Changes of Spasticity across Time in Prolonged Disorders of Consciousness: A Retrospective Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Study Design
2.3. Outcome Measures
2.3.1. Spasticity
2.3.2. Level of Consciousness
2.3.3. Medical and Demographic Data
2.4. Statistical Analysis
3. Result
3.1. Participants
3.2. Upper Limb Spasticity and Brain Injury Etiology
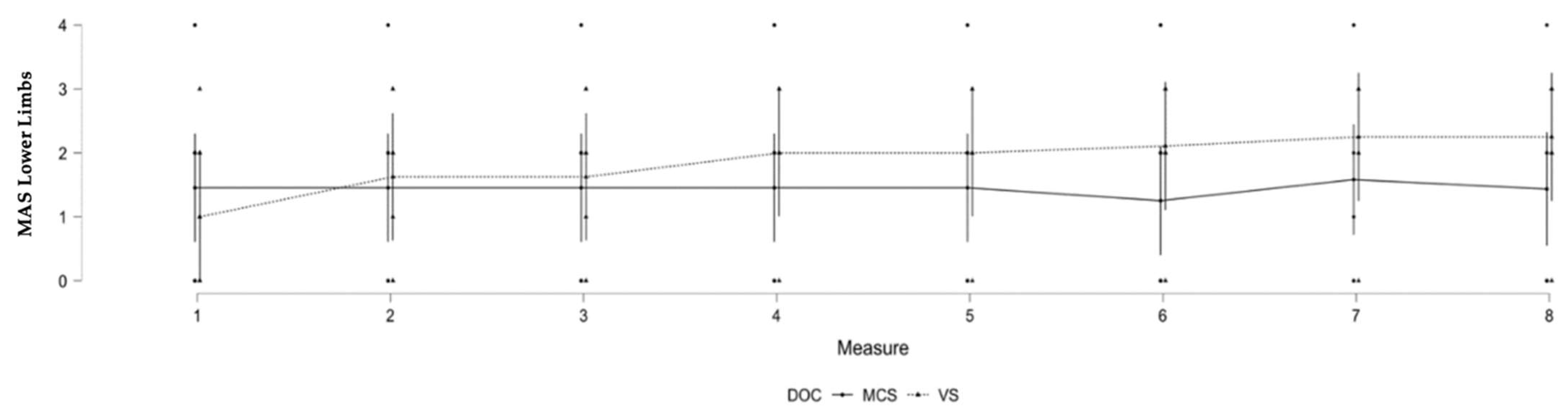
3.3. Lower Limb Spasticity and Level of Consciousness
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lance, J.W. The Control of Muscle Tone, Reflexes, and Movement: Robert Wartenberg Lecture. Neurology 1980, 30, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Martens, G.; Laureys, S.; Thibaut, A. Spasticity Management in Disorders of Consciousness. Brain Sci. 2017, 7, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracies, J.M. Pathophysiology of Spastic Paresis. I: Paresis and Soft Tissue Changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Guernon, A.; Chalcraft, L.; Harton, B.; Smith, B.; Louise-Bender Pape, T. Medical Comorbidities in Disorders of Consciousness Patients and Their Association with Functional Outcomes. Arch. Phys. Med. Rehabil. 2013, 94, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.M.; Vanhaudenhuyse, A.; Coleman, M.R.; Boly, M.; Pickard, J.D.; Tshibanda, L.; Owen, A.M.; Laureys, S. Willful Modulation of Brain Activity in Disorders of Consciousness. N. Engl. J. Med. 2010, 362, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruse, D.; Chennu, S.; Chatelle, C.; Bekinschtein, T.A.; Fernández-Espejo, D.; Pickard, J.D.; Laureys, S.; Owen, A.M. Bedside Detection of Awareness in the Vegetative State: A Cohort Study. Lancet 2011, 378, 2088–2094. [Google Scholar] [CrossRef] [Green Version]
- Thibaut, F.A.; Chatelle, C.; Wannez, S.; Deltombe, T.; Stender, J.; Schnakers, C.; Laureys, S.; Gosseries, O. Spasticity in Disorders of Consciousness: A Behavioral Study. Eur. J. Phys. Rehabil. Med. 2015, 51, 389–397. [Google Scholar] [PubMed]
- Kent, C.N.; Park, C.; Lindsley, C.W. Classics in Chemical Neuroscience: Baclofen. ACS Chem. Neurosci. 2020, 11, 1740–1755. [Google Scholar] [CrossRef] [PubMed]
- Synnot, A.; Chau, M.; Pitt, V.; O’Connor, D.; Gruen, R.L.; Wasiak, J.; Clavisi, O.; Pattuwage, L.; Phillips, K. Interventions for Managing Skeletal Muscle Spasticity Following Traumatic Brain Injury. Cochrane Database Syst. Rev. 2017, 22, CD008929. