Brain Structure and Optimism Bias: A Voxel-Based Morphometry Approach
Abstract
:1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Experimental Paradigm
2.3. Magnetic Resonance Imaging (MRI) Acquisition
2.4. Image Processing
2.5. Voxel-Based Morphometry
2.6. Statistical Analysis
3. Results
3.1. Behavioral Data
3.2. GMV for Optimism Biases
4. Discussion
Implications and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carver, C.S.; Scheier, M.F. Dispositional optimism. Trends Cogn. Sci. 2014, 18, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Nes, L.S.; Segerstrom, S.C. Dispositional optimism and coping: A meta-analytic review. Personal. Soc. Psychol. Rev. 2006, 10, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Peleg, G.; Barak, O.; Harel, Y.; Rochberg, J.; Hoofien, D. Hope dispositional optimism and severity of depression following traumatic brain injury. Brain Inj. 2009, 23, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.S.; Vogeltanz, N.D. Dispositional optimism as a predictor of depressive symptoms over time. Personal. Individ. Differ. 2000, 28, 259–272. [Google Scholar] [CrossRef]
- Weinstein, N.D. Unrealistic optimism about future life events. J. Personal. Soc. Psychol. 1980, 39, 806. [Google Scholar] [CrossRef]
- Sharot, T. The optimism bias. Curr. Biol. 2011, 21, R941–R945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dricu, M.; Kress, L.; Aue, T. The neurophysiological basis of optimism bias. In Cognitive Biases in Health and Psychiatric Disorders: Neurophysiological Foundations; Elsevier: San Diego, CA, USA, 2020. [Google Scholar]
- Perloff, L.S. Social comparison and illusions of invulnerability to negative life events. In Coping with Negative Life Events; Springer: Boston, MA, USA, 1987; pp. 217–242. [Google Scholar]
- Rasmussen, H.N.; Scheier, M.F.; Greenhouse, J.B. Optimism and physical health: A meta-analytic review. Ann. Behav. Med. 2009, 37, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Conversano, C.; Rotondo, A.; Lensi, E.; Della, V.O.; Arpone, F.; Reda, M.A. Optimism and its impact on mental and physical well-being. Clin. Pract. Epidemiol. Ment. Health 2010, 6, 25. [Google Scholar] [CrossRef]
- Rius-Ottenheim, N.; van der Mast, R.C.; Zitman, F.G.; Giltay, E.J. The role of dispositional optimism in physical and mental well-being. In A Positive Psychology Perspective on Quality of Life; Springer: Dordrecht, The Netherlands, 2012; pp. 149–173. [Google Scholar]
- Aue, T.; Nusbaum, H.C.; Cacioppo, J.T. Neural correlates of wishful thinking. Soc. Cogn. Affect. Neurosci. 2012, 7, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Dricu, M.; Bührer, S.; Hesse, F.; Eder, C.; Posada, A.; Aue, T. Warmth and competence predict overoptimistic beliefs for out-group but not in-group members. PLoS ONE 2018, 13, e0207670. [Google Scholar] [CrossRef]
- Dricu, M.; Schüpbach, L.; Bristle, M.; Wiest, R.; Moser, D.; Aue, T. Group membership dictates the neural correlates of social optimism biases. Sci. Rep. 2020, 10, 1139. [Google Scholar] [CrossRef]
- Simmons, J.P.; Massey, C. Is optimism real? J. Exp. Psychol. Gen. 2012, 141, 630. [Google Scholar] [CrossRef] [PubMed]
- Massey, C.; Simmons, J.P.; Armor, D.A. Hope over experience: Desirability and the persistence of optimism. Psychol. Sci. 2011, 22, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Price, P.C. Wishful thinking in the prediction of competitive outcomes. Think. Reason. 2000, 6, 161–172. [Google Scholar] [CrossRef]
- Babad, E. Wishful thinking among voters: Motivational and cognitive influences. Int. J. Public Opin. Res. 1997, 9, 105–125. [Google Scholar] [CrossRef]
