Disrupted Resting State Attentional Network Connectivity in Adolescent and Young Adult Cannabis Users following Two-Weeks of Monitored Abstinence
Abstract
:1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.3.1. Detailed Phone Screen
2.3.2. Study Session
2.4. MRI Scan Acquisition and Pre-Processing
2.5. Data Analysis
3. Results
3.1. Demographic and Substance Use Characteristics
3.2. RSFC Differences between Cannabis Users and Controls
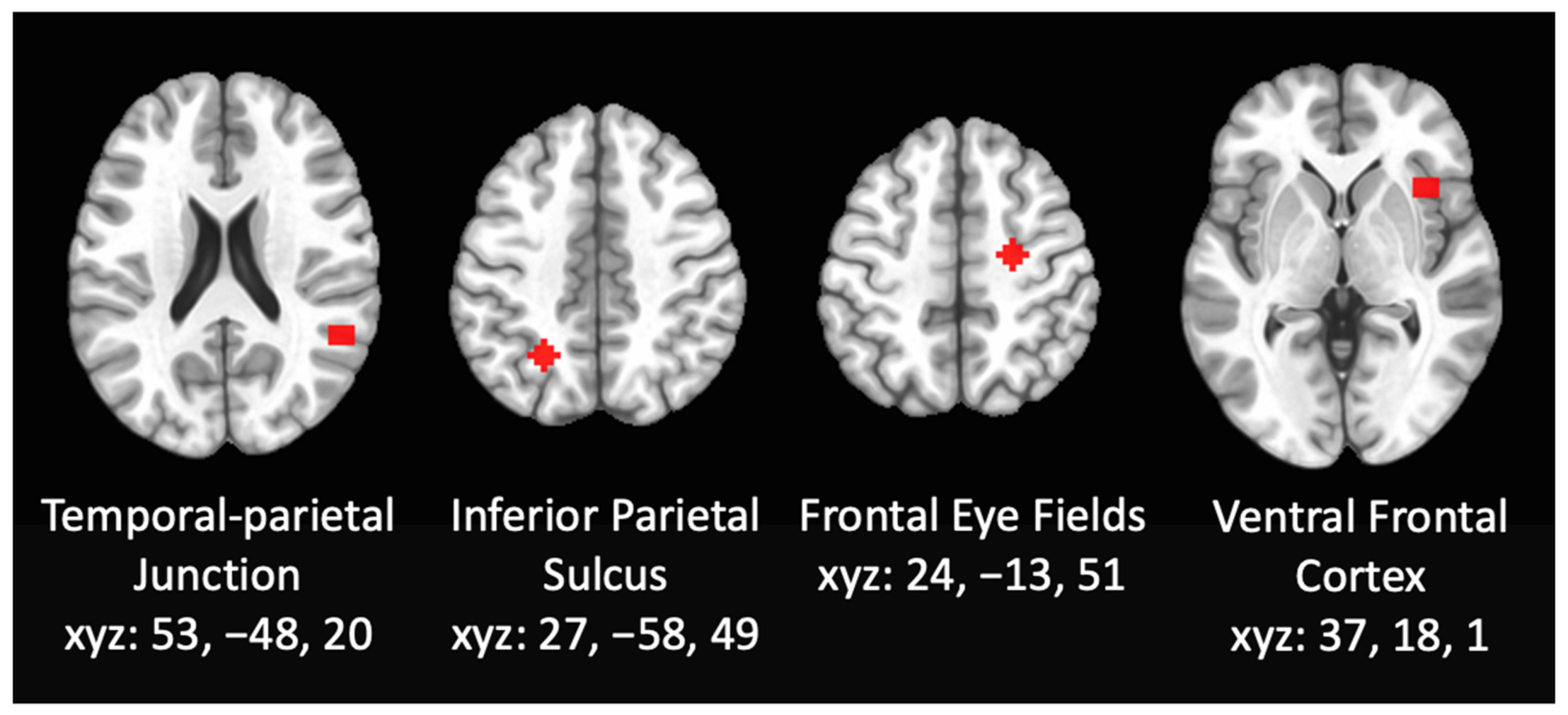
3.2.1. RSFC Differences within DAN
3.2.2. RSFC Differences within the VAN
