Features of Psychomotor Coordination in Adolescents with Neuropsychiatric Pathology Enrolled in a Standard Educational Program †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Equipment
2.3. Examination Procedure
2.4. Statistical Analysis
3. Results
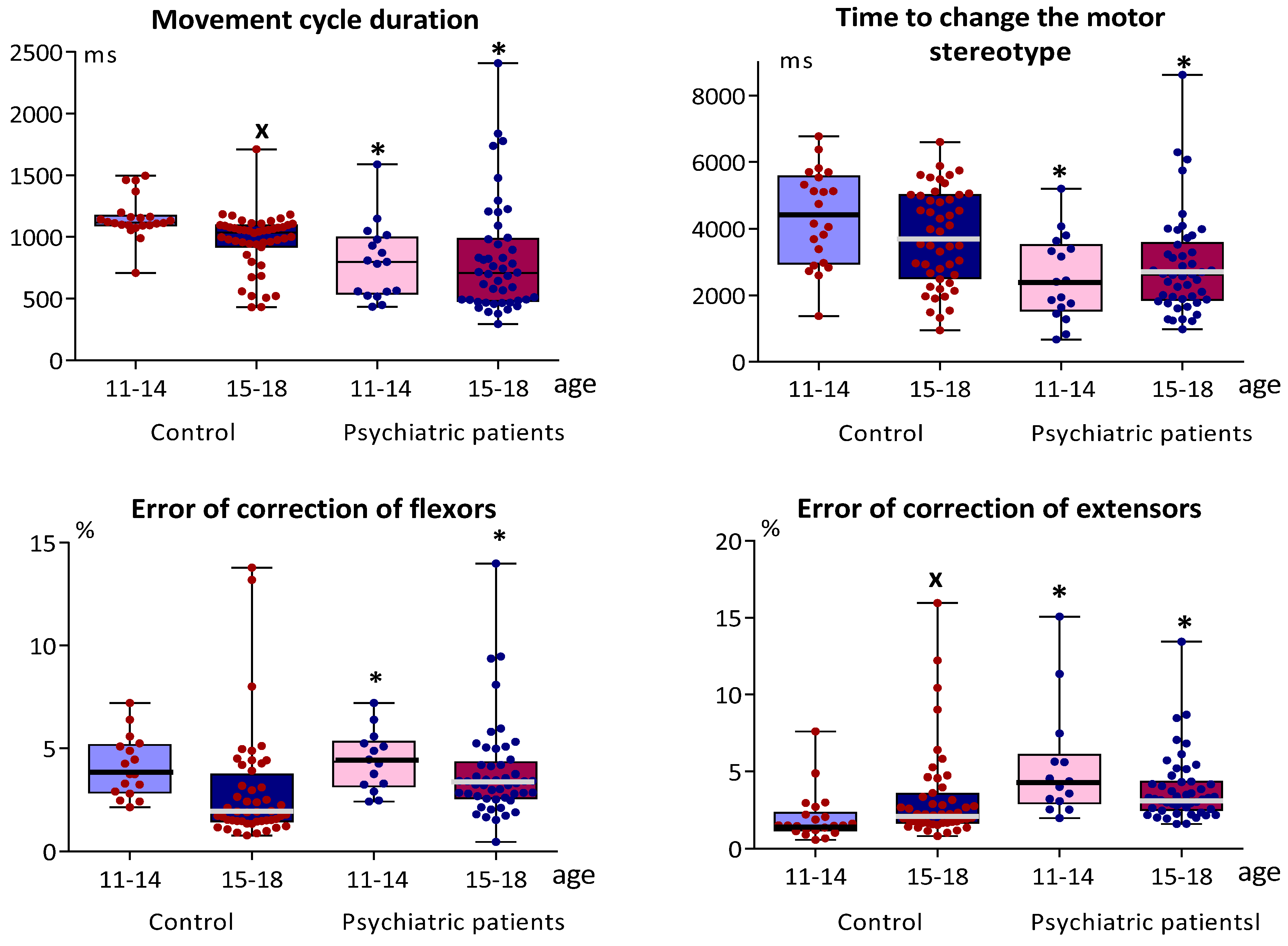
3.1. Indicators of Sensorimotor Coordination and Simple Sensorimotor Reaction in Adolescents with Typical Development and in Adolescents with Mental Disorders
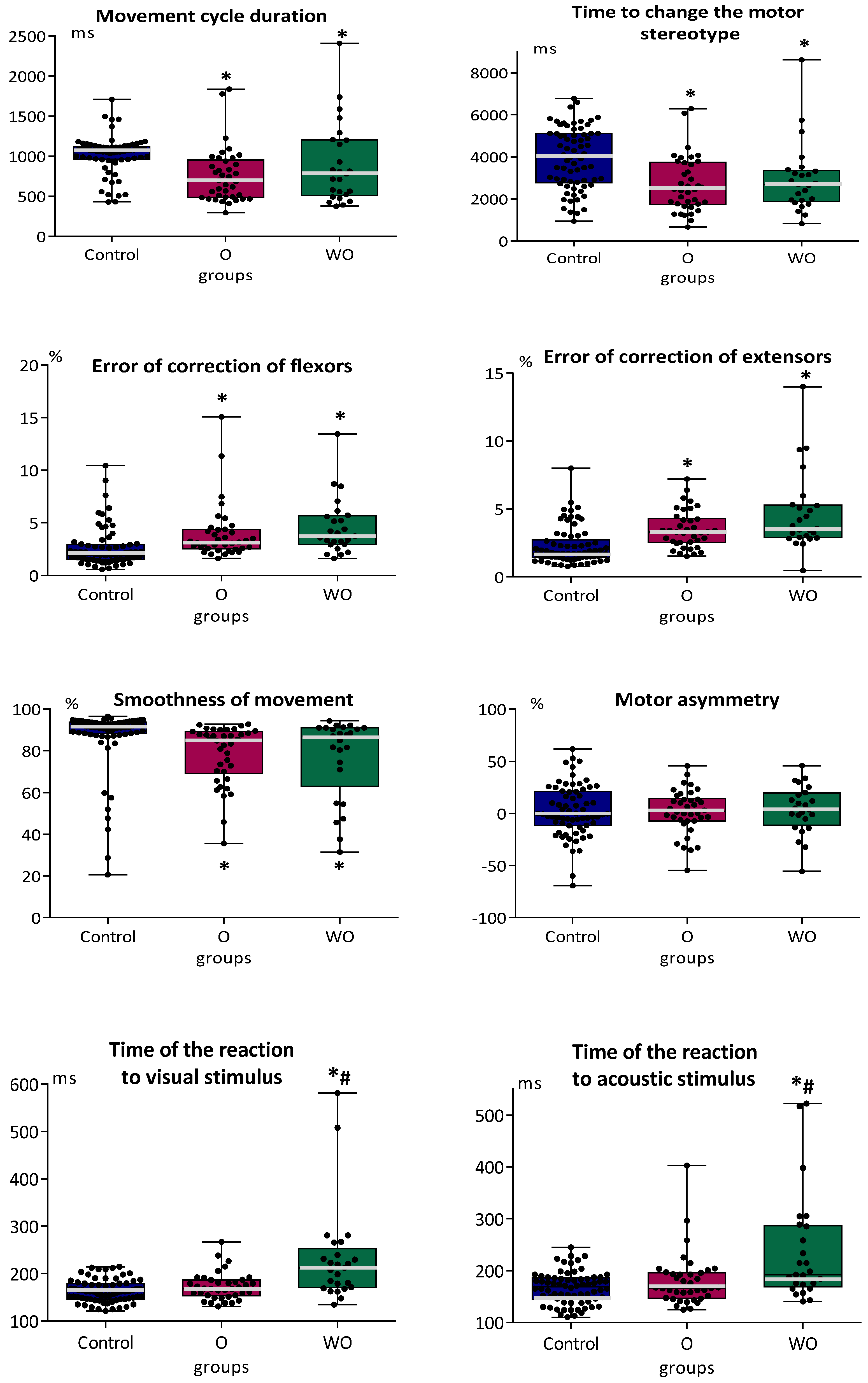
3.2. Indicators of Sensorimotor Coordination and Simple Sensorimotor Reaction in Adolescents with Typical Development and in Adolescents with or without Organic Brain Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kieling, C.; Baker-Henningham, H.; Belfer, M.; Conti, G.; Ertem, I.; Omigbodun, O.; Rohde, L.A.; Srinath, S.; Ulkuer, N.; Rahman, A. Child and adolescent mental health worldwide: Evidence for action. Lancet 2011, 378, 1515–1525. [Google Scholar] [CrossRef]
- Belfer, M.L. Child and adolescent mental disorders: The magnitude of the problem across the globe. J. Child Psychol. Psychiatry 2008, 49, 226–236. [Google Scholar] [CrossRef]
- Kessler, R.; Berglund, O.; Demler, R.; Jin, R.; Walters, E. Life-time prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Monaco, A.P. An epigenetic, transgenerational model of increased mental health disorders in children, adolescents and young adults. Eur. J. Hum. Genet. 2021, 29, 387–395. [Google Scholar] [CrossRef]
