. [Google Scholar] [CrossRef]
- Patrick, P.D.; Blackman, J.A.; Mabry, J.L.; Buck, M.L.; Gurka, M.J.; Conaway, M.R. Dopamine Agonist Therapy in Low-Response Children Following Traumatic Brain Injury. J. Child Neurol. 2006, 21, 879–885. [Google Scholar] [CrossRef]
- Patrick, P.D.; Buck, M.L.; Conaway, M.R.; Blackman, J.A. The Use of Dopamine Enhancing Medications with Children in Low Response States Following Brain Injury. Brain Inj. 2003, 17, 497–506. [Google Scholar] [CrossRef]
- Hornyak, J.E.; Nelson, V.S.; Hurvitz, E.A. The Use of Methylphenidate in Paediatric Traumatic Brain Injury. Dev. Neurorehabilit. 1997, 1, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Dhamapurkar, S.K.; Wilson, B.A.; Rose, A.; Watson, P.; Shiel, A. Does Modafinil Improve the Level of Consciousness for People with a Prolonged Disorder of Consciousness? A Retrospective Pilot Study. Disabil. Rehabil. 2017, 39, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Snyman, N.; Egan, J.R.; London, K.; Howman-Giles, R.; Gill, D.; Gillis, J.; Scheinberg, A. Zolpidem for Persistent Vegetative State—A Placebo-Controlled Trial in Pediatrics. Neuropediatrics 2010, 41, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Appu, M.; Noetzel, M. Clinically Significant Response to Zolpidem in Disorders of Consciousness Secondary to Anti-N-Methyl-d-Aspartate Receptor Encephalitis in a Teenager: A Case Report. Pediatric Neurol. 2014, 50, 262–264. [Google Scholar] [CrossRef]
- Bobele, G.B.; Bale, J. Subacute Encephalopathy in a 5-Year-Old Boy. Semin. Pediatric Neurol. 1999, 6, 168–172. [Google Scholar] [CrossRef]
- Chiaretti, A.; Conti, G.; Falsini, B.; Buonsenso, D.; Crasti, M.; Manni, L.; Soligo, M.; Fantacci, C.; Genovese, O.; Calcagni, M.L.; et al. Intranasal Nerve Growth Factor Administration Improves Cerebral Functions in a Child with Severe Traumatic Brain Injury: A Case Report. Brain Inj. 2017, 31, 1538–1547. [Google Scholar] [CrossRef]
- Chiaretti, A.; Eftimiadi, G.; Buonsenso, D.; Rendeli, C.; Staccioli, S.; Conti, G. Intranasal Nerve Growth Factor Administration Improves Neurological Outcome after GBS Meningitis. Child’s Nerv. Syst. 2020, 36, 2083–2088. [Google Scholar] [CrossRef]
- Jensen, A.; Hamelmann, E. First Autologous Cell Therapy of Cerebral Palsy Caused by Hypoxic-Ischemic Brain Damage in a Child after Cardiac Arrest—Individual Treatment with Cord Blood. Case Rep. Transplant. 2013, 2013, 951827. [Google Scholar] [CrossRef] [PubMed]
- Estraneo, A.; Pascarella, A.; Moretta, P.; Loreto, V.; Trojano, L. Clinical and Electroencephalographic on–off Effect of Amantadine in Chronic Non-Traumatic Minimally Conscious State. J. Neurol. 2015, 262, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Rajan, R.; Rosenbaum, A.; Katz, D.; Kalmar, K.; Seel, R.; Greenwald, B.; Zafonte, R.; Demarest, D.; Brunner, R.; et al. Zolpidem and Restoration of Consciousness. Am. J. Phys. Med. Rehabil. 2014, 93, 101–113. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.H.; Lee, Y.; Lee, J. Additive Effect of Cerebrolysin and Amantadine on Disorders of Consciousness Secondary to Acquired Brain Injury: A Retrospective Case-Control Study. J. Rehabil. Med. 2020, 52, jrm00025. [Google Scholar] [CrossRef] [PubMed]