- Babad, E. Can accurate knowledge reduce wishful thinking in voters’ predictions of election outcomes? J. Psychol. 1995, 129, 285–300. [Google Scholar] [CrossRef]
- Krizan, Z.; Windschitl, P.D. Wishful thinking about the future: Does desire impact optimism? Soc. Personal. Psychol. Compass 2009, 3, 227–243. [Google Scholar] [CrossRef]
- Lench, H.C. Automatic optimism: The affective basis of judgments about the likelihood of future events. J. Exp. Psychol. Gen. 2009, 138, 187–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kress, L.; Aue, T. The link between optimism bias and attention bias: A neurocognitive perspective. Neurosci. Biobehav. Rev. 2017, 80, 688–702. [Google Scholar] [CrossRef]
- Hogg, M.A.; Abrams, D. Social motivation, self-esteem and social identity. Soc. Identity Theory Constr. Crit. Adv. 1990, 28, 47. [Google Scholar]
- Tajfel, H.; Billig, M.G.; Bundy, R.P.; Flament, C. Social categorization and intergroup behaviour. Eur. J. Soc. Psychol. 1971, 1, 149–178. [Google Scholar] [CrossRef]
- Abrams, D.E.; Hogg, M.A. Social Identity Theory: Constructive and Critical Advances; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Turner, J.C.; Onorato, R.S. Social identity, personality, and the self-concept: A self-categorization perspective. In The Psychology of the Social Self; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 11–46. [Google Scholar]
- Tyler, T.R.; Kramer, R.M.; John, O.P. The Psychology of the Social Self; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999. [Google Scholar]
- Berridge, K.C.; Kringelbach, M.L. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 2008, 199, 457–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, K.C.; Kringelbach, M.L. Building a neuroscience of pleasure and well-being. Psychol. Well-Being Theory Res. Pract. 2011, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Sharot, T.; Guitart-Masip, M.; Korn, C.W.; Chowdhury, R.; Dolan, R.J. How dopamine enhances an optimism bias in humans. Curr. Biol. 2012, 22, 1477–1481. [Google Scholar] [CrossRef] [Green Version]
- Guitart-Masip, M.; Huys, Q.; Fuentemilla, L.; Dayan, P.; Duzel, E.; Dolan, R. Go and no-go learning in reward and punishment: Interactions between affect and effect. NeuroImage 2012, 62, 154–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charpentier, C.J.; Bromberg-Martin, E.S.; Sharot, T. Valuation of knowledge and ignorance in mesolimbic reward circuitry. Proc. Natl. Acad. Sci. USA 2018, 115, E7255–E7264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharot, T.; Riccardi, A.M.; Raio, C.M.; Phelps, E.A. Neural mechanisms mediating optimism bias. Nature 2007, 450, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Yang, J.; Yang, W.; Wei, D.; Qiu, J.; Zhang, D. The association between resting functional connectivity and dispositional optimism. PLoS ONE 2017, 12, e0180334. [Google Scholar] [CrossRef] [Green Version]
- Blair, K.S.; Otero, M.; Teng, C.; Jacobs, M.; Odenheimer, S.; Pine, D.S.; Blair, R. Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias. NeuroImage 2013, 78, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Heller, A.S.; Johnstone, T.; Shackman, A.J.; Light, S.N.; Peterson, M.J.; Kolden, G.G.; Kalin, N.H.; Davidson, R.J. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc. Natl. Acad. Sci. USA 2009, 106, 22445–22450. [Google Scholar] [CrossRef] [Green Version]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wager, T.D.; Barrett, L.F.; Bliss-Moreau, E.; Lindquist, K.; Duncan, S.; Kober, H. The neuroimaging of emotion. Handb. Emot. 2008, 3, 249–271. [Google Scholar]