- The World Health Organization (WHO) (2016) Mental Health: Strengthening Our Response. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-health-strengthening-our-response (accessed on 8 February 2022).
- Read, H.; Roush, S.; Downing, D. Early Intervention in Mental Health for Adolescents and Young Adults: A Systematic Review. Am. J. Occup. Ther. 2018, 72, 7205190040p1–7205190040p8. [Google Scholar] [CrossRef]
- O’Reilly, M.; Svirydzenka, N.; Adams, S.; Dogra, N. Review of mental health promotion interventions in schools. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Rosello-Miranda, B.; Berenguer-Forner, C.; Miranda-Casas, A. Adaptive behaviour and learning in children with neurodevelopmental disorders (autism spectrum disorders and attention deficit hyperactivity disorder). Effects of executive functioning. Rev. Neurol. 2018, 66, S127–S132. (In Spanish) [Google Scholar]
- Vacheron, M.N.; Veyrat-Masson, H.; Wehbe, E. What support of young presenting a first psychotic episode, when schooling is being challenged? Encephale 2017, 43, 570–576. (In French) [Google Scholar] [CrossRef]
- Eldreth, D.; Hardin, M.G.; Pavletic, N.; Ernst, M. Adolescent transformations of behavioral and neural processes as potential targets for prevention. Prev. Sci. 2013, 14, 257–266. [Google Scholar] [CrossRef]
- Iznak, A.F.; Iznak, E.V.; Sorokin, S.A. Dynamics and relationships of parameters of cognitive evoked potentials and sensorimotor reactions in the treatment of apathic depression. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 2011, 111, 52–57. (In Russian) [Google Scholar]