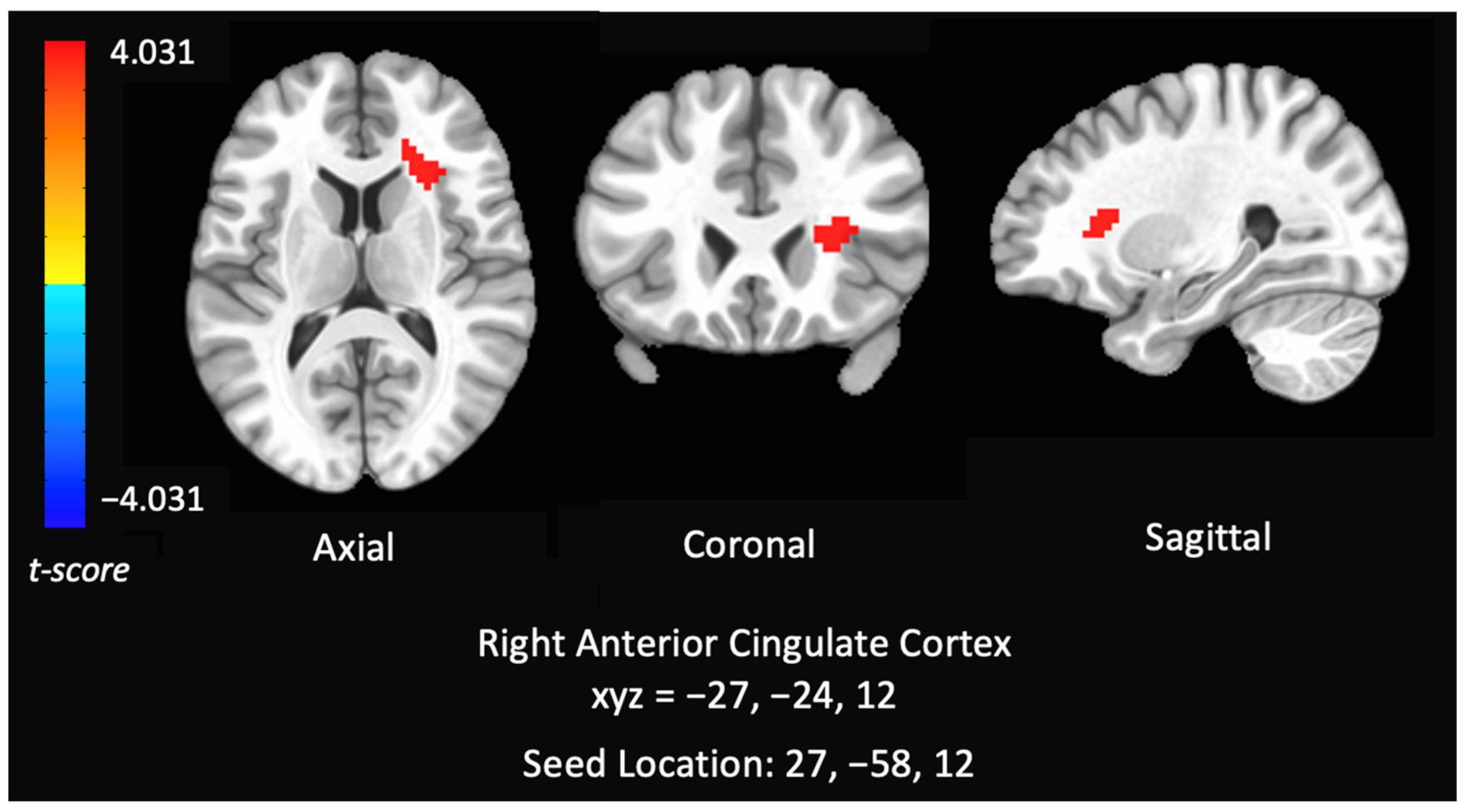
3.3.3. Correlations between RSFC Networks (DAN) and Cannabis Use Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, B.H.; Funk-White, M.; Ko, R.; Al-Rousan, T.; Palamar, J.J. Decreasing perceived risk associated with regular cannabis use among older adults in the United States from 2015 to 2019. J. Am. Geriatr. Soc. 2021, 69, 2591–2597. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration (SAMHSA). National Survey on Drug Use and Health. 2020. Available online: https://www.samhsa.gov/data/ (accessed on 14 August 2020).
