Interdisciplinary Approach to Biological and Health Implications in Selected Professional Competences
Abstract
:1. Introduction
- (i)
- Knowledge: business processes, financial accounting and law, use of IT tools, companies environment;
- (ii)
- Skills: analytical thinking, interpretation of financial and non-financial data, communication skills, ability to work in a team, strategic thinking;
- (iii)
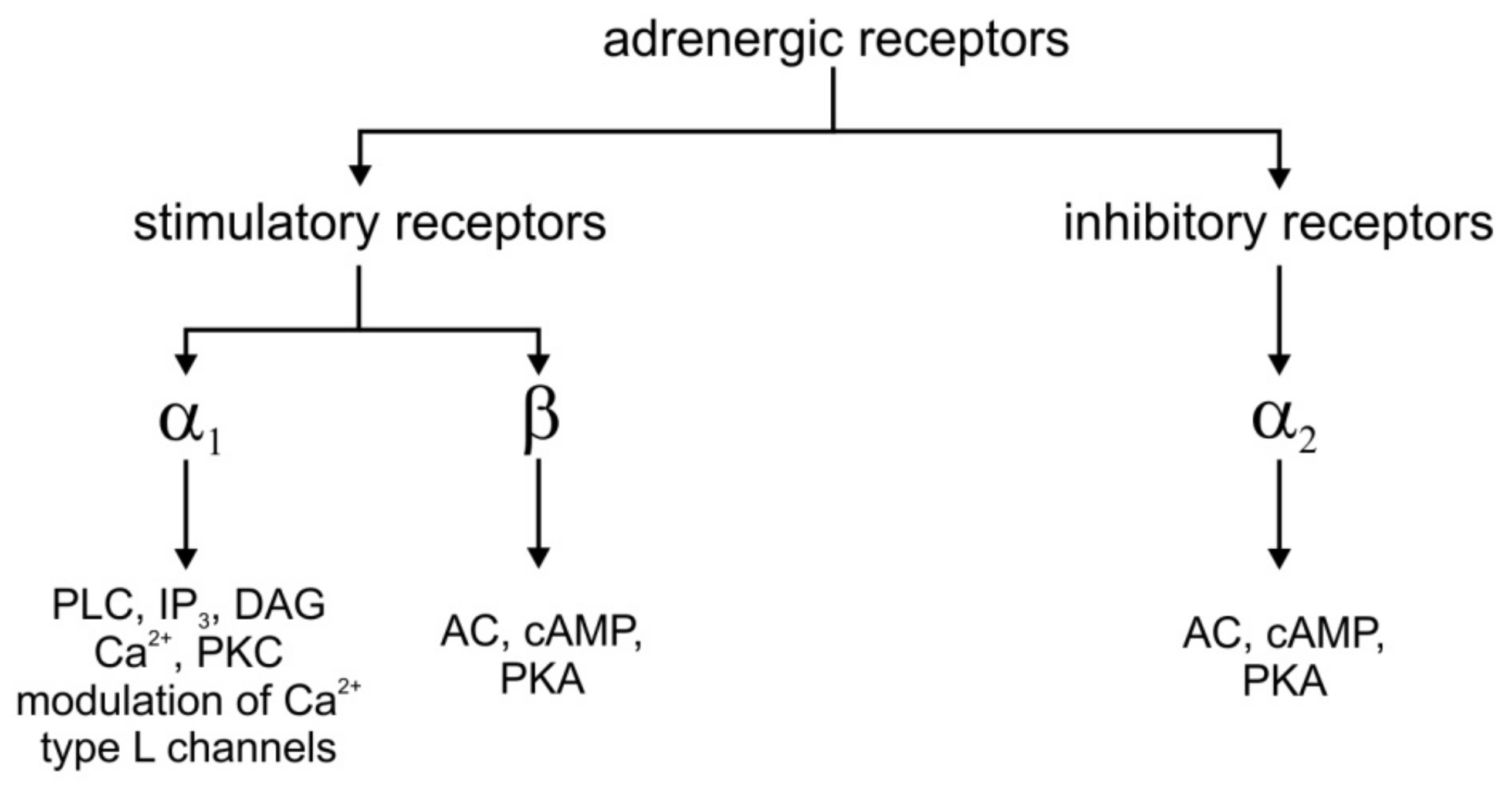
2. The Biological Process of Decision-Making
3. Behavior and Personality Type
4. Behavioral Genetics and Trait Inheritance
5. Stress Resistance
6. Human Emotions and Genotype
7. Intelligence and Genotype
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Szychta, A. Richard Mattessich’s Accounting Theory in the Light of the Basic Directions of Development of Accounting Science. Methodological Study; Foundation for the Development of Accountancy in Poland: Warsaw, Poland, 1996. (In Polish) [Google Scholar]
- Artienwicz, N. Neuroaccounting as a prospective but difficult direction of accounting development. Zeszyty Teoretyczne Rachunkowości 2015, 82, 9–18. (In Polish) [Google Scholar] [CrossRef]
- Artienwicz, N. Behavioral Stream in Accounting: Its Relation to Behavioral Finance and the Perspectives for Neuroaccounting Development in Poland. In Neuroeconomics and the Decision-Making Process; Business Science Reference; Christiansen, B., Lechman, E., Eds.; IGI Global: Hershey, PA, USA, 2016. [Google Scholar]
- Dickhaut, J.; Basu, S.; McCabe, K.; Waymire, G. Neuroaccounting: Consilience between the Biologically Evolved Brain and Culturally Evolved Accounting Principles. Account. Horiz. 2010, 24, 221–255. [Google Scholar] [CrossRef]
- Tank, A.K.; Farrell, A.M. Is Neuroaccounting Taking a Place on the Stage? A Review of the Influence of Neuroscience on Accounting Research. Eur. Account. Rev. 2021. [Google Scholar] [CrossRef]
- Waymire, G.B. Neuroscience and Ultimate Causation in Accounting Research. Account. Rev. 2014, 89, 2011–2019. [Google Scholar] [CrossRef]
- Cesarini, D.; Dawes, C.T.; Johannesson, M.; Lichtenstein, P.; Wallace, B. Genetic variation in preferences forgiving and risk-taking. Q. J. Econ. 2009, 124, 809–842. [Google Scholar] [CrossRef]
- Cesarini, D.; Johannesson, M.; Lichtenstein, P.; Sandewall, Ö.; Wallace, B. Genetic variation in financial decision making. J. Financ. 2010, 65, 1725–1754. [Google Scholar] [CrossRef]
- Contini, V.; Marques, F.; Garcia, C.; Hutz, M.; Bau, C. MAOA-uVNTR polymorphism in a brazilian sample: Further support for the association with impulsive behaviors and alcohol dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Blackwell, A.; Clark, L.; Menzies, L.; Cox, S.; Robbins, T. Tryptophandepletion disrupts the motivational guidance of goal directed behavior as a function of trait impulsivity. Neuropsychopharmacology 2005, 30, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Craig, I. The importance of stress and genetic variation in human aggression. Bioessays 2007, 29, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Damberg, M.; Garpenstrand, H.; Hallman, J.; Oreland, L. Genetic mechanisms of behavior don’t forget about the transcription factors. Mol. Psychiatry 2001, 6, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Neve, J.E. Functional Polymorphism (5-HTTLPR) in the Serotonin Transporter Gene Is Associated with Subjective Well-Being: Evidence from a US Nationally Representative Sample. J. Hum. Genet. 2011, 56, 456–459. [Google Scholar] [CrossRef] [PubMed]
- De Neve, J.E.; Christakis, N.; Fowler, J.H.; Frey, B.S. Genes, economics, and happiness. J. Neurosci. Psychol. Econ. 2012, 5, 193–211. [Google Scholar] [CrossRef] [Green Version]
- De Neve, J.E.; Fowlerca, J.H. Credit card borrowing and the monoamine oxidase A (MAOA) gene. J. Econ. Behav. Organ. 2014, 107, 428–439. [Google Scholar] [CrossRef]
- Wang, B. Analysis of Corporate Social Responsibility Decision-making Behavior Based on Cognitive Neuroscience. NeuroQuantology 2018, 16, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Della Penna, S.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage 2013, 80, 360–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara, E.; Rodriguez-Fornells, A.; Ye, Z.; Münte, T.F. Reward networks in the brain as captured by connectivity measures. Front. Neurosci. 2009, 3, 350–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, J.D.; Petersen, S.E. Control-related systems in the human brain. Curr. Opin. Neurobiol. 2013, 23, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waytz, A.; Mason, M. Your brain at work: What a new approach to neuroscience can teach us about management. Harv. Bus. Rev. 2013, 91, 102–111. [Google Scholar] [PubMed]
- Belkaoui, A. Accounting Theory; Thomson Learning: London, UK, 2004. [Google Scholar]
- Hońko, S. Self-Portrait of Accountants in Poland 2017. Report from the Nationwide Study ‘Portraits of Accountants’; Accountants Association in Poland: Warsaw, Poland, 2018. (In Polish) [Google Scholar]
- Burns, J.; Ezzamel, M.; Scapens, R. Management accounting change in the UK. Manag. Account. 1999, 77, 28–30. [Google Scholar]
- Damasiotisa, V.; Trivellasb, P.; Santouridisa, I.; Nikolopoulosa, S.; Tsiforaa, E. IT Competences for Professional Accountants. A Review. Procedia-Soc. Behav. Sci. 2015, 175, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak, Ż.; Wnuk-Pel, T. The roles and qualities of management accountants in organizations–evidence from the field. Procedia-Soc. Behav. Sci. 2015, 213, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Hageman, A.M. A Review of the Strengths and Weaknesses of Archival, Behavioral, and Qualitative Research Methods: Recognizing the Potential Benefits of Triangulation. Adv. Account. Behav. Res. 2008, 11, 1–30. [Google Scholar] [CrossRef]
- Zaleśkiewicz, T. Economic Psychology; PWN: Warsaw, Poland, 2011. (In Polish) [Google Scholar]
