Zonisamide Ameliorates Microglial Mitochondriopathy in Parkinson’s Disease Models
Abstract
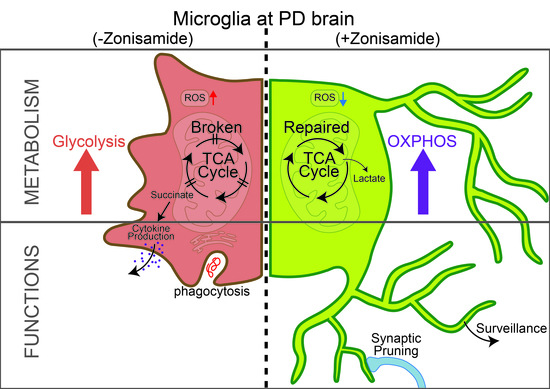
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cells
2.3. Flow Cytometry
2.4. Mitochondrial Bioenergetic Assay
2.5. Quantitative Real-Time RT-PCR (qPCR)
2.6. Statistical Analysis
3. Results
3.1. ZNS Inhibited mitROS Generation in the Microglia of In Vivo PD Models
3.2. ZNS Abolished mitROS Generation and Phagocytic Activity in LPS-Treated BV2 Cells
3.3. ZNS Ameliorated Mitochondrial Dysfunction of LPS-Treated BV2 Cells but Not MPP+-Treated Neuro 2A Cells
3.4. ZNS Reversed LPS Gene Expression in Treated BV2 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Barcia, C.; Ros, C.M.; Annese, V.; Gomez, A.; Ros-Bernal, F.; Aguado-Llera, D.; Martinez-Pagan, M.E.; de Pablos, V.; Fernandez-Villalba, E.; Herrero, M.T. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2012, 3, e379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcia, C.; Ros, C.M.; Ros-Bernal, F.; Gomez, A.; Annese, V.; Carrillo-de Sauvage, M.A.; Yuste, J.E.; Campuzano, C.M.; de Pablos, V.; Fernandez-Villalba, E.; et al. Persistent phagocytic characteristics of microglia in the substantia nigra of long-term Parkinsonian macaques. J. Neuroimmunol. 2013, 261, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Oliva-Martin, M.J.; Brown, G.C. Primary phagocytosis of viable neurons by microglia activated with LPS or Abeta is dependent on calreticulin/LRP phagocytic signalling. J. Neuroinflamm. 2012, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Aono, H.; Choudhury, M.E.; Higaki, H.; Miyanishi, K.; Kigami, Y.; Fujita, K.; Akiyama, J.I.; Takahashi, H.; Yano, H.; Kubo, M.; et al. Microglia may compensate for dopaminergic neuron loss in experimental Parkinsonism through selective elimination of glutamatergic synapses from the subthalamic nucleus. Glia 2017, 65, 1833–1847. [Google Scholar] [CrossRef]
- Croisier, E.; Moran, L.B.; Dexter, D.T.; Pearce, R.K.; Graeber, M.B. Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J. Neuroinflamm. 2005, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Neal, M.L.; Fleming, S.M.; Budge, K.M.; Boyle, A.M.; Kim, C.; Alam, G.; Beier, E.E.; Wu, L.J.; Richardson, J.R. Pharmacological inhibition of CSF1R by GW2580 reduces microglial proliferation and is protective against neuroinflammation and dopaminergic neurodegeneration. FASEB J. 2020, 34, 1679–1694. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Johnson, A.; Kim, J.H.; Lukowicz, A.; Svedberg, D.; Cvetanovic, M. Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice. J. Neuroinflamm. 2017, 14, 107. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Wood, K.; Li, M.; Qiu, S.; Shi, F.D.; Ma, C.; Liu, Q. Depletion of microglia augments the dopaminergic neurotoxicity of MPTP. FASEB J. 2018, 32, 3336–3345. [Google Scholar] [CrossRef] [Green Version]
- Hung, A.Y.; Schwarzschild, M.A. Approaches to Disease Modification for Parkinson’s Disease: Clinical Trials and Lessons Learned. Neurotherapeutics 2020, 17, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Hasegawa, K.; Kanazawa, I.; Fukasaka, J.; Kochi, K.; Shimazu, R.; The Japan Zonisamide on PD Study Group. Zonisamide improves wearing-off in Parkinson’s disease: A randomized, double-blind study. Mov. Disord. 2015, 30, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Fujita, H.; Matsubara, T.; Haruyama, Y.; Kadowaki, T.; Funakoshi, K.; Watanabe, Y.; Hirata, K. Zonisamide effects on sleep problems and depressive symptoms in Parkinson’s disease. Brain Behav. 2021, 11, e02026. [Google Scholar] [CrossRef] [PubMed]