- Britton, J.C.; Phan, K.L.; Taylor, S.; Welsh, R.C.; Berridge, K.; Liberzon, I. Neural correlates of social and nonsocial emotions: An fMRI study. NeuroImage 2006, 31, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.A.; Dricu, M.; Kotikalapudi, R.; Doucet, G.E.; Aue, T. Reduced network integration in default mode and executive networks is associated with social and personal optimism biases. Hum. Brain Mapp. 2021, 42, 2893–2906. [Google Scholar] [CrossRef] [PubMed]
- Samson, D.; Apperly, I.A.; Chiavarino, C.; Humphreys, G.W. Left temporoparietal junction is necessary for representing someone else’s belief. Nat. Neurosci. 2004, 7, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Saxe, R.; Kanwisher, N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. NeuroImage 2003, 19, 1835–1842. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. NeuroImage 2000, 11, 805–821. [Google Scholar] [CrossRef] [Green Version]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Hensonac, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 2001, 14, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sharot, T.; Wolfe, T.; Düzel, E.; Dolan, R.J. Optimistic update bias increases in older age. Psychol. Med. 2014, 44, 2003–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wei, D.; Wang, K.; Qiu, J. Gray matter correlates of dispositional optimism: A voxel-based morphometry study. Neurosci. Lett. 2013, 553, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Wang, S.; Zhao, Y.; Qiu, C.; Gong, Q. Neurostructural correlates of optimism: Gray matter density in the putamen predicts dispositional optimism in late adolescence. Hum. Brain Mapp. 2019, 41, 1459–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolcos, S.; Hu, Y.; Iordan, A.D.; Moore, M.; Dolcos, F. Optimism and the brain: Trait optimism mediates the protective role of the orbitofrontal cortex gray matter volume against anxiety. Soc. Cogn. Affect. Neurosci. 2016, 11, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, D.A.; Dricu, M.; Wiest, R.; Schüpbach, L.; Aue, T. Social optimism biases are associated with cortical thickness. Soc. Cogn. Affect. Neurosci. 2020, 15, 745–754. [Google Scholar] [CrossRef]
- Aue, T.; Dricu, M.; Moser, D.A.; Mayer, B.; Bührer, S. Comparing personal and social optimism biases: Magnitude, overlap, modifiability, and links with social identification and expertise. Humanit. Soc. Sci. Commun. 2021, 8, 233. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama 2013, 310, 2191–2194. [Google Scholar]
- Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Rolls, E.T.; Joliot, M.; Tzourio-Mazoyer, N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. NeuroImage 2015, 122, 1–5. [Google Scholar] [CrossRef]
- Mazoyera, N.-; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Tzourio-Mazoyer, N.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, K.; Kumada, S.; Weitemier, A.; Jo, T.; Inoue, M. Correction: Reward-Induced Phasic Dopamine Release in the Monkey Ventral Striatum and Putamen. PLoS ONE 2015, 10, e0135592. [Google Scholar] [CrossRef] [PubMed]
- Flagel, S.B.; E Robinson, T.; Clark, J.J.; Clinton, S.M.; Watson, S.J.; Seeman, P.; Phillips, P.; Akil, H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology 2010, 35, 388–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cromwell, H.C.; Schultz, W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J. Neurophysiol. 2003, 89, 2823–2838. [Google Scholar] [CrossRef]
- Schultz, W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000, 1, 199–207. [Google Scholar] [CrossRef]