- Birnberg, J.G.; Ganguly, A.R. Is Neuroaccounting Waiting in the Wings? An Essay. Account. Organ. Soc. 2012, 37, 1–13. [Google Scholar] [CrossRef]
- Bauer, A.J.; Just, M.A. Brain reading and behavioral methods provide complementary perspectives on the representation of concepts. Neuroimage 2019, 186, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Na, J.; Agres, P.F.; Savalia, N.K.; Park, D.C.; Wig, G.S. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc. Natl. Acad. Sci. USA 2018, 115, E5144–E5153. [Google Scholar] [CrossRef] [Green Version]
- Maksimenko, V.A.; Runnova, A.E.; Zhuravlev, M.O.; Protasov, P.; Kulanin, R.; Khramova, M.V.; Pisarchik, A.N.; Hramov, A.E. Human personality reflects spatio-temporal and time-frequency EEG structure. PLoS ONE 2018, 13, e0197642. [Google Scholar] [CrossRef] [Green Version]
- Shakeschaft, N.; Trzaskowski, M.; McMillan, A.; Krapohol, E.; Simpson, M.; Reichenberg, A.; Cederloef, M.; Larsson, H.; Lichtenstein, P.; Plomin, R. Thinking Positively: The genetics of high intelligence. Intelligence 2015, 48, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweig, J. Your Brain, Your Money; MT Biznes: Warsaw, Poland, 2008. (In Polish) [Google Scholar]
- Strelau, J. Temperament, Personality, Action; PWN: Warsaw, Poland, 1985. (In Polish) [Google Scholar]
- Zdrojewski, E.; Gajewski, D. The personality concepts and its application of a process of employes recruitment. Sci. Noteb. Inst. Econ. Manag. 2009, 13, 171–196. (In Polish) [Google Scholar]
- Cloninger, C.R. Temperament and personality. Curr. Opin. Neurobiol. 1994, 4, 266–273. [Google Scholar] [CrossRef]
- Cloninger, C.R. The genetic structure of personality and learning: A phylogenetic model. Clin. Genet. 1994, 46, 124–137. [Google Scholar] [CrossRef]
- Cloninger, C.R. A psychobiological model of personality and psychopathology. Jpn. J. Psychosom. Med. 1997, 37, 91–102. [Google Scholar]
- Svrakic, D.M.; Whitehead, C.; Przybeck, T.R.; Cloninger, C.R. Differential Diagnosis of Personality Disorders by the Seven-Factor Model of Temperament and Character. Arch. Gen. Psychiatry 1993, 50, 991–999. [Google Scholar] [CrossRef]
- Jang, K.L.; Vernon, P.A.; Livesley, W.J. Behavioural-Genetic Perspectives on Personality Function. Can. J. Psychiatry 2001, 46, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrick, M.R.; Mount, M.K. Yes, Personality Matters: Moving on to More Important Matters. Hum. Perform. 2005, 18, 359–372. [Google Scholar] [CrossRef]
- Barrick, M.R.; Mount, M.K.; Judge, T.A. Personality and performance at the beginning of the new millennium: What do we know and where do we go next? Int. J. Select. Assess. 2001, 9, 9–30. [Google Scholar] [CrossRef]
- Cewińska, J.; Grzesiak, L.; Kabalski, P.; Kusidel, E. Accounting students’ preonality verus contemporary accounting system. Zesz. Teor. Rachun. 2016, 90, 87–104. (In Polish) [Google Scholar]
- Holland, J.L. Making Vocational Choices: A Theory of Careers; Prentice-Hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Holland, J.L. Making Vocational Choices: A Theory of Vocational Personalities and Work Environments, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1985. [Google Scholar]
- Holland, J.L. Making Vocational Choices: A Theory of Vocational Personalities and Work Environments, 3rd ed.; Psychological Assessment Resources; William Michael Books: Cave Springs, AR, USA, 1997. [Google Scholar]
- Duszkiewicz, A.J.; McNamara, C.G.; Takeuchi, T.; Genzel, L. Novelty and Dopaminergic Modulation of Memory Persistence: A Tale of Two Systems. Trends Neurosci. 2019, 42, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernández, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef]
- Gray, J.A. The neuropsychology of temperament. In Explorations in Temperament: International Prespecitves on Theory and Measurement; Strelau, J., Angeleitner, A., Eds.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Moreira, A.S.P.; Inman, R.A.; Cloninger, K.; Cloninger, C.R. Student engagement with school and personality: A biopsychosocial and person-centred approach. Br. J. Educ. Psychol. 2021, 91, 691–713. [Google Scholar] [CrossRef]
- Farrell, S.M.; Tunbridge, E.M.; Braeutigam, S.; Harrison, P.J. COMT Val158Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol. Psychiatry 2012, 71, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, B.; Vasquez, A.A.; Johansson, S.; Hoogman, M.; Romanos, J.; Boreatti-Hümmer, A.; Heine, M.; Jacob, C.P.; Lesch, K.P.; Casas, M.; et al. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology 2010, 35, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Kohn, P.D.; Kolachana, B.; Kippenhan, S.; McInerney-Leo, A.; Nussbaum, R.; Weinberger, D.R.; Berman, K.F. Midbrain dopamine and prefrontal function in humans: Interaction and modulation by COMT genotype. Nat. Neurosci. 2005, 8, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Tunbridge, M.E.; Narajos, M.; Harrison, C.H.; Beresford, C.; Cipriani, A.; Harrison, P.J. Which Dopamine Polymorphisms Are Functional? Systematic Review and Meta-analysis of COMT, DAT, DBH, DDC, DRD1-5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biol. Psychiatry 2019, 86, 608–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.J.; Minneman, K.P. Recent progress in alpha1-adrenergic receptor research Recent progress in alpha1-adrenergic receptor research. Acta Pharmacol. Sin. 2005, 26, 1281–1287. [Google Scholar] [CrossRef]
- Vilar, S.; Sobarzo-Sanchez, E.; Santana, L.; Uriarte, E. Molecular Docking and Drug Discovery in β-Adrenergic Receptors. Curr. Med. Chem. 2017, 24, 4340–4359. [Google Scholar] [CrossRef] [PubMed]
- Wachter, S.B.; Gilbert, E.M. Beta-adrenergic receptors, from their discovery and characterization through their manipulation to beneficial clinical application. Cardiology 2012, 122, 104–112. [Google Scholar] [CrossRef]