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Deltombe, T.; Wannez, S.; Gosseries, O.; Ziegler, E.; Dieni, C.; Deroy, M.; Laureys, S. Impact of Soft Splints on Upper Limb Spasticity in Chronic Patients with Disorders of Consciousness: A Randomized, Single-Blind, Controlled Trial. Brain Inj. 2015, 29, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Wannez, S.; Deltombe, T.; Martens, G.; Laureys, S.; Chatelle, C. Physical Therapy in Patients with Disorders of Consciousness: Impact on Spasticity and Muscle Contracture. Neuro Rehabil. 2018, 42, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusick, A.; Lannin, N.A.; Hanssen, R.; Allaous, J. Validating the Western Neuro Sensory Stimulation Profile for Patients with Severe Traumatic Brain Injury Who Are Slow-To-Recover. Aust. Occup. Ther. J. 2014, 61, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.-B.; Zafonte, R.; et al. Assessment Scales for Disorders of Consciousness: Evidence-Based Recommendations for Clinical Practice and Research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Medical Aspects of the Persistent Vegetative State. N. Engl. J. Med. 1994, 330, 1499–1508. [CrossRef] [PubMed]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or APALLIC syndrome. BMC Med. 2010, 1, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, E.; Bell, M. Chapter 3: Diffuse Axonal Injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press; Taylor and Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kesar, T.M.; Belagaje, S.R.; Pergami, P.; Haut, M.W.; Hobbs, G.; Buetefisch, C.M. Effects of Monoaminergic Drugs on Training-Induced Motor Cortex Plasticity in Older Adults. Brain Res. 2017, 1670, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibaut, A.; Piarulli, A.; Martens, G.; Chatelle, C.; Laureys, S. Effect of Multichannel Transcranial Direct Current Stimulation to Reduce Hypertonia in Individuals with Prolonged Disorders of Consciousness: A Randomized Controlled Pilot Study. Ann. Phys. Rehabil. Med. 2019, 62, 418–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, A.; Boltzmann, M.; Schmidt, S.B.; Gutenbrunner, C.; Krauss, J.K.; Stangel, M.; Höglinger, G.U.; Wallesch, C.W.; Rollnik, J.D. Treatment of Upper Limb Spasticity with Inhibitory Repetitive Transcranial Magnetic Stimulation: A Randomized Placebo-Controlled Trial. Neuro Rehabil. 2021, 49, 425–434. [Google Scholar] [CrossRef]

| Gender | Age | TSI | Etio | DOC | WNSSP | Medication | Number Measures |
|---|---|---|---|---|---|---|---|
| M | 34 | 99 | Traumatic | MCS | 46 | 1 | 7 |
| M | 47 | 66 | Traumatic | VS | 0 | 0 | 8 |
| M | 23 | 404 | Non-traumatic (anoxia) | MCS | 15 | 0 | 8 |
| M | 25 | 1006 | Traumatic | VS | 13 | 1 | 8 |