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Harkany, T.; Guzmán, M.; Galve-Roperh, I.; Berghuis, P.; Devi, L.A.; Mackie, K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007, 28, 83–92. [Google Scholar] [CrossRef]
- Heng, L.; Beverley, J.A.; Steiner, H.; Tseng, K.Y. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse 2011, 65, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellgren, M.; Artmann, A.; Tkalych, O.; Gupta, A.; Hansen, H.S.; Hansen, S.H.; Devi, L.A.; Hurd, Y.L. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur. Neuropsychopharmacol. 2008, 18, 826–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology 2018, 43, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, S.; Scorticati, C.; García-Arencibia, M.; de Miguel, R.; Ramos, J.A.; Fernández-Ruiz, J. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson’s disease. Brain Res. 2006, 1073–1074, 209–219. [Google Scholar] [CrossRef]
- Belue, R.C.; Howlett, A.C.; Westlake, T.M.; Hutchings, D.E. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology Teratol. 1995, 17, 25–30. [Google Scholar] [CrossRef]
- Glass, M.; Dragunow, M.; Faull, R.L. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997, 77, 299–318. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Behrmann, M.; Geng, J.J.; Shomstein, S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004, 14, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.K.; Mencl, W.E.; Westerveld, M.; Pugh, K.R. Impact of cannabis use on brain function in adolescents. Ann. N. Y. Acad. Sci. 2004, 1021, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Wade, N.E.; Bagot, K.S.; Tapert, S.F.; Gruber, S.A.; Filbey, F.M.; Lisdahl, K.M. Cognitive Functioning Related to Binge Alcohol and Cannabis Co-Use in Abstinent Adolescents and Young Adults. J. Stud. Alcohol Drugs 2020, 81, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.L.; Wade, N.E.; Lisdahl, K.M. Impact of 2 Weeks of Monitored Abstinence on Cognition in Adolescent and Young Adult Cannabis Users. J. Int. Neuropsychol. Soc. JINS 2020, 26, 776–784. [Google Scholar] [CrossRef]
- Abdullaev, Y.; Posner, M.I.; Nunnally, R.; Dishion, T.J. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav. Brain Res. 2010, 215, 45–57. [Google Scholar] [CrossRef]
- Pardini, D.; White, H.R.; Xiong, S.; Bechtold, J.; Chung, T.; Loeber, R.; Hipwell, A. Unfazed or Dazed and Confused: Does Early Adolescent Marijuana Use Cause Sustained Impairments in Attention and Academic Functioning? J. Abnorm. Child Psychol. 2015, 43, 1203–1217. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, D.M.; Mathias, C.W.; Dawes, M.A.; Furr, R.M.; Charles, N.E.; Liguori, A.; Shannon, E.E.; Acheson, A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology 2013, 226, 307–319. [Google Scholar] [CrossRef]
- Price, J.S.; McQueeny, T.; Shollenbarger, S.; Browning, E.L.; Wieser, J.; Lisdahl, K.M. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology 2015, 232, 2939–2950. [Google Scholar] [CrossRef] [Green Version]
- Ritchay, M.M.; Huggins, A.A.; Wallace, A.L.; Larson, C.L.; Lisdahl, K.M. Resting state functional connectivity in the default mode network: Relationships between cannabis use, gender, and cognition in adolescents and young adults. NeuroImage Clin. 2021, 30, 102664. [Google Scholar] [CrossRef]
- Lisdahl, K.M.; Wright, N.E.; Kirchner-Medina, C.; Maple, K.E.; Shollenbarger, S. Considering Cannabis: The Effects of Regular Cannabis Use on Neurocognition in Adolescents and Young Adults. Curr. Addict. Rep. 2014, 1, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, N.E.; Wallace, A.L.; Swartz, A.M.; Lisdahl, K.M. Aerobic Fitness Level Moderates the Association Between Cannabis Use and Executive Functioning and Psychomotor Speed Following Abstinence in Adolescents and Young Adults. J. Int. Neuropsychol. Soc. 2019, 25, 134–145. [Google Scholar] [CrossRef]