- Cromwell, H.C.; Hassani, O.K.; Schultz, W. Relative reward processing in primate striatum. Exp. Brain Res. 2005, 162, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Haruno, M.; Kawato, M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J. Neurophysiol. 2006, 95, 948–959. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [Green Version]
- Delgado, M.R. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007, 1104, 70–88. [Google Scholar] [CrossRef]
- Elliott, R.; Newman, J.L.; A Longe, O.; Deakin, J.W. Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. NeuroImage 2004, 21, 984–990. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, H.; Han, D.; Mo, Y.; Li, Z.; Cheng, Y.; Xu, X.; Shen, Z.; Tan, C.; Zhao, W. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. NeuroImage Clin. 2016, 11, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Savitz, J.; Drevets, W.C. Bipolar and major depressive disorder: Neuroimaging the developmental-degenerative divide. Neurosci. Biobehav. Rev. 2009, 33, 699–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexton, C.E.; Mackay, C.E.; Ebmeier, K.P. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am. J. Geriatr. Psychiatry 2013, 21, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Parashos, I.A.; Tupler, L.A.; Blitchington, T.; Krishnan, K.R. Magnetic-resonance morphometry in patients with major depression. Psychiatry Res. Neuroimaging 1998, 84, 7–15. [Google Scholar] [CrossRef]
- Strunk, D.R.; Lopez, H.; DeRubeis, R.J. Depressive symptoms are associated with unrealistic negative predictions of future life events. Behav. Res. Ther. 2006, 44, 861–882. [Google Scholar] [CrossRef] [PubMed]
- Strunk, D.R.; Adler, A.D. Cognitive biases in three prediction tasks: A test of the cognitive model of depression. Behav. Res. Ther. 2009, 47, 34–40. [Google Scholar] [CrossRef]
- Korn, C.W.; Sharot, T.; Walter, H.; Heekeren, H.R.; Dolan, R.J. Depression is related to an absence of optimistically biased belief updating about future life events. Psychol. Med. 2014, 44, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Boorman, E.D.; Behrens, T.E.; Woolrich, M.W.; Rushworth, M. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron 2009, 62, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Kovach, C.K.; Daw, N.D.; Rudrauf, D.; Tranel, D.; O’Doherty, J.P.; Adolphs, R. Anterior prefrontal cortex contributes to action selection through tracking of recent reward trends. J. Neurosci. 2012, 32, 8434–8442. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, A.R.; Fellows, L.K. The Neuropsychology of Decision-Making: A View From the Frontal Lobes. In Decision Neuroscience; Elsevier: Amsterdam, The Netherlands, 2017; pp. 277–289. [Google Scholar]
- Koechlin, E.; Hyafil, A. Anterior prefrontal function and the limits of human decision-making. Science 2007, 318, 594–598. [Google Scholar] [CrossRef]
- Pollmann, S. Frontopolar resource allocation in human and nonhuman primates. Trends Cogn. Sci. 2016, 20, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Badre, D.; Doll, B.B.; Long, N.M.; Frank, M.J. Rostrolateral prefrontal cortex and individual differences in uncertainty-driven exploration. Neuron 2012, 73, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.J.; Doll, B.B.; Oas-Terpstra, J.; Moreno, F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat. Neurosci. 2009, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Beharelle, A.R.; Polanía, R.; Hare, T.A.; Ruff, C.C. Transcranial stimulation over frontopolar cortex elucidates the choice attributes and neural mechanisms used to resolve exploration-exploitation trade-offs. J. Neurosci. 