- Bender Pape, T.L.; Herrold, A.A.; Livengood, S.L.; Guernon, A.; Weaver, J.A.; Higgins, J.P.; Rosenow, J.M.; Walsh, E.; Bhaumik, R.; Pacheco, M.; et al. A Pilot Trial Examining the Merits of Combining Amantadine and Repetitive Transcranial Magnetic Stimulation as an Intervention for Persons with Disordered Consciousness after TBI. J. Head Trauma Rehabil. 2020, 35, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Passler, M.A.; Riggs, R.V. Positive Outcomes in Traumatic Brain Injury-Vegetative State: Patients Treated with Bromocriptine. Arch. Phys. Med. Rehabil. 2001, 82, 311–315. [Google Scholar] [CrossRef]
- Matsuda, W.; Komatsu, Y.; Yanaka, K.; Matsumura, A. Levodopa Treatment for Patients in Persistent Vegetative or Minimally Conscious States. Neuropsychol. Rehabil. 2005, 15, 414–427. [Google Scholar] [CrossRef]
- Ugoya, S.O.; Akinyemi, R.O. The Place of L-Dopa/Carbidopa in Persistent Vegetative State. Clin. Neuropharmacol. 2010, 33, 279–284. [Google Scholar] [CrossRef]
- Fridman, E.A.; Calvar, J.; Bonetto, M.; Gamzu, E.; Krimchansky, B.Z.; Meli, F.; Leiguarda, R.C.; Zafonte, R. Fast Awakening from Minimally Conscious State with Apomorphine. Brain Inj. 2009, 23, 172–177. [Google Scholar] [CrossRef]
- Fridman, E.A.; Krimchansky, B.Z.; Bonetto, M.; Galperin, T.; Gamzu, E.R.; Leiguarda, R.C.; Zafonte, R. Continuous Subcutaneous Apomorphine for Severe Disorders of Consciousness after Traumatic Brain Injury. Brain Inj. 2010, 24, 636–641. [Google Scholar] [CrossRef]
- Masotta, O.; Trojano, L.; Loreto, V.; Moretta, P.; Estraneo, A. Selegiline in Patients With Disorder of Consciousness: An Open Pilot Study. Can. J. Neurol. Sci. 2018, 45, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.T.; Whyte, J. The Effects of Methylphenidate on Command Following and Yes/No Communication in Persons with Severe Disorders of Consciousness: A Meta-Analysis of n-of-1 Studies. Am. J. Phys. Med. Rehabil. 2007, 86, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Herrold, A.A.; Pape, T.L.B.; Guernon, A.; Mallinson, T.; Collins, E.; Jordan, N. Prescribing Multiple Neurostimulants during Rehabilitation for Severe Brain Injury. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Barra, M.E.; Izzy, S.; Sarro-Schwartz, A.; Hirschberg, R.E.; Mazwi, N.; Edlow, B.L. Stimulant Therapy in Acute Traumatic Brain Injury: Prescribing Patterns and Adverse Event Rates at 2 Level 1 Trauma Centers. J. Intensive Care Med. 2020, 35, 1196–1202. [Google Scholar] [CrossRef]
- Wroblewski, B.; Glenn, M.B.; Cornblatt, R.; Joseph, A.B.; Suduikis, S. Protriptyline as an Alternative Stimulant Medication in Patients with Brain Injury: A Series of Case Reports. Brain Inj. 1993, 7, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, D.L.; Whyte, J.; Sandel, M.E. Improved Arousal and Initiation Following Tricyclic Antidepressant Use in Severe Brain Injury. Arch. Phys. Med. Rehabil. 1996, 77, 80–83. [Google Scholar] [CrossRef]
- Whyte, J.; Myers, R. Incidence of Clinically Significant Responses to Zolpidem among Patients with Disorders of Consciousness: A Preliminary Placebo Controlled Trial. Am. J. Phys. Med. Rehabil. 2009, 88, 410–418. [Google Scholar] [CrossRef]