- Jager, G.; Ramsey, N.F. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Curr. Drug Abuse Rev. 2008, 1, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.; Vittinghoff, E.; Yaffe, K.; Künzi, A.; Kertesz, S.G.; Levine, D.A.; Albanese, E.; Whitmer, R.A.; Jacobs, D.R.; Sidney, S.; et al. Association Between Lifetime Marijuana Use and Cognitive Function in Middle Age: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern. Med. 2016, 176, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, H.G., Jr.; Yurgelun-Todd, D. The residual cognitive effects of heavy marijuana use in college students. JAMA 1996, 275, 521–527. [Google Scholar] [CrossRef]
- Harvey, M.A.; Sellman, J.D.; Porter, R.J.; Frampton, C.M. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007, 26, 309–319. [Google Scholar] [CrossRef]
- Medina, K.L.; Hanson, K.L.; Schweinsburg, A.D.; Cohen-Zion, M.; Nagel, B.J.; Tapert, S.F. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. 2007, 13, 807–820. [Google Scholar] [CrossRef] [Green Version]
- Tapert, S.F.; Baratta, M.V.; Abrantes, A.M.; Brown, S.A. Attention Dysfunction Predicts Substance Involvement in Community Youths. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 680–686. [Google Scholar] [CrossRef]
- Solowij, N.; Grenyer, B.F. Are the adverse consequences of cannabis use age-dependent? Addiction 2002, 97, 1083–1086. [Google Scholar] [CrossRef]
- Pope, H.G.; Gruber, A.J.; Hudson, J.I.; Cohane, G.; Huestis, M.A.; Yurgelun-Todd, D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug Alcohol Depend. 2003, 69, 303–310. [Google Scholar] [CrossRef]
- Lisdahl, K.M.; Price, J.S. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J. Int. Neuropsychol. Soc. 2012, 18, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, K.L.; Winward, J.L.; Schweinsburg, A.D.; Medina, K.L.; Brown, S.A.; Tapert, S.F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010, 35, 970–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, S.A.; Dahlgren, M.K.; Sagar, K.A.; Gönenc, A.; Killgore, W.D. Age of onset of marijuana use impacts inhibitory processing. Neurosci. Lett. 2012, 511, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dafters, R.I.; Hoshi, R.; Talbot, A.C. Contribution of cannabis and MDMA (“ecstasy”) to cognitive changes in long-term polydrug users. Psychopharmacology 2004, 173, 405–410. [Google Scholar] [CrossRef]
- Indlekofer, F.; Piechatzek, M.; Daamen, M.; Glasmacher, C.; Lieb, R.; Pfister, H.; Tucha, O.; Lange, K.W.; Wittchen, H.U.; Schütz, C.G. Reduced memory and attention performance in a population-based sample of young adults with a moderate lifetime use of cannabis, ecstasy and alcohol. J. Psychopharmacol. 2009, 23, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.C.; Slomiak, S.T.; Jones, J.D.; Rosen, A.F.G.; Moore, T.M.; Gur, R.C. Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 585–595. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Cortes-Briones, J.A.; Ranganathan, M.; Thurnauer, H.; Creatura, G.; Surti, T.; Planeta, B.; Neumeister, A.; Pittman, B.; Normandin, M.D.; et al. Rapid Changes in CB1 Receptor Availability in Cannabis Dependent Males after Abstinence from Cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 60–67. [Google Scholar]
- Gonzalez, R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol. Rev. 2007, 17, 347–361. [Google Scholar] [CrossRef]
- Schweinsburg, A.D.; Schweinsburg, B.C.; Medina, K.L.; McQueeny, T.; Brown, S.A.; Tapert, S.F. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J. Psychoact. Drugs 2010, 42, 401–412. [Google Scholar] [CrossRef]