- Molenaar, P.; Chen, L.; Semmler, A.B.; Parsonage, W.A.; Kaumann, A.J. Human heart beta-adrenoceptors: Beta1-adrenoceptor diversification through ‘affinity states’ and polymorphism. Clin. Exp. Pharm. Physiol. 2007, 34, 1020–1028. [Google Scholar] [CrossRef]
- de Jong, J.M.A.; Wouters, R.T.F.; Boulet, N.; Cannon, B.; Nedergaard, J.; Petrovic, N. The β3-adrenergic receptor is dispensable for browning of adipose tissues. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E508–E518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheibenbogen, C.; Loebel, M.; Freitag, H.; Krueger, A.; Bauer, S.; Antelmann, M.; Doehner, W.; Scherbakov, N.; Heidecke, H.; Reinke, P.; et al. Immunoadsorption to remove ß2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS ONE 2018, 13, e0193672. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.P.; Carey, R.J.; Huston, J.P.; De Souza Silva, M.A. Serotonin and psychostimulant addiction: Focus on 5-HT1A-receptors. Prog. Neurobiol. 2007, 81, 133–178. [Google Scholar] [CrossRef]
- Müller, C.P.; Homberg, J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015, 277, 146–192. [Google Scholar] [CrossRef]
- Levran, O.; Peles, E.; Randesi, M.; Correa da Rosa, J.; Ott, J.; Rotrosen, J.; Adelson, M.; Kreek, M.J. Susceptibility loci for heroin and cocaine addiction in the serotonergic and adrenergic pathways in populations of different ancestry. Pharmacogenomics 2015, 16, 1329–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Q.; Lin, W.W.; Wu, N.; Wang, S.Y.; Chen, M.Z.; Lin, Z.H.; Xie, X.Q.; Feng, Z.W. Structural insight into the serotonin (5-HT) receptor family by molecular docking, molecular dynamics simulation and systems pharmacology analysis. Acta Pharm. Sin. 2019, 40, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Shimizu, S.; Tokudome, K.; Kunisawa, N.; Sasa, M. New insight into the therapeutic role of the serotonergic system in Parkinson’s disease. Prog. Neurobiol. 2015, 134, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Mizuno, M.; Nabeshima, T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002, 70, 735–744. [Google Scholar] [CrossRef]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar] [CrossRef]
- Siuda, J.; Patalong-Ogiewa, M.; Żmuda, W.; Targosz-Gajniak, M.; Niewiadomska, E.; Matuszek, I.; Jędrzejowska-Szypułka, H.; Lewin-Kowalik, J.; Rudzińska-Bar, M. Cognitive impairment and BDNF serum levels. Neurol. Neurochir. Pol. 2017, 51, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Diaz-delCastillo, M.; Woldbye, D.P.D.; Heegaard, A.M. Neuropeptide Y and its Involvement in Chronic Pain. Neuroscience 2018, 387, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.A.; Moran, T.D.; Abdulla, F.; Tumber, K.K.; Taylor, B.K. Spinal mechanisms of NPY analgesia. Peptides 2007, 28, 464–474. [Google Scholar] [CrossRef]
- Ito, M.; Dumont, Y.; Quirion, R. Mood and memory-associated behaviors in neuropeptide Y5 knockout mice. Neuropeptides 2013, 47, 75–84. [Google Scholar] [CrossRef]
- Alves, H.; Voss, M.W.; Boot, W.R.; Deslandes, A.; Cossich, V.; Salles, J.I.; Kramer, A.F. Perceptual-cognitive expertise in elite volleyball players. Front. Psychol. 2013, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, S.; Kida, N.; Oda, S. Central and peripheral visual reaction time of soccer players and nonathletes. Percept. Mot. Skill 2001, 92, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, M.; Ando, S.; Kida, N.; Oda, S. Interference effects between saccadic and key-press reaction times of volleyball players and nonathletes. Percept. Mot. Skill 2006, 103, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.T.Y.; Williams, A.M.; Ward, P.; Janelle, C.M. Perceptual-cognitive expertise in sport: A meta-analysis. J. Sport Exerc. Psychol. 2007, 29, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Muiños, M.; Ballesteros, S. Peripheral vision and perceptual asymmetries in young and older martial arts athletes and non-athletes. Atten. Percept. Psychophys. 2014, 76, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Kramer, A.F.; Basak, C.; Prakash, R.S.; Roberts, B. Are expert athletes “expert” in the cognitive laboratory? A meta-analytic review of basic attention and perception and sport expertise. Appl. Cogn. Psychol. 2010, 24, 812–826. [Google Scholar]
- Miązek, A. Neuroaccounting as a modern approach in accounting. Stud. Oeconomica Posnaniensia 2014, 4, 75–83. (In Polish) [Google Scholar]
- Anokhin, A.P.; Muller, V.; Lindenberger, U.; Heath, A.C.; Myers, E. Genetic influences on dynamic complexity of brain oscillations. Neurosci. Lett. 2006, 397, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Oniszczenko, W.; Jakubowska, U. Genetic determinants and personality correlates of sociopolitical attitudes in a Polish sample. Twin Res. Hum. Genet. 2005, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Oniszczenko, W.; Dragan, W.Ł. Behavioral Genetics in Psychology and Psychiatry; Scholar: Warsaw, Poland, 2008. (In Polish) [Google Scholar]
- Oniszczenko, W.; Dragan, W.Ł. Association between temperament in terms of the Regulative Theory of Temperament and DRD4 and DAT1 gene polymorphisms. Compr. Psychiatry 2012, 53, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Oniszczenko, W. Behavior genetics; What does it bring to the knowledge of man? Pol. Psychol. Forum 2017, 22, 5–19. (In Polish) [Google Scholar] [CrossRef]
- Finne, L.B.; Christensen, J.O.; Knardahl, S. Psychological and social work factors as predictors of mental distress: A prospective study. PLoS ONE 2014, 9, e102514. [Google Scholar] [CrossRef] [Green Version]