- Epstein, J.; Perez, D.L.; Ervin, K.; Pan, H.; Kocsis, J.H.; Butler, T.; Stern, E.; Silbersweig, D.A. Failure to segregate emotional processing from cognitive and sensorimotor processing in major depression. Psychiatry Res. 2011, 93, 144–150. [Google Scholar] [CrossRef]
- Qin, J.; Shen, H.; Zeng, L.L.; Jiang, W.; Liu, L.; Hu, D. Predicting clinical responses in major depression using intrinsic functional connectivity. Neuroreport 2015, 26, 675–680. [Google Scholar] [CrossRef]
- Bo, Q.; Mao, Z.; Tian, Q.; Wen, Y.; Dong, F.; Li, X.; Wang, Z.; Ma, X.; Wang, C. Deficits of perceived spatial separation-induced prepulse inhibition in patients with bipolar disorder compared to healthy controls. J. Affect. Disord. 2018, 240, 63–71. [Google Scholar] [CrossRef]
- Chen, L.C.; Su, W.C.; Ho, T.L.; Lu, L.; Tsai, W.C.; Chiu, Y.N.; Jeng, S.F. Postural control and interceptive skills in children with autism spectrum disorder. Phys. Ther. 2019, 99, 1231–1241. [Google Scholar] [CrossRef]
- Dickson, H.; Roberts, R.E.; To, M.; Wild, K.; Loh, M.; Laurens, K.R. Adolescent trajectories of fine motor and coordination skills and risk for schizophrenia. Schizophr. Res. 2020, 215, 263–269. [Google Scholar] [CrossRef]
- Davies, P.L.; Rose, J.D. Motor skills of typically developing adolescents. Phys. Occup. Ther. Pediatr. 2000, 20, 19–42. [Google Scholar]
- Walther, S.; Strik, W. Motor symptoms and schizophrenia. Neuropsychobiology 2012, 66, 77–92. [Google Scholar] [CrossRef]