- Wallace, A.L.; Wade, N.E.; Hatcher, K.F.; Lisdahl, K.M. Effects of Cannabis Use and Subclinical ADHD Symptomology on Attention Based Tasks in Adolescents and Young Adults. Arch. Clin. Neuropsychol. 2018, 34, 700–705. [Google Scholar] [CrossRef]
- Allsop, D.J.; Norberg, M.M.; Copeland, J.; Fu, S.; Budney, A.J. The Cannabis Withdrawal Scale development: Patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011, 119, 123–129. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Wallace, A.L.; Wade, N.E.; Swartz, A.M.; Lisdahl, K.M. Cannabis Use and Brain Volume in Adolescent and Young Adult Cannabis Users: Effects Moderated by Sex and Aerobic Fitness. J. Int. Neuropsychol. Soc. JINS 2021, 27, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Patel, G.; Shulman, G.L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbetta, M.; Kincade, J.M.; Ollinger, J.M.; McAvoy, M.P.; Shulman, G.L. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 2000, 3, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.L.; d'Avossa, G.; Tansy, A.P.; Corbetta, M. Two attentional processes in the parietal lobe. Cereb. Cortex 2002, 12, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Taki, Y.; Sato, K.; Hashizume, H.; Sassa, Y.; Takeuchi, H.; Thyreau, B.; He, Y.; Evans, A.C.; Li, X.; et al. Topological organization of functional brain networks in healthy children: Differences in relation to age, sex, and intelligence. PLoS ONE 2013, 8, e55347. [Google Scholar] [CrossRef] [Green Version]
- Casey, B.J.; Giedd, J.N.; Thomas, K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000, 54, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Huttenlocher, P.R. Morphometric study of human cerebral cortex development. Neuropsychologia 1990, 28, 517–527. [Google Scholar] [CrossRef]
- Jolles, D.D.; van Buchem, M.A.; Crone, E.A.; Rombouts, S.A. Functional brain connectivity at rest changes after working memory training. Hum. Brain Mapp. 2013, 34, 396–406. [Google Scholar] [CrossRef]
- Farrant, K.; Uddin, L.Q. Asymmetric development of dorsal and ventral attention networks in the human brain. Dev. Cogn. Neurosci. 2015, 12, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dijk, K.R.; Hedden, T.; Venkataraman, A.; Evans, K.C.; Lazar, S.W.; Buckner, R.L. Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization. J. Neurophysiol. 2009, 103, 297–321. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, J.; Wang, L.; Chen, Z.J.; Yan, C.; Yang, H.; Tang, H.; Zhu, C.; Gong, Q.; Zang, Y.; et al. Uncovering Intrinsic Modular Organization of Spontaneous Brain Activity in Humans. PLoS ONE 2009, 4, e5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. From The Cover: The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Corbetta, M.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. USA 2006, 103, 10046–10051. [Google Scholar] [CrossRef] [Green Version]
- He, B.J.; Snyder, A.Z.; Vincent, J.L.; Epstein, A.; Shulman, G.L.; Corbetta, M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007, 53, 905–918. [Google Scholar] [CrossRef] [Green Version]