- Gilbert-Ouimet, M.; Brisson, C.; Vézina, M. Psychosocial work stressors, high family responsibilities, and psychological distress among women: A 5-year prospective study. Am. J. Ind. Med. 2020, 63, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Magee, C.; Biesanz, J.C. Toward understanding the relationship between personality and well-being states and traits. J. Personal. 2019, 87, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; He, J.G. Effects of environmental stress on shrimp innate immunity and white spot syndrome virus infection. Fish Shellfish Immunol. 2019, 84, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Dragoş, D.; Tănăsescu, M.D. The effect of stress on the defense systems. J. Med. Life 2010, 3, 10–18. [Google Scholar] [PubMed]
- Friedrich, T.; Schalla, M.A.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Inflammatory Stress Induced by Intraperitoneal Injection of LPS Increases Phoenixin Expression and Activity in Distinct Rat Brain Nuclei. Brain Sci. 2022, 12, 135. [Google Scholar] [CrossRef]
- Malik, S.; Alnaji, O.; Malik, M.; Gambale, T.; Rathbone, M.P. Correlation between Mild Traumatic Brain Injury-Induced Inflammatory Cytokines and Emotional Symptom Traits: A Systematic Review. Brain Sci. 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.P.; Sertil, A.R. A stressful microenvironment: Opposing effects of the endoplasmic reticulum stress response in the suppression and enhancement of adaptive tumor immunity. Int. Rev. Immunol. 2015, 34, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Segerstrom, S.C. Resources, stress, and immunity: An ecological perspective on human psychoneuroimmunology. Ann. Behav. Med. 2010, 40, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Burns, V.E.; Edwards, K.M.; Ring, C.; Drayson, M.; Carroll, D. Complement cascade activation after an acute psychological stress task. Psychosom. Med. 2008, 70, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A. Neuroscience and the emergence of neuroeconomics. In Neuroeconomics Decision Making and the Brain; Academic Press: Cambridge, MA, USA, 2009; pp. 207, 209–213. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleshner, M.; Frank, M.; Maier, S.F. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 2017, 42, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, T.C.; Xu, C.; Duman, R.S. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav. Immun. 2018, 72, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Enoch, M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology 2011, 214, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Nieman, D.C. Exercise immunology: Practical applications. Int. J. Sport. Med. 1997, 18 (Suppl. 1), S91–S100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, H.Y.; Liu, W.X.; Jia, X.X.; Zhang, J.G.; Ma, C.L.; Zhang, X.J.; Yu, F.; Cong, B. Prostaglandin E2 restrains human Treg cell differentiation via E prostanoid receptor 2-protein kinase A signaling. Immunol. Lett. 2017, 191, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P. Recommendations to maintain immune health in athletes. Eur. J. Sport Sci. 2018, 18, 820–831. [Google Scholar] [CrossRef] [Green Version]
- Walsh, N.P.; Oliver, S.J. Exercise, immune function and respiratory infection: An update on the influence of training and environmental stress. Immunol. Cell Biol. 2016, 94, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhou, S.; Davie, A.; Su, Q. Effects of moderate and high intensity exercise on T1/T2 balance. Exerc. Immunol. Rev. 2012, 18, 98–114. [Google Scholar]
- Seyle, H. The General Adaptation Syndrome and the Diseases of Adaptation. J. Clin. Endocrinol. Metab. 1946, 6, 117–230. [Google Scholar] [CrossRef]
- Landowski, J. Neurobiology of stress. Neuropsychiatry Neuropsychol. 2007, 2, 26–36. (In Polish) [Google Scholar]
- Turecki, G.; Meaney, M.J. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol. Psychiatry 2016, 79, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starcke, K.; Brand, M. Decision making under stress: A selective review. Neurosci. Biobehav. Rev. 2012, 36, 1228–1248. [Google Scholar] [CrossRef]
- Funder, J.W. Mineralocorticoid receptors: Distribution and activation. Heart Fail. Rev. 2005, 10, 15–22. [Google Scholar] [CrossRef]
- Williams, S.; Ghosh, C. Neurovascular glucocorticoid receptors and glucocorticoids: Implications in health, neurological disorders and drug therapy. Drug Discov. Today 2020, 25, 89–106. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joels, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, T.W.; Lovallo, W.R. The role of genetics in stress effects on health and addiction. Curr. Opin. Psychol. 2019, 27, 72–76. [Google Scholar] [CrossRef]
- Wang, X.; Pongrac, J.L.; DeFranco, D.B. Glucocorticoid Receptors in Hippocampal Neurons that Do Not Engage Proteasomes Escape from Hormone-Dependent Down-Regulation but Maintain Transactivation Activity. Mol. Endocrinol. 2002, 16, 1987–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonko, M.; Ausserlechner, M.J.; Bernhard, D.; Helmberg, A.; Kofler, R. Gene Expression Profiles of Proliferating vs. G1/G0 Arrested Human Leukemia Cells Suggest a Mechanism for Glucocorticoid-Induced Apoptosis. FASEB J. 2001, 15, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Malkoski, S.P.; Dorin, R.I. Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Mol. Endocrinol. 1999, 13, 1629–1644. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R. Cortisol secretion patterns in addiction and addiction risk. Int. J. Psychophysiol. 2006, 59, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, O.C. Understanding stress through the genome. Stress 2006, 9, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gatt, J.M.; Burton, K.L.; Williams, L.M.; Schofield, P.R. Specific and common genes implicated across major mental disorders: A review of meta-analysis studies. J. Psychiatr. Res. 2015, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.H.; Scoriels, L.; Munafo, M.R. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol. Psychiatry 2008, 64, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R.; Enoch, M.A.; Sorocco, K.H.; Vincent, A.S.; Acheson, A.; Cohoon, A.J.; Hodgkinson, C.A.; Goldman, D. Joint impact of early life adversity and COMT Val158Met (rs4680) genotypes on the adult cortisol response to psychological stress. Psychosom. Med. 2017, 79, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Bukh, J.D.; Bock, C.; Vinberg, M.; Werge, T.; Gether, U.; Vedel Kessing, L. Interaction between genetic polymorphisms and stressful life events in first episode depression. J. Affec. Disord. 2009, 119, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bondy, B.; Kuznik, J.; Baghai, T.; Schüle, C.; Zwanzger, P.; Minov, C.; de Jonge, S.; Rupprecht, R.; Meyer, H.; Engel, R.R.; et al. Lack of association of serotonin-2A receptor gene polymorphism (T102C) with suicidal ideation and suicide. Am. J. Med. Genet. 2000, 96, 831–835. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Dragan, W.Ł.; Oniszczenko, W. Association of a functional polymorphism in the serotonin transporter gene with personality traits in females in a Polish population. Neuropsychobiology 2006, 54, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Strobel, A.; Gutknecht, L.; Rothe, C.; Reif, A.; Mossner, R.; Zeng, Y.; Brocke, B.; Lesch, K.P. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J. Neural Transm. 2003, 110, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Skalkidou, A.; Poromaa, I.S.; Iliadis, S.I.; Huizink, A.C.; Hellgren, C.; Freyhult, E.; Comasco, E. Stress-related genetic polymorphisms in association with peripartum depression symptoms and stress hormones: A longitudinal population-based study. Psychoneuroendocrinology 2019, 103, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Kohlberg, L. A cognitive developmental approach to moral education. Humanist 1972, 4, 13–16. [Google Scholar]
- Kohlberg, L. Stages and sequences: The cognitive developmental approach to socialization. In Handbook of Socialization Theory and Research; Goslin, D., Ed.; Rand McNally: Chicago, IL, USA, 1969. [Google Scholar]
- Bloom, B. Taxonomy of Educational Objectives, the Classification of Educational Goals-Handbook 1: Cognitive Domain; David McKay: New York, NY, USA, 1956. [Google Scholar]
- Antonakis, J.; Ashkanasy, N.M.; Dasborough, M.T. Does leadership need emotional intelligence? Leadersh. Q. 2009, 20, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Cook, G.L.; Bay, D.; Visser, B.; Myburgh, J.E.; Njoroge, J. Emotional Intelligence: The Role of Accounting Education and Work Experience. Issue Account. Educ. 2011, 26, 267–286. [Google Scholar] [CrossRef]
- McPhail, K. An emotional response to the state of accounting education: Developing accounting students’ emotional intelligence. Crit. Perspect. Account. 2004, 15, 629–648. [Google Scholar] [CrossRef]
- Bay, D.; McKeage, K. Emotional Intelligence in Undergraduate Accounting Students: Preliminary Assessment. Account. Educ. Int. J. 2006, 15, 439–454. [Google Scholar] [CrossRef]
- Strelau, J. About Human Intelligence; Wiedza Powszechna: Warsaw, Poland, 1987. (In Polish) [Google Scholar]
- Strelau, J. Psychology of Individual Differences; Scholar: Warsaw, Poland, 2008. (In Polish) [Google Scholar]
- Horta, M.; Kaylor, K.; Feifel, D.; Ebner, N.C. Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neurosci. Biobehav. Rev. 2020, 108, 1–23. [Google Scholar] [CrossRef]
- Festante, F.; Ferrari, P.F.; Thorpe, S.G.; Buchanan, R.W.; Fox, N.A. Intranasal oxytocin enhances EEG mu rhythm desynchronization during execution and observation of social action: An exploratory study. Psychoneuroendocrinology 2019, 111, 104467. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.L.; Dal Monte, O.; Noble, P.; Averbeck, B.B. Intranasal oxytocin effects on social cognition: A critique. Brain Res. 2014, 1580, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, H.E.; Young, L.E. Oxytocin and the neural mechanism regulating social cognition and affiliative behavior. Front. Neuroendocr. 2009, 30, 534–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dębiec, J. Peptides of Love and Fear: Vasopressin and Oxytocin Modulate the Integration of Information in the Amygdala. Bioessays 2005, 27, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Anzani, S.; Cannito, L.; Bellia, F.; Domenico, A.D.; Dell’Osso, B.; Palumbo, R.; D’Addario, C. OXTR Gene DNA Methylation Levels Are Associated with Discounting Behavior with Untrustworthy Proposers. Brain Sci. 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Netherton, E.; Schatte, D. Potential for oxytocin use in children and adolescents with mental illness. Hum. Psychopharmacol. 2011, 26, 271–281. [Google Scholar] [CrossRef]
- Theodoridou, A.; Rowe, A.C.; Penton-Voak, I.S.; Rogers, P.J. Oxytocin and Social Perception: Oxytocin Increases Perceived Facial Trustworthiness and Attractiveness. Horm. Behav. 2009, 56, 128–132. [Google Scholar] [CrossRef]
- Saphire-Bernstein, S.; Way, B.M.; Kim, H.S.; Sherman, D.K.; Taylor, S.E. Oxytocin receptor gene (OXTR) is related to psychological resources. Proc. Natl. Acad. Sci. USA 2011, 108, 15118–15122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsubo, Y.; Matsunaga, M.; Komiya, A.; Mifune, N.; Yagi, A. Oxytocin receptor gene (OXTR) polymorphism and self-punishment after an unintentional transgression. Personal. Individ. Differ. 2014, 69, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Israel, S.; Lerer, E.; Shalev, I.; Uzefovsky, F.; Reibold, M.; Bachner-Melman, R.; Granot, R.; Bornstein, G.; Knafo, A.; Yirmiya, N.; et al. Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: From autism to altruism with some notes in between. Prog. Brain Res. 2008, 170, 435–449. [Google Scholar] [CrossRef]
- Hopkin, M. ‘Ruthlessness Gene’ Discovered. Nature. 2008. Available online: https://www.nature.com/articles/news.2008.738 (accessed on 15 December 2019).