- Pankova, N.B.; Mavrenkova, P.V.; Lebedeva, M.A.; Karganov, M.Y. Parameters of heart rate and blood pressurevariability in adolescents with neuropsychiatric disorders during functional tests. Patogenez 2020, 18, 64–70. (In Russian) [Google Scholar] [CrossRef]
- Noskin, L.; Pivovarov, V.; Landa, S. Methodology, hard- and software of polysystemic monitoring. In Polysystemic Approach to School, Sport and Environment Medicine; OMICS Group Incorporation: Hyderabad, India, 2014; pp. 13–22. [Google Scholar]
- Das, J.K.; Salam, R.A.; Thornburg, K.L.; Prentice, A.M.; Campisi, S.; Lassi, Z.S.; Koletzko, B.; Bhutta, Z.A. A Nutrition in adolescents: Physiology, metabolism, and nutritional needs. N. Y. Acad. Sci. 2017, 1393, 21–33. [Google Scholar] [CrossRef]
- Patton, G.C.; Sawyer, S.M.; Santelli, J.S.; Ross, D.A.; Afifi, R.; Allen, N.B.; Arora, M.; Azzopardi, P.; Baldwin, W.; Bonell, C.; et al. Our future: A Lancet commission on adolescent health and wellbeing. Lancet 2016, 387, 2423–2478. [Google Scholar] [CrossRef] [Green Version]
- Quatman-Yates, C.C.; Quatman, C.E.; Meszaros, A.J.; Paterno, M.V.; Hewett, T.E. A systematic review of sensorimotor function during adolescence: A developmental stage of increased motor awkwardness? Br. J. Sports Med. 2012, 46, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Pivovarov, V.V. The computerized motion meter. Biomed. Eng. 2006, 40, 74–77. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lenhard, W.; Lenhard, A. Computation of Effect Sizes. Psychometrica. 2016. Available online: https://www.psychometrica.de/effect_size.html (accessed on 8 February 2022).