- Visintin, E.; De Panfilis, C.; Antonucci, C.; Capecci, C.; Marchesi, C.; Sambataro, F. Parsing the intrinsic networks underlying attention: A resting state study. Behav. Brain Res. 2015, 278, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yin, X.; Ge, H.; Han, Y.; Pang, Z.; Tang, Y.; Liu, B.; Liu, S. Attentional performance is correlated with the local regional efficiency of intrinsic brain networks. Frontiers in behavioral neuroscience 2015, 9, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitko, A.; Rothlein, D.; Poole, V.; Robinson, M.; McGlinchey, R.; DeGutis, J.; Salat, D.; Esterman, M. Individual differences in sustained attention are associated with cortical thickness. Hum. Brain Mapp. 2019, 40, 3243–3253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterman, M.; Noonan, S.K.; Rosenberg, M.; Degutis, J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb. Cortex 2013, 23, 2712–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortenbaugh, F.C.; DeGutis, J.; Germine, L.; Wilmer, J.B.; Grosso, M.; Russo, K.; Esterman, M. Sustained Attention Across the Life Span in a Sample of 10,000: Dissociating Ability and Strategy. Psychol. Sci. 2015, 26, 1497–1510. [Google Scholar] [CrossRef] [Green Version]
- Esterman, M.; Liu, G.; Okabe, H.; Reagan, A.; Thai, M.; DeGutis, J. Frontal eye field involvement in sustaining visual attention: Evidence from transcranial magnetic stimulation. Neuroimage 2015, 111, 542–548. [Google Scholar] [CrossRef]
- Rohr, C.S.; Arora, A.; Cho, I.; Katlariwala, P.; Dimond, D.; Dewey, D.; Bray, S. Functional network integration and attention skills in young children. Dev. Cogn. Neurosci. 2018, 30, 200–211. [Google Scholar] [CrossRef]
- Kelly, A.M.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008, 39, 527–537. [Google Scholar] [CrossRef]
- Basseer Sami MMcCutcheon, R.A.; Ettinger, U.; Williams, S.; Lythgoe, D.; McGuire, P.; Bhattacharyya, S. Cannabis Use Linked to Altered Functional Connectivity of the Visual Attentional Connectivity in Patients With Psychosis and Controls. Schizophr. Bull. Open 2020, 1, sgaa018. [Google Scholar]
- Peeters, S.C.; van Bronswijk, S.; van de Ven, V.; Gronenschild, E.H.; Goebel, R.; van Os, J.; Marcelis, M.; Genetic Risk and Outcome of Psychosis (G.R.O.U.P.). Cognitive correlates of frontoparietal network connectivity ‘at rest’ in individuals with differential risk for psychotic disorder. Eur. Neuropsychopharmacol. 2015, 25, 1922–1932. [Google Scholar] [CrossRef]
- Orr, C.; Spechler, P.; Cao, Z.; Albaugh, M.; Chaarani, B.; Mackey, S.; D’Souza, D.; Allgaier, N.; Banaschewski, T.; Bokde, A.; et al. Grey Matter Volume Differences Associated with Extremely Low Levels of Cannabis Use in Adolescence. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 1817–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, D.G.; Brown, S.A. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction 1995, 90, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Myers, M.G.; Lippke, L.; Tapert, S.F.; Stewart, D.G.; Vik, P.W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J. Stud. Alcohol 1998, 59, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33, quiz 34–57. [Google Scholar]
- Medina, K.L.; Shear, P.K.; Corcoran, K. Ecstasy (MDMA) exposure and neuropsychological functioning: A polydrug perspective. J. Int. Neuropsychol. Soc. 2005, 11, 753–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fals-Stewart, W.; O'Farrell, T.J.; Freitas, T.T.; McFarlin, S.K.; Rutigliano, P. The Timeline Followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. J. Consult. Clin. Psychol. 2000, 68, 134–144. [Google Scholar] [CrossRef]
- Sobell, L.C.; Maisto, S.A.; Sobell, M.B.; Cooper, A.M. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav. Res. Ther. 1979, 17, 157–160. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. Int. J. 1996, 29, 162. [Google Scholar] [CrossRef]
- Biswal, B.B.; Mennes, M.; Zuo, X.N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef] [Green Version]
- Gusnard, D.A.; Raichle, M.E.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- AFNI Program: 3dClustSim. 2019. Available online: https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html (accessed on 16 February 2022).