2015, 35, 14544–14556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespi, C.; Laureiro-Martínez, D.; Dodich, A.; Cappa, S.; Brusoni, S.; Zollo, M.; Falini, A.; Canessa, N. Improving innovative decision-making: Training-induced changes in fronto-parietal networks. Brain Cogn. 2018, 128, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Soutschek, A.; Kang, P.; Ruff, C.C.; Hare, T.; Tobler, P.N. Brain stimulation over the frontopolar cortex enhances motivation to exert effort for reward. Biol. Psychiatry 2018, 84, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glascher, J.; Adolphs, R.; Damasio, H.; Bechara, A.; Rudrauf, D.; Calamia, M.; Paul, L.; Tranel, D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2012, 109, 14681–14686. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.G.; Bourgeois-Gironde, S.; Ullsperger, M. Short-term reward experience biases inference despite dissociable neural correlates. Nat. Commun. 2017, 8, 1690. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.; Gallate, J. The function of the anterior temporal lobe: A review of the empirical evidence. Brain Res. 2012, 1449, 94–116. [Google Scholar] [CrossRef]
- OlsonI, R.; McCoy, D.; Klobusicky, E.; Ross, L.A. Social cognition and the anterior temporal lobes: A review and theoretical framework. Soc. Cogn. Affect. Neurosci. 2013, 8, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Binney, R.J.; Embleton, K.V.; Jefferies, E.; Parker, G.; Ralph, M.L. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb. Cortex 2010, 20, 2728–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.J.; Heggs, A.; Antoun, N.; Hodges, J.R. Progressive prosopagnosia associated with selective right temporal lobe atrophy: A new syndrome? Brain 1995, 118 Pt 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.; Avanzini, P.; Pelliccia, V.; Lorusso, G.; Sartori, I.; Orban, G.A. Rapid and specific processing of person-related information in human anterior temporal lobe. Commun. Biol. 2019, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Hodges, J.R.; Piguet, O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain 2014, 137, 1241–1253. [Google Scholar] [CrossRef] [Green Version]
- Zahn, R.; Moll, J.; Iyengar, V.; Huey, E.D.; Tierney, M.; Krueger, F.; Grafman, J.H. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain 2009, 132, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Tusche, A.; Böckler, A.; Kanske, P.; Trautwein, F.-M.; Singer, T. Decoding the charitable brain: Empathy, perspective taking, and attention shifts differentially predict altruistic giving. J. Neurosci. 2016, 36, 4719–4732. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, T.; Schiller, B.; Rieskamp, J.; Gianotti, L.R.; Knoch, D. Diminishing parochialism in intergroup conflict by disrupting the right temporo-parietal junction. Soc. Cogn. Affect. Neurosci. 2014, 9, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Bardi, L.; Six, P.; Brass, M. Repetitive TMS of the temporo-parietal junction disrupts participant’s expectations in a spontaneous Theory of Mind task. Soc. Cogn. Affect. Neurosci. 2017, 12, 1775–1782. [Google Scholar] [CrossRef] [Green Version]
- Paracampo, R.; Pirruccio, M.; Costa, M.; Borgomaneri, S.; Avenanti, A. Visual, sensorimotor and cognitive routes to understanding others’ enjoyment: An individual differences rTMS approach to empathic accuracy. Neuropsychologia 2018, 116, 86–98. [Google Scholar] [CrossRef]