- Yabe, H.; Choudhury, M.E.; Kubo, M.; Nishikawa, N.; Nagai, M.; Nomoto, M. Zonisamide increases dopamine turnover in the striatum of mice and common marmosets treated with MPTP. J. Pharmacol. Sci. 2009, 110, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, M.E.; Moritoyo, T.; Yabe, H.; Nishikawa, N.; Nagai, M.; Kubo, M.; Matsuda, S.; Nomoto, M. Zonisamide attenuates MPTP neurotoxicity in marmosets. J. Pharmacol. Sci. 2010, 114, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, M.E.; Moritoyo, T.; Kubo, M.; Kyaw, W.T.; Yabe, H.; Nishikawa, N.; Nagai, M.; Matsuda, S.; Nomoto, M. Zonisamide-induced long-lasting recovery of dopaminergic neurons from MPTP-toxicity. Brain Res. 2011, 1384, 170–178. [Google Scholar] [CrossRef]
- Choudhury, M.E.; Sugimoto, K.; Kubo, M.; Iwaki, H.; Tsujii, T.; Kyaw, W.T.; Nishikawa, N.; Nagai, M.; Tanaka, J.; Nomoto, M. Zonisamide up-regulated the mRNAs encoding astrocytic anti-oxidative and neurotrophic factors. Eur. J. Pharmacol. 2012, 689, 72–80. [Google Scholar] [CrossRef]
- Hossain, M.M.; Weig, B.; Reuhl, K.; Gearing, M.; Wu, L.J.; Richardson, J.R. The anti-parkinsonian drug zonisamide reduces neuroinflammation: Role of microglial Nav 1.6. Exp. Neurol. 2018, 308, 111–119. [Google Scholar] [CrossRef]
- Black, J.A.; Liu, S.; Waxman, S.G. Sodium channel activity modulates multiple functions in microglia. Glia 2009, 57, 1072–1081. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sonsalla, P.K.; Richardson, J.R. Coordinated role of voltage-gated sodium channels and the Na+/H+ exchanger in sustaining microglial activation during inflammation. Toxicol. Appl. Pharmacol. 2013, 273, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Liu, J.; Richardson, J.R. Pyrethroid Insecticides Directly Activate Microglia through Interaction with Voltage-Gated Sodium Channels. Toxicol. Sci. 2017, 155, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, H.; Ohkawara, B.; Nakashima, H.; Ota, K.; Kanbara, S.; Inoue, T.; Tomita, H.; Sayo, A.; Kiryu-Seo, S.; Konishi, H.; et al. Zonisamide ameliorates neuropathic pain partly by suppressing microglial activation in the spinal cord in a mouse model. Life Sci. 2020, 263, 118577. [Google Scholar] [CrossRef] [PubMed]
- Higaki, H.; Choudhury, M.E.; Kawamoto, C.; Miyamoto, K.; Islam, A.; Ishii, Y.; Miyanishi, K.; Takeda, H.; Seo, N.; Sugimoto, K.; et al. The hypnotic bromovalerylurea ameliorates 6-hydroxydopamine-induced dopaminergic neuron loss while suppressing expression of interferon regulatory factors by microglia. Neurochem. Int. 2016, 99, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Choudhury, M.E.; Watanabe, M.; Kawasaki, S.; Nishihara, T.; Yano, H.; Matsumoto, S.; Kunieda, T.; Kumon, Y.; Yorozuya, T.; et al. Comparison of the detrimental features of microglia and infiltrated macrophages in traumatic brain injury: A study using a hypnotic bromovalerylurea. Glia 2018, 66, 2158–2173. [Google Scholar] [CrossRef]
- Choudhury, M.E.; Mikami, K.; Nakanishi, Y.; Matsuura, T.; Utsunomiya, R.; Yano, H.; Kubo, M.; Ando, R.; Iwanami, J.; Yamashita, M.; et al. Insomnia and depressive behavior of MyD88-deficient mice: Relationships with altered microglial functions. J. Neuroimmunol. 2022, 363, 577794. [Google Scholar] [CrossRef]
- Ando, R.; Choudhury, M.E.; Yamanishi, Y.; Kyaw, W.T.; Kubo, M.; Kannou, M.; Nishikawa, N.; Tanaka, J.; Nomoto, M.; Nagai, M. Modafinil alleviates levodopa-induced excessive nighttime sleepiness and restores monoaminergic systems in a nocturnal animal model of Parkinson’s disease. J. Pharmacol. Sci. 2018, 136, 266–271. [Google Scholar] [CrossRef]