- Cox, R.W.; Chen, G.; Glen, D.R.; Reynolds, R.C.; Taylor, P.A. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017, 7, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Slotnick, S.D. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn. Neurosci. 2017, 8, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.C.; Blankenburg, F.; Bjoertomt, O.; Bestmann, S.; Freeman, E.; Haynes, J.D.; Rees, G.; Josephs, O.; Deichmann, R.; Driver, J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr. Biol. 2006, 16, 1479–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressler, S.L.; Tang, W.; Sylvester, C.M.; Shulman, G.L.; Corbetta, M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J. Neurosci. 2008, 28, 10056–10061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droutman, V.; Bechara, A.; Read, S.J. Roles of the Different Sub-Regions of the Insular Cortex in Various Phases of the Decision-Making Process. Front. Behav. Neurosci. 2015, 9, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Larson, M.P.; Bogorodzki, P.; Rogowska, J.; McGlade, E.; King, J.B.; Terry, J.; Yurgelun-Todd, D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav. Brain Res. 2011, 220, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, C.; Rubin-Kahana, D.S.; Pushparaj, A.; Musiol, M.; Blumberger, D.M.; Daskalakis, Z.J.; Zangen, A.; Le Foll, B. The Insula: A Brain Stimulation Target for the Treatment of Addiction. Front. Pharmacol. 2019, 10, 720. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Lalumiere, R.T.; Knackstedt, L.; Shen, H. Glutamate transmission in addiction. Neuropharmacology 2009, 56, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Shollenbarger, S.; Thomas, A.M.; Wade, N.E.; Gruber, S.A.; Tapert, S.F.; Filbey, F.M.; Lisdahl, K.M. Intrinsic Frontolimbic Connectivity and Mood Symptoms in Young Adult Cannabis Users. Front. Public Health 2019, 7, 311. [Google Scholar] [CrossRef] [Green Version]
- Maple, K.E.; Thomas, A.M.; Kangiser, M.M.; Lisdahl, K.M. Anterior cingulate volume reductions in abstinent adolescent and young adult cannabis users: Association with affective processing deficits. Psychiatry Res. Neuroimaging 2019, 288, 51–59. [Google Scholar] [CrossRef]
- Meier, M.H.; Caspi, A.; Ambler, A.; Harrington, H.; Houts, R.; Keefe, R.S.; McDonald, K.; Ward, A.; Poulton, R.; Moffitt, T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA 2012, 109, E2657–E2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, J.T.; Sweitzer, M.M.; Tunno, A.M.; Kollins, S.H.; McClernon, F.J. “I Use Weed for My ADHD”: A Qualitative Analysis of Online Forum Discussions on Cannabis Use and ADHD. PLoS ONE 2016, 11, e0156614. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.; Epstein, J.N.; Peugh, J.L.; Nakonezny, P.A.; Hughes, C.W. Preliminary data suggesting the efficacy of attention training for school-aged children with ADHD. Dev. Cogn. Neurosci. 2013, 4, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Fried, P.A.; Watkinson, B.; Gray, R. Neurocognitive consequences of marihuana—A comparison with pre-drug performance. Neurotoxicol. Teratol. 2005, 27, 231–239. [Google Scholar] [CrossRef]