- Machado, C.; Estévez, M.; Rodríguez, R.; Pérez-Nellar, J.; Chinchilla, M.; DeFina, P.; Leisman, G.; Carrick, F.R.; Melillo, R.; Schiavi, A.; et al. Zolpidem Arousing Effect in Persistent Vegetative State Patients: Autonomic, EEG and Behavioral Assessment. Curr. Pharm. Des. 2014, 20, 4185–4202. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Aricò, I.; De Salvo, S.; Conti-Nibali, V.; Bramanti, P. Transient awakening from vegetative state: Is high-dose zolpidem more effective? Psychiatry Clin. Neurosci. 2014, 69, 122–123. [Google Scholar] [CrossRef]
- Delargy, M.; O’Connor, R.; McCann, A.; Galligan, I.; Cronin, H.; Gray, D.; O’Toole, C. An Analysis of the Effects of Using Zolpidem and an Innovative Multimodal Interdisciplinary Team Approach in Prolonged Disorders of Consciousness (PDOC). Brain Inj. 2019, 33, 242–248. [Google Scholar] [CrossRef]
- Sayadnasiri, M.; Rezvani, F. Treatment of Catatonia in Frontotemporal Dementia: A Lesson from Zolpidem Test. Clin. Neuropharmacol. 2019, 42, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; O’brien, K.; Won, W.; Li, S. A Retrospective Analysis on Clinical Practice-Based Approaches Using Zolpidem and Lorazepam in Disorders of Consciousness. Brain Sci. 2021, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Carboncini, M.C.; Piarulli, A.; Virgillito, A.; Arrighi, P.; Andre, P.; Tomaiuolo, F.; Frisoli, A.; Bergamasco, M.; Rossi, B.; Bonfiglio, L. A Case of Post-Traumatic Minimally Conscious State Reversed by Midazolam: Clinical Aspects and Neurophysiological Correlates. Restor. Neurol. Neurosci. 2014, 32, 767–787. [Google Scholar] [CrossRef]
- Margetis, K.; Korfias, S.I.; Gatzonis, S.; Boutos, N.; Stranjalis, G.; Boviatsis, E.; Sakas, D.E. Intrathecal Baclofen Associated with Improvement of Consciousness Disorders in Spasticity Patients. Neuromodulation 2014, 17, 699–704. [Google Scholar] [CrossRef]
- Sarà, M.; Pistoia, F.; Mura, E.; Onorati, P.; Govoni, S. Intrathecal Baclofen in Patients With Persistent Vegetative State: 2 Hypotheses. Arch. Phys. Med. Rehabil. 2009, 90, 1245–1249. [Google Scholar] [CrossRef]
- Formica, F.; Pozzi, M.; Avantaggiato, P.; Molteni, E.; Arrigoni, F.; Giordano, F.; Clementi, E.; Strazzer, S. Disordered Consciousness or Disordered Wakefulness? The Importance of Prolonged Polysomnography for the Diagnosis, Drug Therapy, and Rehabilitation of an Unresponsive Patient with Brain Injury. J. Clin. Sleep Med. 2017, 13, 1477–1481. [Google Scholar] [CrossRef]
- Kondratieva, E.A.; Kondratev, S.A.; Denisova, A.A.; Ivanova, N.E.; Kondratiev, A.N. Results of treatment with intravenous amantadine sulfate (PK-Merz) patients with chronic disorders of consciousness. Zh Nevrol Psikhiatr Im S S Korsakova 2020, 120, 102–108. (In Russian) [Google Scholar] [CrossRef]
- Lanzillo, B.; Loreto, V.; Calabrese, C.; Estraneo, A.; Moretta, P.; Trojano, L. Does pain relief influence recovery of consciousness? A case report of a patient treated with ziconotide. EJPRM 2016, 52, 263–266. [Google Scholar]