- Lyle, M.A.; Valero-Cuevas, F.J.; Gregor, R.J.; Powers, C.M. Control of dynamic foot-ground interactions in male and female soccer athletes: Females exhibit reduced dexterity and higher limb stiffness during landing. J. Biomech. 2014, 47, 512–517. [Google Scholar] [CrossRef]
- Gur, R.C.; Richard, J.; Calkins, M.E.; Chiavacci, R.; Hansen, J.A.; Bilker, W.B.; Loughead, J.; Connolly, J.J.; Qiu, H.; Mentch, F.D.; et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology 2012, 26, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Schilaty, N.D.; Bates, N.A.; Bies, N.J.; McPherson, A.L.; Hewett, T.E. High school female basketball athletes exhibit decreased knee-specific choice visual-motor reaction time. Scand. J. Med. Sci. Sports 2021, 31, 1699–1707. [Google Scholar] [CrossRef]
- Gasser, T.; Rousson, V.; Caflisch, J.; Jenni, O.G. Development of motor speed and associated movements from 5 to 18 years. Dev. Med. Child. Neurol. 2010, 52, 256–263. [Google Scholar] [CrossRef]
- Dorfberger, S.; Adi-Japha, E.; Karni, A. Sex differences in motor performance and motor learning in children and adolescents: An increasing male advantage in motor learning and consolidation phase gains. Behav. Brain Res. 2009, 198, 165–171. [Google Scholar] [CrossRef]
- Bezrukikh, M.M.; Kiselev, M.F.; Komarov, G.D.; Kozlov, A.P.; Kurneshova, L.E.; Landa, S.B.; Noskin, L.A.; Noskin, V.A.; Pivovarov, V.V. The age-related characteristics of the organization of motor activity in 6- to 16-year-old children. Hum. Physiol. 2000, 26, 337–344. [Google Scholar] [CrossRef]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F., 3rd; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef] [Green Version]
- Alchinova, I.; Karganov, M. Physiological Balance of the Body: Theory, Algorithms, and Results. Mathematics 2021, 9, 209. [Google Scholar] [CrossRef]
- McKinney, T.L.; Euler, M.J. Neural anticipatory mechanisms predict faster reaction times and higher fluid intelligence. Psychophysiology 2019, 56, e13426. [Google Scholar] [CrossRef]
- Svoboda, K.; Li, N. Neural mechanisms of movement planning: Motor cortex and beyond. Curr. Opin. Neurobiol. 2018, 49, 33–41. [Google Scholar] [CrossRef]
- Goodale, M.A. Transforming vision into action. Vis. Res. 2011, 51, 1567–1587. [Google Scholar] [CrossRef] [Green Version]
- Niechwiej-Szwedo, E.; Meier, K.; Christian, L.; Nouredanesh, M.; Tung, J.; Bryden, P.; Giaschi, D. Concurrent maturation of visuomotor skills and motion perception in typically-developing children and adolescents. Dev. Psychobiol. 2020, 62, 353–367. [Google Scholar] [CrossRef]