- Hirvonen, J.; Goodwin, R.S.; Li, C.T.; Terry, G.E.; Zoghbi, S.S.; Morse, C.; Pike, V.W.; Volkow, N.D.; Huestis, M.A.; Innis, R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 2012, 17, 642–649. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Prescot, A.P.; Renshaw, P.F.; Yurgelun-Todd, D.A. γ-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend. 2013, 129, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Lisdahl, K.M.; Sher, K.J.; Conway, K.P.; Gonzalez, R.; Feldstein Ewing, S.W.; Nixon, S.J.; Tapert, S.; Bartsch, H.; Goldstein, R.Z.; Heitzeg, M. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev. Cogn. Neurosci. 2018, 32, 80–96. [Google Scholar] [CrossRef]

| Controls | Cannabis Users | |
|---|---|---|
| (n = 39) | (n = 36) | |
| Age M (SD) | 20.95 (2.67) | 21.25 (2.16) |
| Education M (SD) | 14.31 (2.32) | 13.75 (1.48) |
| Gender (% Female) | ||
| Male n (%) | 17 (43.6) | 23 (63.9) |
| Female n (%) | 22 (56.4) | 13 (36.1) |
| Race (%) | ||
| American Indian/Alaska Native | 0 (0.0) | 1 (2.8) |
| Asian | 6 (15.3) | 3 (8.4) |
| Native Hawaiian/Other Pacific Islander | 1 (2.6) | 0 (0) |
| Black or AA | 2 (5.1) | 4 (11.1) |
| White, Caucasian, not of Hispanic Origin | 28 (71.8) | 22 (61.1) |
| More than on race | 1 (2.6) | 5 (13.8) |
| Unknown | 1 (2.6) | 1 (2.8) |
| Ethnicity % | ||
| Hispanic/Latino | 4 (10.2) | 7 (19.4) |
| Not Hispanic | 34 (87.2) | 29 (80.6) |
| Unknown | 1 (2.6) | 0 (0.0) |
| Past Year Cannabis Use (joints) M (SD) | 0.40 (1.16) | 421.69 (443.50) |
| Length of cannabis abstinence (days) M (SD) | 168.33 (132.57) | 32.06 (23.24) |
| Minimum | 31 | 17 |
| Maximum | 313 | 150 |
| Past Year Alcohol Use (standard drinks) M (SD) | 93.65 (143.59) | 315.36 (294.10) |
| Past Year Cigarette Use (cigarettes) M (SD) | 0.55 (2.00) | 213.59 (484.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, J.C.; Wallace, A.L.; Thomas, A.M.; Wirtz, H.G.; Kaiver, C.M.; Lisdahl, K.M. Disrupted Resting State Attentional Network Connectivity in Adolescent and Young Adult Cannabis Users following Two-Weeks of Monitored Abstinence. Brain Sci. 2022, 12, 287. https://doi.org/10.3390/brainsci12020287
Harris JC, Wallace AL, Thomas AM, Wirtz HG, Kaiver CM, Lisdahl KM. Disrupted Resting State Attentional Network Connectivity in Adolescent and Young Adult Cannabis Users following Two-Weeks of Monitored Abstinence. Brain Sciences. 2022; 12(2):287. https://doi.org/10.3390/brainsci12020287
Chicago/Turabian StyleHarris, Julia C., Alexander L. Wallace, Alicia M. Thomas, Hailey G. Wirtz, Christine M. Kaiver, and Krista M. Lisdahl. 2022. "Disrupted Resting State Attentional Network Connectivity in Adolescent and Young Adult Cannabis Users following Two-Weeks of Monitored Abstinence" Brain Sciences 12, no. 2: 287. https://doi.org/10.3390/brainsci12020287
APA StyleHarris, J. C., Wallace, A. L., Thomas, A. M., Wirtz, H. G., Kaiver, C. M., & Lisdahl, K. M. (2022). Disrupted Resting State Attentional Network Connectivity in Adolescent and Young Adult Cannabis Users following Two-Weeks of Monitored Abstinence. Brain Sciences, 12(2), 287. https://doi.org/10.3390/brainsci12020287