- Wang, Z.; Hart, S.A.; Kovas, Y.; Lukowski, S.; Soden, B.; Thompson, L.A.; Petrill, S.A. Who Is Afraid of Math? Two Sources of Genetic Variance for Mathematical Anxiety. J. Child Psychol. Psychiatry 2014, 55, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Z. The relationship between MAOA gene polymorphism and test anxiety. Twin Res. Hum. Genet. 2013, 16, 1103–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Israel, S.; Uzefovsky, F.; Ebstein, R.P.; Knafo-Noam, A. Dopamine D4 receptor polymorphism and sex interact to predict children’s affective knowledge. Front. Psychol. 2015, 6, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzefovsky, F.; Shalev, I.; Israel, S.; Edelman, S.; Raz, Y.; Perach-Barzilay, N.; Mankuta, D.; Shamay-Tsoory, S.G.; Knafo, A.; Ebstein, R.P. The Dopamine D4 Receptor Gene Shows a Gender-Sensitive Association with Cognitive Empathy: Evidence from Two Independent Samples. Emotion 2014, 14, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Mayseless, N.; Uzefovsky, F.; Shalev, I.; Ebstein, R.P.; Shamay-Tsoory, S.G. The association between creativity and 7R polymorphism in the dopamine receptor D4 gene (DRD4). Front. Hum. Neurosci. 2013, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Drewa, G.; Ferenc, T. Medical Genetics; Edra Urban & Partner: Wrocław, Poland, 2015. (In Polish) [Google Scholar]
- Bolton, T.J.; Marioni, R.E.; Deary, I.J.; Harris, S.E.; Steward, M.C.; Murray, G.D.; Fowkes, F.G.; Price, J.F. Association between polymorphism of the dopamine receptor D2 and catechol-o-methyl Transferase genes and cognitive functions. Behav. Genet. 2010, 40, 630–638. [Google Scholar] [CrossRef]
- Kleifield, E.I.; Sunday, S.; Hurt, S.; Halmi, K.A. The Tridimensional Personality Questionaire: An exploration of personality traits in eating disorders. J. Psychiatr. Res. 1994, 28, 413–423. [Google Scholar] [CrossRef]
- Ebstein, R.P.; Segman, R.; Benjamin, J.; Osher, Y.; Nemanov, L.; Belmaker, R.H. 5-HT2C (HTR2C) serotonin receptor gene polymorphism associated with the human personality trait of reward dependence: Interaction with dopamine D4 receptor (D4DR) and dopamine D3 receptor (D3DR) polymorphisms. Am. J. Med. Genet. 1994, 74, 65–72. [Google Scholar] [CrossRef]
- Samochowiec, J.; Fiszer-Piosik, E.; Kucharska-Mazur, J.; Horodnicki, J. The Influence of Genes on the Development of Personality. Psychiatr. Pol. 2000, 34, 99–109. (In Polish) [Google Scholar]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Hanson, I.; Seawright, A.; van Heyningen, V. The human BDNF gene maps between FSHB and HVBS1 at the boundary of 11p13–p14. Genomics 1992, 13, 1331–1333. [Google Scholar] [CrossRef]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.J.; Hong, C.J.; Yu, Y.W.; Chen, T.J. Association Study of a Brain-Derived Neurotrophic Factor (BDNF) Val66Met Polymorphism and Personality Trait and Intelligence in Healthy Young Females. Neuropsychobiology 2004, 49, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Hoft, N.R.; Ehringer, M.A. The genetic components of alcohol and nicotine co-addiction:from genes to behavior. Curr. Drug Abus. Rev 2008, 1, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Goleman, D. Emotional Intelligence; Media Rodzina: Poznań, Poland, 1999. (In Polish) [Google Scholar]
- Eysenck, H.J. Psychiatric Diagnosis as a Psychological and Statistical Problem. Psychol. Rep. 1955, 1, 3–17. [Google Scholar] [CrossRef]
- Eysenck, H.J. Personality Factors in a Random Sample of the Population. Psychol. Rep. 1979, 44 (Suppl. 3), 1023–1027. [Google Scholar] [CrossRef]
- Eysenck, H.J. The Biological Basis of Cross-Cultural Differences in Personality: Blood Group Antigens. Psychol. Rep. 1982, 51, 531–540. [Google Scholar] [CrossRef]
- Gardner, H. Taking a multiple intelligences (MI) perspective. Behav. Brain Sci. 2017, 40, e203. [Google Scholar] [CrossRef]
- Colom, R.; Karama, S.; Jung, R.E.; Haier, R.J. Human intelligence and brain networks. Dialogues Clin. Neurosci. 2010, 12, 489–501. [Google Scholar] [PubMed]
- Haier, R.J.; Jung, R.E.; Yeo, R.A.; Head, K.; Alkire, M.T. The neuroanatomy of general intelligence: Sex matters. NeuroImage 2005, 25, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Ramchandran, K.; Tranel, D.; Duster, K.; Denburg, N.L. The Role of Emotional vs. Cognitive Intelligence in Economic Decision-Making Amongst Older Adults. Front. Neurosci. 2020, 14, 497. [Google Scholar] [CrossRef] [PubMed]
- Plomin, R.; McClearn, G.E.; Smith, D.L.; Vignetti, S.; Chorney, M.J.; Chorney, K.; Venditti, C.P.; Kasarda, S.; Thompson, L.A.; Detterman, D.K.; et al. DNA markers associated with high versus low IQ: The IQ quantitative trait loci (QTL) project. Behav. Genet. 1994, 24, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Plomin, R.; Mcclearn, G.E.; Smith, D.L.; Skuder, P.; Vignetti, S.; Chorney, M.J.; Chorney, K.; Kasarda, S.; Thompson, L.A.; Detterman, D.K.; et al. Allelic associations between 100 DNA markers and high versus low IQ. Intelligence 1995, 21, 31–48. [Google Scholar] [CrossRef]
- Plomin, R.; DeFries, J.C.; McClearn, G.E.; McGuffin, P. Behavioral Genetics; PWN: Warsaw, Poland, 2001. (In Polish) [Google Scholar]
- Plomin, R. Genetics: How Intelligence Changes with Age. Nature 2012, 482, 165–166. [Google Scholar] [CrossRef]
- Bouchard, T.J., Jr.; Lykken, D.T.; McGue, M.; Segal, N.L.; Tellegen, A. Sources of human psychological differences: The Minesota study of twins reared apart. Science 1990, 250, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.C. Twin studies of ability, personality and interests. Homo 1978, 29, 158–173. [Google Scholar]
- Trzaskowski, M.; Young, J.; Visscher, P.M.; Plomin, R. DNA evidence for strong genetic stability and increasing heritability of intelligence from age 7 to 12. Mol. Psychiatry 2014, 19, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzaskowski, M.; Harlaar, N.; Arden, R.; Krapohl, E.; Rimfeld, K.; McMillan, A.; Dale, P.S.; Plomin, R. Genetic Influence on Family Socioeconomic Status and Children’s Intelligence. Intelligence 2014, 42, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, T.J., Jr. The Wilson effect: The increase in heritability of IQ with age. Twin Res. Hum. Genet. 2013, 16, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S. Synchronies in mental development: Epigenetic perspective. Science 1978, 202, 939–948. [Google Scholar] [CrossRef]