- Vidal, P.P.; Lacquaniti, F. Perceptual-motor styles. Exp. Brain Res. 2021, 239, 1359–1380. [Google Scholar] [CrossRef]
- Kostrubiec, V.; Huys, R.; Jas, B.; Kruck, J. Age-dependent Relationship Between Socio-adaptability and Motor Coordination in High Functioning Children with Autism Spectrum Disorder. J. Autism. Dev. Disord. 2018, 48, 209–224. [Google Scholar] [CrossRef]
- Burton, B.K.; Hjorthøj, C.; Jepsen, J.R.; Thorup, A.; Nordentoft, M.; Plessen, K.J. Research Review: Do motor deficits during development represent an endophenotype for schizophrenia? A meta-analysis. J. Child Psychol. Psychiatry 2016, 57, 446–456. [Google Scholar] [CrossRef]
- Saunders, K.E.; Goodwin, G.M.; Rogers, R.D. Borderline personality disorder, but not euthymic bipolar I disorder, is associated with prolonged post-error slowing in sensorimotor performance. J. Affect. Disord. 2016, 198, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Doumas, M.; Smolders, C.; Brunfaut, E.; Bouckaert, F.; Krampe, R.T. Dual task performance of working memory and postural control in major depressive disorder. Neuropsychology 2012, 26, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Van Damme, T.; Fransen, E.; Simons, J.; van West, D.; Sabbe, B. Motor impairment among different psychiatric disorders: Can patterns be identified? Hum. Mov. Sci. 2015, 44, 317–326. [Google Scholar] [CrossRef]
- Brenner, E.; Smeets, J.B.J. How Can You Best Measure Reaction Times? J. Mot. Behav. 2019, 51, 486–495. [Google Scholar] [CrossRef]
- Haith, A.M.; Pakpoor, J.; Krakauer, J.W. Independence of Movement Preparation and Movement Initiation. J. Neurosci. 2016, 36, 3007–3015. [Google Scholar] [CrossRef]
- Inui, N.; Yamanishi, M.; Tada, S. Simple reaction times and timing of serial reactions of adolescents with mental retardation, autism, and Down syndrome. Percept. Mot. Skills 1995, 81, 739–745. [Google Scholar] [CrossRef]
- Klotz, J.M.; Johnson, M.D.; Wu, S.W.; Isaacs, K.M.; Gilbert, D.L. Relationship between reaction time variability and motor skill development in ADHD. Child. Neuropsychol. 2012, 18, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Mansoubi, M.; Weedon, B.D.; Esser, P.; Mayo, N.; Fazel, M.; Wade, W.; Ward, T.E.; Kemp, S.; Delextrat, A.; Dawes, H. Cognitive Performance, Quality and Quantity of Movement Reflect Psychological Symptoms in Adolescents. J. Sports Sci. Med. 2020, 19, 364–373. [Google Scholar]