- Islam, A.; Choudhury, M.E.; Kigami, Y.; Utsunomiya, R.; Matsumoto, S.; Watanabe, H.; Kumon, Y.; Kunieda, T.; Yano, H.; Tanaka, J. Sustained anti-inflammatory effects of TGF-beta1 on microglia/macrophages. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 721–734. [Google Scholar] [CrossRef]
- Costa, T.; Fernandez-Villalba, E.; Izura, V.; Lucas-Ochoa, A.M.; Menezes-Filho, N.J.; Santana, R.C.; de Oliveira, M.D.; Araujo, F.M.; Estrada, C.; Silva, V.; et al. Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. J. Neuroimmune Pharmacol. 2021, 16, 390–402. [Google Scholar] [CrossRef]
- Grunewald, A.; Kumar, K.R.; Sue, C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef]
- Leston Pinilla, L.; Ugun-Klusek, A.; Rutella, S.; De Girolamo, L.A. Hypoxia Signaling in Parkinson’s Disease: There Is Use in Asking “What HIF?”. Biology 2021, 10, 723. [Google Scholar] [CrossRef]
- Franco-Iborra, S.; Cuadros, T.; Parent, A.; Romero-Gimenez, J.; Vila, M.; Perier, C. Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death Dis. 2018, 9, 1122. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Cai, S.; Li, Y.; Li, G.; Cao, Y.; Ai, C.; Gao, Y.; Li, T. Drp-1 as Potential Therapeutic Target for Lipopolysaccharide-Induced Vascular Hyperpermeability. Oxid. Med. Cell Longev. 2020, 2020, 5820245. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, L.; Liu, Y.; Yang, Z.; Chi, S.; Xie, A. Zonisamide for the Treatment of Parkinson Disease: A Current Update. Front. Neurosci. 2020, 14, 574652. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, Y.; Wang, T.; Wei, S.J.; Block, M.L.; Wilson, B.; Liu, B.; Hong, J.S. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004, 279, 1415–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Hao, W.; Dawson, A.; Liu, S.; Fassbender, K. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J. Biol. Chem. 2009, 284, 3691–3699. [Google Scholar] [CrossRef] [Green Version]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Crews, F.T. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J. Neuroinflamm. 2012, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.C.; Teismann, P.; Tieu, K.; Vila, M.; Jackson-Lewis, V.; Ischiropoulos, H.; Przedborski, S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 6145–6150. [Google Scholar] [CrossRef] [Green Version]
- Franco-Iborra, S.; Vila, M.; Perier, C. The Parkinson Disease Mitochondrial Hypothesis: Where Are We at? Neuroscientist 2016, 22, 266–277. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tozzi, A.; Luchetti, E.; Siliquini, S.; Belcastro, V.; Tantucci, M.; Picconi, B.; Ientile, R.; Calabresi, P.; Pisani, F. Electrophysiological actions of zonisamide on striatal neurons: Selective neuroprotection against complex I mitochondrial dysfunction. Exp. Neurol. 2010, 221, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, S.; Choudhury, M.E.; Kubo, M.; Ando, R.; Tanaka, J.; Nagai, M. Zonisamide Ameliorates Microglial Mitochondriopathy in Parkinson’s Disease Models. Brain Sci. 2022, 12, 268. https://doi.org/10.3390/brainsci12020268
Tada S, Choudhury ME, Kubo M, Ando R, Tanaka J, Nagai M. Zonisamide Ameliorates Microglial Mitochondriopathy in Parkinson’s Disease Models. Brain Sciences. 2022; 12(2):268. https://doi.org/10.3390/brainsci12020268
Chicago/Turabian StyleTada, Satoshi, Mohammed E. Choudhury, Madoka Kubo, Rina Ando, Junya Tanaka, and Masahiro Nagai. 2022. "Zonisamide Ameliorates Microglial Mitochondriopathy in Parkinson’s Disease Models" Brain Sciences 12, no. 2: 268. https://doi.org/10.3390/brainsci12020268
APA StyleTada, S., Choudhury, M. E., Kubo, M., Ando, R., Tanaka, J., & Nagai, M. (2022). Zonisamide Ameliorates Microglial Mitochondriopathy in Parkinson’s Disease Models. Brain Sciences, 12(2), 268. https://doi.org/10.3390/brainsci12020268