- Kovas, Y.; Garon-Carrier, G.; Boivin, M.; Petrill, S.A.; Plomin, R.; Malykh, S.B.; Spinath, F.; Murayama, K.; Ando, J.; Bogdanova, O.Y.; et al. Why children differ in motivation to learn: Insights over 13,000 twins from 6 countries. Personal. Individ. Differ. 2015, 80, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deary, I.J.; Harris, S.E.; Fox, H.C.; Hayward, C.; Whalley, L.J.; Wright, A.F.; Starr, J.M. KLOTHO genotype and cognitive ability in childhood and old age in the same individuals. Neurosci. Lett. 2005, 378, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.A.; Fox, N.A. The relations between frontal brain electrical activity and cognitive development during infancy. Child Dev. 1992, 63, 1142–1163. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Greenstein, D.; Lerch, J.; Clasen, L.; Lenroot, R.; Gogtay, N.; Evans, A.; Rapoport, J.; Giedd, J. Intellectual ability and cortical development in children and adolescents. Nature 2006, 440, 676–679. [Google Scholar] [CrossRef]
- Hulshoff Pol, H.E.; Schnack, H.G.; Posthuma, D.; Mandl, R.C.W.; Baare, W.F.; van Oel, C.; van Haren, N.E.; Collins, D.L.; Evans, A.C.; Amunts, K.; et al. Genetic contributions to human brain morphology and intelligence. J. Neurosci. 2006, 26, 10235–10242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennington, B.F.; Filipek, P.A.; Lefly, D.; Chhabildas, R.; Kennedy, D.N.; Simon, J.H.; Filley, C.M.; Galaburda, A.; DeFries, J.C. A Twin MRI study of size variation in the human brain. J. Cogn. Neurosci. 2000, 12, 223–323. [Google Scholar] [CrossRef] [PubMed]
- Peper, J.S.; Brouwer, R.M.; Boomsma, D.I.; Kahn, R.S.; Poll, H.E.H. Genetic influences on human brain and structure: A review of brain imaging studies in twins. Hum. Brain Mapp. 2007, 28, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, D.; De Geus, E.J.C.; Baare, W.F.C.; Pol, H.E.H.; Kahn, R.S.; Boomsma, D.I. The Association between brain volume and intelligence is of genetic origin. Nat. Neurosci. 2012, 5, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.M.; Hibar, D.P.; Stein, J.; Prasad, G.; Jahanshad, N. Micro-, Meso- and Macro-Connectomics of the Brain. In Genetics of the Connectome and the ENIGMA Project; Springer International Publishing AG: Cham, Switzerland, 2016. [Google Scholar]
- Deary, I.J.; Johnson, W.; Houlihan, L.M. Genetic foundation of human intelligence. Hum. Genet. 2009, 126, 215–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addington, A.M.; Gornick, M.; Duckworth, J.; Sporn, A.; Gogtay, N.; Bobb, A.; Greenstein, D.; Lenane, M.; Gochman, P.; Baker, N.; et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol. Psychiatry 2005, 10, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Shimokata, H.; Ando, F.; Niino, N.; Miyasaka, K.; Funakoshi, A. Cholecystokinin A receptor gene promoter polymorphism and intelligence. Ann. Epidemiol. 2005, 15, 196–201. [Google Scholar] [CrossRef]
- Lidelli, M.; Williams, J.; Bayer, A. Confirmation of association between the e4 allele of apolipoprotein E and Alzheimer disease. J. Med. Genet. 1994, 31, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Strittmatter, W.J.; Weisgraber, K.H.; Huang, D.Y.; Dong, L.M.; Salvesen, G.S.; Pericak-Vance, M.; Schmechel, D.; Saunders, A.M.; Goldgaber, D.; Roses, A.D. Binding of Human Apolipoprotein E to Synthetic Amyloid Beta Peptide: Isoform-Specific Effects and Implications for Late-Onset Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1993, 90, 8098–8102. [Google Scholar] [CrossRef] [Green Version]
- Junkiert-Czarnecka, A.; Haus, O. Genetical Beckground of intelligence. Postep. Hig. Med. Dosw. 2006, 70, 590–598. [Google Scholar] [CrossRef]
- Djalali, S.; Höltje, M.; Grosse, G.; Rothe, T.; Stroh, T.; Grosse, J.; Deng, D.R.; Hellweg, R.; Grantyn, R.; Hörtnagl, H.; et al. Effects of brain-derived neurotrophic factor (BDNF) on glial cells and serotonergic neurones during development. J. Neurochem. 2005, 92, 616–627. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comings, D.E.; Wu, S.; Rostamkhani, M.; McGue, M.; Lacono, W.G.; Cheng, L.S.; MacMurray, J.P. Role of cholinergic muscarinic 2 receptor (CHRM2) gene in cognition. Mol. Psychiatry 2003, 8, 10–11. [Google Scholar] [CrossRef]
- Dick, D.M.; Aliev, F.; Kramer, J.; Wang, J.C.; Hinrichs, A.; Bertelsen, S.; Kuperman, S.; Schuckit, M.; Nurnberger, J., Jr.; Edenberg, H.J.; et al. Association of CHRM2 with IQ: Converging evidence for a gene influencing intelligence. Behav. Genet. 2007, 37, 265–272. [Google Scholar] [CrossRef]
- Gosso, M.; van Belzen, M.; de Geus, E.J.C.; Polderman, J.C.; Heutink, P.; Boomsma, D.I.; Posthuma, D. Association between the CHRM2 gene and intelligence in a sample of 304 Dutch families. Genes Brain Behav. 2006, 5, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, S.E.; Fox, H.; Wright, A.F.; Hayward, C.; Starr, J.M.; Whalley, L.J.; Deary, I.J. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, G.; Tenesa, A.; Payton, A.; Yang, J.; Harris, S.E.; Liewald, D.; Ke, X.; Le Hellard, S.; Christoforou, A.; Luciano, M.; et al. Genome Wide Association studies establish that human intelligence is highly heritable and polygenic. Mol. Psychiatry 2011, 16, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Butcher, L.M.; Meaburn, E.; Dale, P.S.; Sham, P.; Schalkwyk, L.C.; Craig, I.W.; Plomin, R. Association analysis of mild mental impairment using DNA pooling to screen 432 brain-expressed single nucleotide polymorphisms. Mol. Psychiatry 2005, 10, 384–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porteous, D.J.; Thomson, P.; Brandon, N.J.; Millar, J.K. The genetics and biology of DISC1: An emerging role in psychosis and cognition. Biol. Psychiatry 2006, 60, 123–131. [Google Scholar] [CrossRef]