- Podnieks, I.; Doust, J.W. Spontaneous rhythms of perceptual motor performance in intact and damaged brain of man. Biol. Psychol. 1975, 3, 201–212. [Google Scholar] [CrossRef]
- Incoccia, C.; Formisano, R.; Muscato, P.; Reali, G.; Zoccolotti, P. Reaction and movement times in individuals with chronic traumatic brain injury with good motor recovery. Cortex 2004, 40, 111–115. [Google Scholar] [CrossRef]

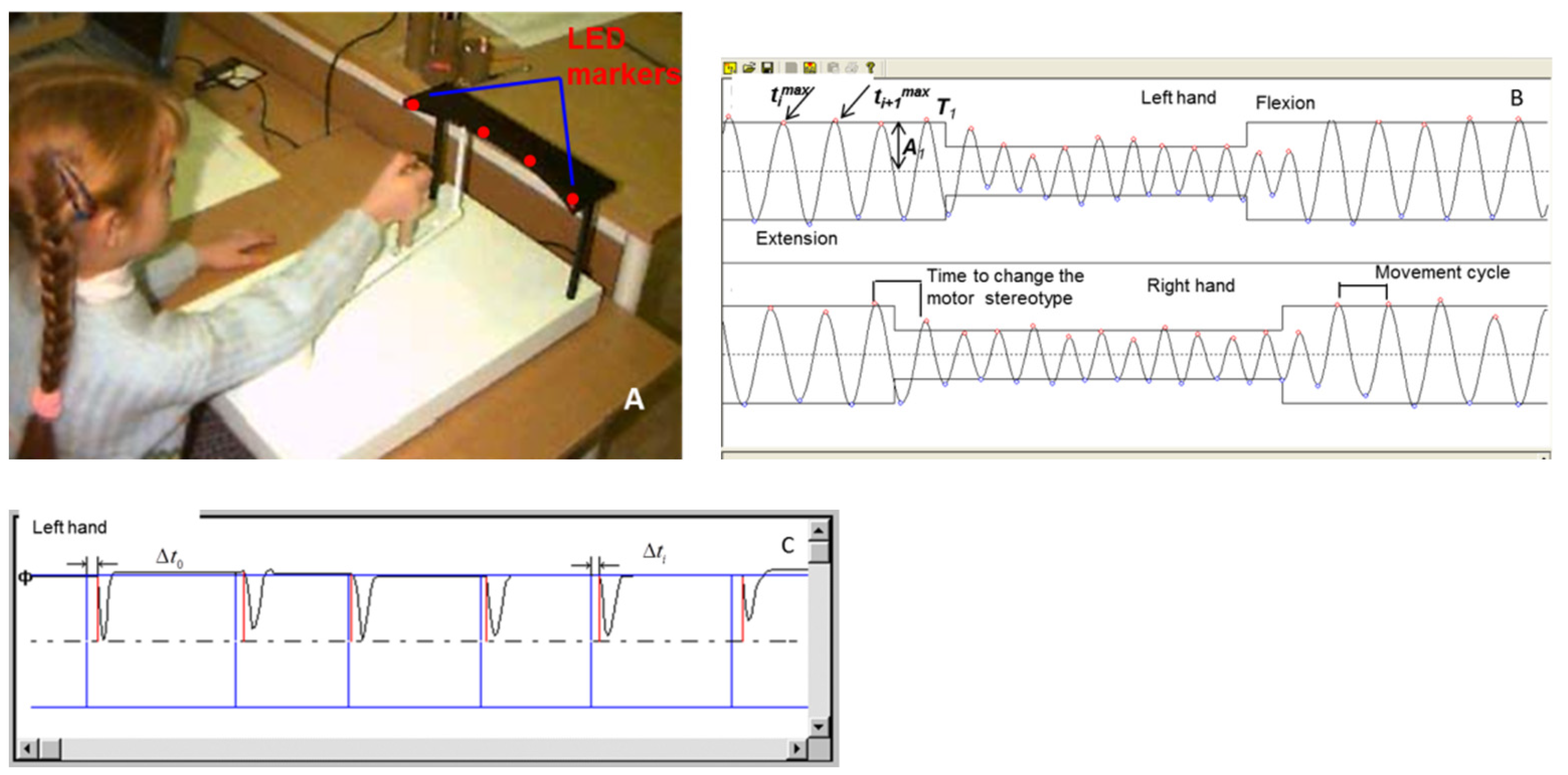

| Group | Control (Typical Development) | Psychiatric Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without Organic Pathology of the Brain | With Organic Pathology of the Brain | |||||||||||
| Sex | Female | Male | Female | Male | Female | Male | ||||||
| Age | Early | Late | Ealy | Late | Early | Late | Early | Late | Early | Late | Early | Late |
| n | 8 | 24 | 14 | 24 | 3 | 8 | 4 | 12 | 3 | 4 | 7 | 22 |
| ICD-10 codes/n | F43.2/1 F84.0/1 F91.9/1 | F20.0/1 F21/1 F32.0/2 F40.8/1 F42.8/1 F98.5/2 | F84.0/3 F20.0/1 | F21/1 F29/2 F30.1/1 F31.0/1 F43.2/1 F84.1/1 F84.9/1 F89/1 F91.9/1 F92.8/1 F98.5/1 | (F06.7/1; F07.8/1 F07.9/1) | F06.6/1 F06.8/2 F07.8/1 | F06.6/3 F06.7/1 F07.0/2 F07.8/1 | F06.6/14 F06.7/2 F06.8/3 F07.0/3 | ||||
| Parameters of Movement | Control | Psychiatric Patients |
|---|---|---|
| Movement cycle duration | −0.54 * | −0.11 |
| Time to change the motor stereotype | −0.19 | 0.01 |
| Time of the reaction to visual stimulus | 0.05 | 0.07 |
| Time of the reaction to acoustic stimulus | −0.27 * | −0.04 |
| Error of correction of flexors | 0.27 * | 0.02 |
| Error of correction of extensors | 0.43 * | 0.07 |
| Smoothness of movement | −0.18 | −0.08 |
| Motor asymmetry | −0.26 * | −0.31 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrenkova, P.; Pankova, N.; Lebedeva, M.; Karganov, M. Features of Psychomotor Coordination in Adolescents with Neuropsychiatric Pathology Enrolled in a Standard Educational Program. Brain Sci. 2022, 12, 245. https://doi.org/10.3390/brainsci12020245
Mavrenkova P, Pankova N, Lebedeva M, Karganov M. Features of Psychomotor Coordination in Adolescents with Neuropsychiatric Pathology Enrolled in a Standard Educational Program. Brain Sciences. 2022; 12(2):245. https://doi.org/10.3390/brainsci12020245
Chicago/Turabian StyleMavrenkova, Polina, Natalia Pankova, Marina Lebedeva, and Mikhail Karganov. 2022. "Features of Psychomotor Coordination in Adolescents with Neuropsychiatric Pathology Enrolled in a Standard Educational Program" Brain Sciences 12, no. 2: 245. https://doi.org/10.3390/brainsci12020245