- Schnakers, C.; Hustinx, R.; Vandewalle, G.; Majerus, S.; Moonen, G.; Boly, M.; Vanhaudenhuyse , A.; Laureys, S. Measuring the effect of amantadine in chronic anoxic minimally conscious state. J. Neurol. Neurosurg. Psychiatry 2008, 79, 225–227. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, L.; Liang, F.; Zhang, Y.; Yang, L.; Liu, X.; Yang, J. The use of amantadine in patients with unresponsive wakefulness syndrome after severe cerebral hemorrhage. Brain Inj. 2020, 34, 1084–1088. [Google Scholar] [CrossRef]
- Valenza, G.; Carboncini, M.C.; Virgillito, A.; Creatini, I.; Bonfiglio, L.; Rossi, B.; Lanata, A.; Scilingo, E.P. EEG complexity drug-induced changes in disorders of consciousness: A preliminary report. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3724–3727. [Google Scholar] [CrossRef]
| State | Definition | Available Epidemiology |
|---|---|---|
| Coma | Complete absence of arousal and awareness | Incidence of non-traumatic coma in UK: 30.8/100,000 children under 16 per year; 6.0/100,000 of general population per year [8]. |
| Vegetative State/Unresponsive Wakefulness Syndrome (VS/UWS; formerly also Apallic State) | Arousal without awareness | Incidence: ~2.6/100,000 people [9]. Prevalence: ~2.0/100,000 to ~5.0/100,000 people, depending on national protocols [9]. |
| Minimally conscious state minus (MCS-) | Minimal, reproducible, but inconsistent awareness without language | Prevalence: ~2.2/100,000 in Europe [2]. |
| Minimally conscious state plus (MCS+) | Minimal, reproducible, but inconsistent awareness with language comprehension and expression (i.e., either command following, intelligible verbalization or intentional communication). | |
| Emergence to consciousness (eMCS), including the Confused-Agitated State (CAS) | Persistent dysfunction across multiple cognitive domains, behavioural dysregulation, disorientation, also with symptom fluctuation. | Indirectly estimated in: ~0.4/100,000 in a single centre study in Europe [10]. |
| Cognitive Motor Dissociation (CMD) * | Volitional brain activity with no behavioural manifestation. | Unknown. |
| Treatments | Exclusion Criteria |
|---|---|
| Neurostimulation |
|
| Neuromodulation |
|
| Drug delivery |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irzan, H.; Pozzi, M.; Chikhladze, N.; Cebanu, S.; Tadevosyan, A.; Calcii, C.; Tsiskaridze, A.; Melbourne, A.; Strazzer, S.; Modat, M.; et al. Emerging Treatments for Disorders of Consciousness in Paediatric Age. Brain Sci. 2022, 12, 198. https://doi.org/10.3390/brainsci12020198
Irzan H, Pozzi M, Chikhladze N, Cebanu S, Tadevosyan A, Calcii C, Tsiskaridze A, Melbourne A, Strazzer S, Modat M, et al. Emerging Treatments for Disorders of Consciousness in Paediatric Age. Brain Sciences. 2022; 12(2):198. https://doi.org/10.3390/brainsci12020198
Chicago/Turabian StyleIrzan, Hassna, Marco Pozzi, Nino Chikhladze, Serghei Cebanu, Artashes Tadevosyan, Cornelia Calcii, Alexander Tsiskaridze, Andrew Melbourne, Sandra Strazzer, Marc Modat, and et al. 2022. "Emerging Treatments for Disorders of Consciousness in Paediatric Age" Brain Sciences 12, no. 2: 198. https://doi.org/10.3390/brainsci12020198
APA StyleIrzan, H., Pozzi, M., Chikhladze, N., Cebanu, S., Tadevosyan, A., Calcii, C., Tsiskaridze, A., Melbourne, A., Strazzer, S., Modat, M., & Molteni, E. (2022). Emerging Treatments for Disorders of Consciousness in Paediatric Age. Brain Sciences, 12(2), 198. https://doi.org/10.3390/brainsci12020198