- Small, B.J.; Rosnick, C.B.; Fratiglioni, L.; Bäckman, L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychol. Aging 2004, 19, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Kachiwala, S.J.; Harris, S.E.; Wright, A.F.; Hayward, C.; Starr, J.M.; Whalley, L.J.; Deary, I.J. Genetic influences on oxidative stress and their association with normal cognitive ageing. Neurosci. Lett. 2005, 386, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.J.; Cesarini, D.; van der Loos, M.J.; Dawes, C.T.; Koellinger, P.D.; Magnusson, P.K.; Chabris, C.F.; Conley, D.; Laibson, D.; Johannesson, M.; et al. The genetic architecture of economic and political preferences. Proc. Natl. Acad. Sci. USA 2012, 109, 8026–8031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFries, J.C.; Fulker, D.W. Multiple regression analysis of twin data. Behav. Genet. 1985, 15, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Yong-Kyu, K. Handbook of Behavior Genetics; Springer: New York, NY, USA, 2009. [Google Scholar]
- Miller, P.; Mulvey, C.; Martin, N. Genetic and environmental contributions to educational attainment in Australia. Econ. Educ. Rev. 2001, 20, 211–224. [Google Scholar] [CrossRef]
- Stein, J.L.; Medland, S.E.; Vasquez, A.A.; Hibar, D.P.; Senstad, R.E.; Winkler, A.M.; Toro, R.; Appel, K.; Bartecek, R.; Bergmann, Ø.; et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat. Genet. 2012, 44, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Protein | Chromosome Localization |
|---|---|---|
| ADH2 | alcohol dehydrogenase 2 | 4q21–q23 |
| ADH3 | alcohol dehydrogenase 3 | 4q21–q23 |
| ADH5 | alcohol dehydrogenase 5 | 4q21–q25 |
| ADRB2 | beta-2 adrenergic receptor | 5q33 |
| AGXT | alanine-glyoxylate and serine-pyruvate aminotransferase | 2p36–q37 |
| ALPP | alkaline phosphatase, placental | 2q37 |
| ALPL | alkaline phosphatase | 1p36.1–p34 |
| APOE | apolipoprotein E | 19q13 |
| ATP1B | sodium-potassium ATPase subunit beta 1 | 1q22 |
| ALDH5A1 | aldehyde dehydrogenase 5 family member A1 | 6p22 |
| ASPM | abnormal spindle-like microcephaly-associated protein | 1q31 |
| BDNF | brain-derived neurotrophic factor | 11p14 |
| BPI | bactericidal permeability-increasing | 20q12 |
| CTG-B37 | atrophin-1 | 12p |
| CHRM2 | cholinergic receptor muscarinic 2 | 7q33 |
| CD27 | CD27 antigen | 12p13 |
| CCKAR | cholecystokinin A receptor | 4p15 |
| CTSD | cathepsin D | 11p15 |
| CHRNA7 | cholinergic receptor nicotinic alpha 7 subunit | 15q14 |
| CYP2D6 | cytochrome P450 family 2 subfamily D member 6 | 22q13 |
| COMT | catechol-O-methyltransferase | 22q11 |
| CKB | brain-type creatine kinase also | 14q32.3 |
| DTNBP1 | dystrobrevin binding protein 1 | 6p22 |
| DISC1 | disrupted-in-dchizophrenia 1 (DISC1) scaffold protein | 1q42.1 |
| DAT1 | dopamine transporter 1 | 5p15.3 |
| DM | myotonic dystrophy | 19q13.3 |
| MCPH1 | microcephalin 1 | 8p23 |
| DRD1 | dopamine receptor D1 | 5q34–q35 |
| DRD2 | dopamine receptor D2 | 11q23 |
| DRD3 | dopamine receptor D3 | 3q13.3 |
| DRD4 | dopamine receptor D4 | 11p15 |
| EST00083 | cDNA sequence from hippocampal library | 15926 bp mtDNA |
| ESR | estrogen receptor | 6q24–q27 |
| FADS2 | fatty acid desaturase 2 | 11q12 |
| FBN1 | fibrillin-1 | 15q15 |
| GLUT2 | glucose transporter 2 | 3q26 1–q26.3 |
| GLUT3 | glucose transporter 3 | 12p13.3 |
| GLUT4 | glucose transporter 4 | 17p13 |
| GAD1 | glutamate decarboxylase 1 | 2q31.1 |
| GAA | acid alpha-glucosidase | 17q23 |
| GRL | glucocorticoid receptor | 5q31–q32 |
| Hsp70 | heat shock protein | 6p21.3 |
| HEXA | beta-hexosaminidase A | 15q23–q24 |
| HEXB | beta-hexosaminidase B | 5q13 |
| HOX2F | Homeobox 2F | 17q21–q22 |
| HOX2G | Homeobox 2G | 17q21–q22 |
| HTR2 | serotonin-receptor 2 | 13q14–q21 |
| HTR2A | serotonin-receptor 2A | 13q14 |
| HLA-H | major histocompatibility complex, class I, H | 6p21.3 |
| HLA-A | major histocompatibility complex, class I, A | 6p21.3 |
| HP | haptoglobin | 16q22.1 |
| INSR | insulin receptor | 19p13.3–p13.2 |
| IGF2R | insulin-like growth factor 2 receptor | 6q26 |
| KL | klotho | 13q13 |
| LAMB1 | laminin subunit beta 1 | 7q31 |
| LAMB2 | laminin subunit beta 2 | 3p21 |
| MYH2 | myosin heavy chain 2 | 17p13.1 |
| MAP2 | microtubule associated protein 2 | 2q34–q35 |
| MAOA | monoamine oxidase A | Xp11.3 |
| NRN1 | neutrin 1 | 6p25.1 |
| NGFB | nerve growth factor-beta | 1p13 |
| NEFM | neurofilament medium polypeptide | 8p21.2 |
| OXTR | oxytocin receptor | 3p25 |
| OTC | ornithine carbamoyltransferase | Xp11.4 |
| PPP1R1B | protein phosphatase 1 regulatory subunit 1B | 17q12 |
| PCCA | propionyl-CoA carboxylase subunit A | 13q31–q34 |
| PCCB | propionyl-CoA carboxylase subunit B | 3q21-–q22 |
| PAH | phenylalanine hydroxylase | 12q22–q24.2 |
| PRNP | prion protein | 20p13 |
| PTH | parathyroid hormone | 11p15.2–p15.1 |
| PCI | protein C inhibitor | 14q32.1 |
| SELE | selectin E | 1q22–q25 |
| SOD2 | superoxide dismutase 2 [Mn], mitochondrial | 6q21 |
| SNAP25 | synaptosomal-associated protein 25kDa | 20p12 |
| S100B | S100 calcium-binding protein B | 21q22 |
| SLC6A3 | sodium-dependent dopamine transporter | 5p15 |
| TIMP2 | tissue inhibitor of metalloproteinases 2 | 17q22–q25 |
| TAT | tyrosine aminotransferase | 16q22.1 |
| TG | thyroglobulin | 8q24 |
| TGFA | transforming growth factor alpha | 2p13 |
| TH | tyrosine hydroxylase | 11p15.5 |
| THRB | thyroid hormone receptor beta | 3p24.1–p22 |
| TPO | thyroid peroxidase | 2p25–p24 |
| UNG | uracil DNA glycosylase | 12q24 |
| WRN | Werner syndrome ATP-dependent helicase | 8p12 |
| VDR | vitamin D receptor | 12q12–q14 |
| ZNF40 | Human Immunodeficiency Virus Type I Enhancer Binding Protein 1 | 6p24.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzewa-Nowak, D.; Nowak, R.; Kubaszewska, J.; Gos, W. Interdisciplinary Approach to Biological and Health Implications in Selected Professional Competences. Brain Sci. 2022, 12, 236. https://doi.org/10.3390/brainsci12020236
Kostrzewa-Nowak D, Nowak R, Kubaszewska J, Gos W. Interdisciplinary Approach to Biological and Health Implications in Selected Professional Competences. Brain Sciences. 2022; 12(2):236. https://doi.org/10.3390/brainsci12020236
Chicago/Turabian StyleKostrzewa-Nowak, Dorota, Robert Nowak, Joanna Kubaszewska, and Waldemar Gos. 2022. "Interdisciplinary Approach to Biological and Health Implications in Selected Professional Competences" Brain Sciences 12, no. 2: 236. https://doi.org/10.3390/brainsci12020236