| F | 31 | 389 | Non-traumatic (anoxia) | VS | 11 | 0.875 | 8 |
| M | 48 | 1022 | Non-traumatic (anoxia) | MCS | 14 | 0 | 6 |
| M | 67 | 37 | Traumatic | MCS | 21 | 0 | 8 |
| M | 57 | 93 | Traumatic | VS | 4 | 0.75 | 8 |
| F | 53 | 397 | Non-traumatic (anoxia) | MCS | 47 | 0 | 8 |
| F | 60 | 1118 | Non-traumatic (anoxia) | MCS | 21 | 0 | 5 |
| M | 56 | 286 | Traumatic | VS | 2 | 0 | 8 |
| M | 48 | 36 | Non-traumatic (anoxia) | MCS | 27 | 0 | 8 |
| M | 64 | 164 | Traumatic | VS | 4 | 0.8 | 5 |
| M | 47 | 87 | Traumatic | MCS | 21 | 0.857 | 7 |
| M | 65 | 91 | Non-traumatic (anoxia) | VS | 6 | 0.875 | 8 |
| F | 26 | 308 | Non-traumatic (anoxia) | MCS | 13 | 1 | 6 |
| M | 34 | 799 | Non-traumatic (anoxia) | MCS | 66 | 1 | 8 |
| M | 62 | 83 | Traumatic | MCS | 89 | 0 | 8 |
| F | 24 | 544 | Traumatic | VS | 7 | 0.125 | 8 |
| MAS Evolution Upper Limbs | |||
|---|---|---|---|
| Effect | df | F | p |
| Etio | 7, 107.29 | 2.226 | 0.038 * |
| TSI | 7, 107.38 | 0.695 | 0.676 |
| Age | 7, 107.13 | 0.159 | 0.992 |
| Medication | 7, 107.20 | 1.129 | 0.350 |
| Gender | 7, 107.54 | 0.359 | 0.924 |
| DOC | 7, 107.19 | 0.569 | 0.780 |
| MAS Evolution Lower Limbs | |||
|---|---|---|---|
| Effect | df | F | p |
| Etio | 7, 107.09 | 1.245 | 0.285 |
| TSI | 7, 107.12 | 0.531 | 0.809 |
| Age | 7, 107.07 | 0.533 | 0.808 |
| Medication | 7, 107.11 | 0.547 | 0.797 |
| Gender | 7, 107.15 | 0.402 | 0.899 |
| DOC | 7, 107.07 | 3.196 | 0.004 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winters, B.; Kuluris, B.; Pathmanaban, R.; Vanderwalt, H.; Thibaut, A.; Schnakers, C. Changes of Spasticity across Time in Prolonged Disorders of Consciousness: A Retrospective Study. Brain Sci. 2022, 12, 295. https://doi.org/10.3390/brainsci12020295
Winters B, Kuluris B, Pathmanaban R, Vanderwalt H, Thibaut A, Schnakers C. Changes of Spasticity across Time in Prolonged Disorders of Consciousness: A Retrospective Study. Brain Sciences. 2022; 12(2):295. https://doi.org/10.3390/brainsci12020295
Chicago/Turabian StyleWinters, Benjamin, Bruce Kuluris, Rita Pathmanaban, Hannelise Vanderwalt, Aurore Thibaut, and Caroline Schnakers. 2022. "Changes of Spasticity across Time in Prolonged Disorders of Consciousness: A Retrospective Study" Brain Sciences 12, no. 2: 295. https://doi.org/10.3390/brainsci12020295
APA StyleWinters, B., Kuluris, B., Pathmanaban, R., Vanderwalt, H., Thibaut, A., & Schnakers, C. (2022). Changes of Spasticity across Time in Prolonged Disorders of Consciousness: A Retrospective Study. Brain Sciences, 12(2), 295. https://doi.org/10.3390/brainsci12020295