- Donaldson, P.H.; Kirkovski, M.; Rinehart, N.J.; Enticott, P.G. Autism-relevant traits interact with temporoparietal junction stimulation effects on social cognition: A high-definition transcranial direct current stimulation and electroencephalography study. Eur. J. Neurosci. 2018, 47, 669–681. [Google Scholar] [CrossRef]
- Koster-Hale, J.; Richardson, H.; Velez, N.; Asaba, M.; Young, L.; Saxe, R. Mentalizing regions represent distributed, continuous, and abstract dimensions of others’ beliefs. NeuroImage 2017, 161, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rilling, J.K.; Dagenais, J.E.; Goldsmith, D.R.; Glenn, A.L.; Pagnoni, G. Social cognitive neural networks during in-group and out-group interactions. NeuroImage 2008, 41, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Telzer, E.H.; Ichien, N.; Qu, Y. The ties that bind: Group membership shapes the neural correlates of in-group favoritism. NeuroImage 2015, 115, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef]
- Parkinson, C.; Wheatley, T. Old cortex, new contexts: Re-purposing spatial perception for social cognition. Front. Hum. Neurosci. 2013, 7, 645. [Google Scholar] [CrossRef] [Green Version]
- Cuddy, A.J.; Fiske, S.T.; Glick, P. Warmth and competence as universal dimensions of social perception: The stereotype content model and the BIAS map. Adv. Exp. Soc. Psychol. 2008, 40, 61–149. [Google Scholar]


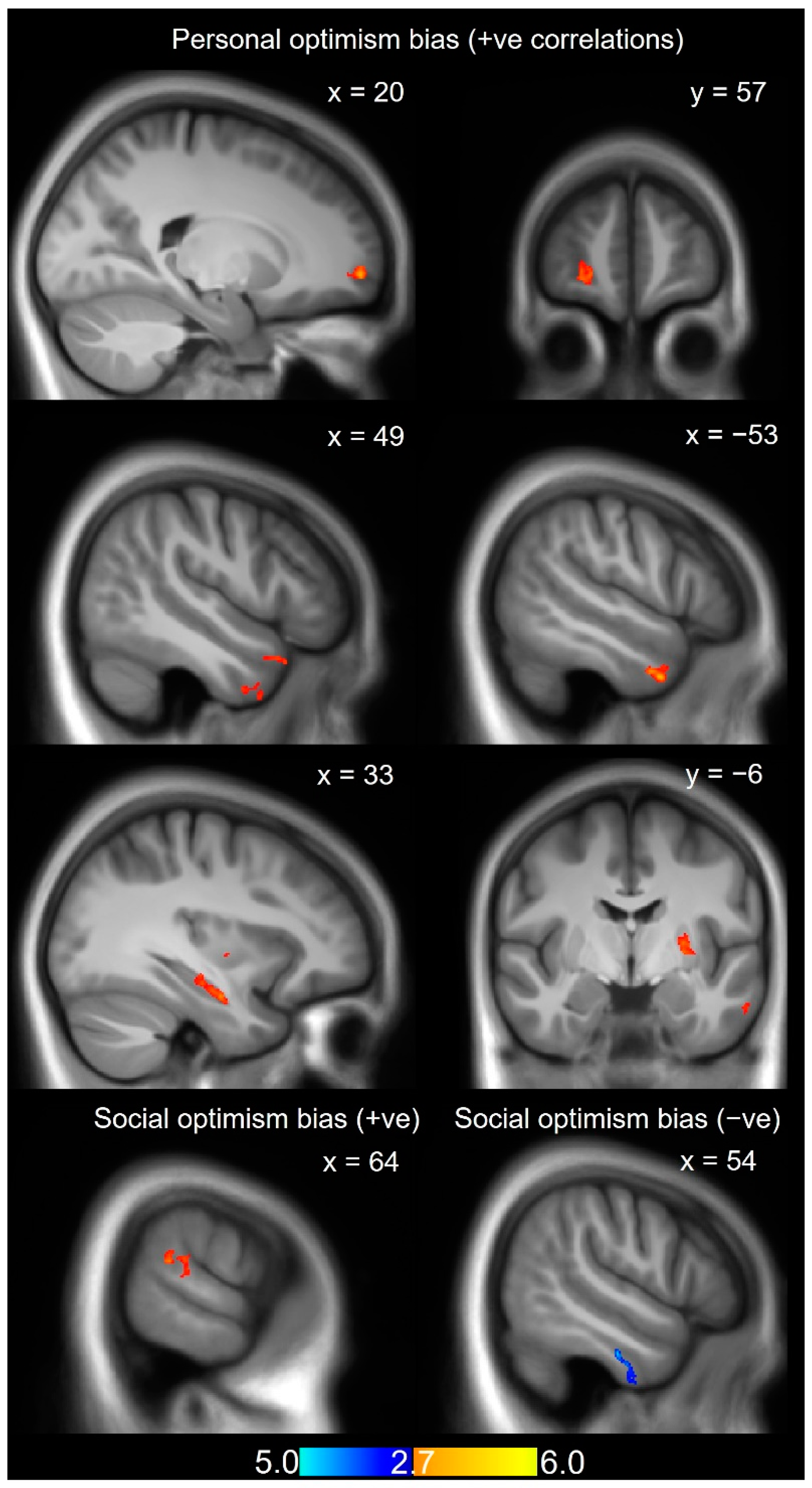
| Contrast | T-max | Peak MNI Coordinate | k | Region (Brodmann Area) | Overlap |
|---|---|---|---|---|---|
| POB | 4.14 | 40, 10, −41 | 499 | R Temporal pole (BA38) | 50.5% Temporal Inf R 18% Temporal Mid R 9.8% Temporal Pole Mid R |
| POB | 5.20 | −53, 4, −34 | 564 | L Temporal pole (BA38) | 20.9% Temporal Inf L 50.2% Temporal Mid L 28% Temporal Pole Mid L 0.9% Temporal Pole Sup L |
| POB | 4.47 | 44, 16, 8 | 592 | R Temporal pole (BA38) | 31.9% Temporal Pole Mid R 66.2% Temporal Pole Sup R |
| POB | 4.36 | −61, −28, −28 | 570 | L Inferior temporal gyrus (BA20) | 86% Temporal Inf L 9.1% Temporal Mid L |
| POB | 5.86 | −32, −10, −21 | 348 | L Hippocampus | 88.5% Hippocampus L 11.5% Para Hippocampal L |
| POB | 5.40 | 23, −96, −16 | 478 | R Visual association area (BA18) | 11.1% Lingual R 6.7% Occipital Inf R 76.8% Occipital Mid R |
| POB | 4.58 | 61, −20, −3 | 363 | R Mid-superior temporal gyrus (BA22) | 100% Temporal Mid R |
| POB | 4.47 | −27, −4, 2 | 499 | L Putamen | 17.4% Pallidum L 79.2% Putamen L |
| POB | 5.02 | 20, 59, −5 | 491 | R Frontal pole (BA10) | 100% Frontal Sup 2 R |
| SOB | 4.50 | 63, −37, 19 | 345 | R Temporoparietal junction (BA22) | 27.8% Supra Marginal R 57.7% Temporal Sup R |
| SOB (negative) | 4.84 | 56, −17, −29 | 377 | R Inferior temporal gyrus (BA20) | 79.3% Temporal Inf R |
| SOB (negative) | 5.23 | −31, 3, 61 | 354 | L Pre-supplementary motor area (BA6) | 42.9% Frontal Mid 2 L 56.5% Frontal Sup 2 L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotikalapudi, R.; Dricu, M.; Moser, D.A.; Aue, T. Brain Structure and Optimism Bias: A Voxel-Based Morphometry Approach. Brain Sci. 2022, 12, 315. https://doi.org/10.3390/brainsci12030315
Kotikalapudi R, Dricu M, Moser DA, Aue T. Brain Structure and Optimism Bias: A Voxel-Based Morphometry Approach. Brain Sciences. 2022; 12(3):315. https://doi.org/10.3390/brainsci12030315
Chicago/Turabian StyleKotikalapudi, Raviteja, Mihai Dricu, Dominik Andreas Moser, and Tatjana Aue. 2022. "Brain Structure and Optimism Bias: A Voxel-Based Morphometry Approach" Brain Sciences 12, no. 3: 315. https://doi.org/10.3390/brainsci12030315
APA StyleKotikalapudi, R., Dricu, M., Moser, D. A., & Aue, T. (2022). Brain Structure and Optimism Bias: A Voxel-Based Morphometry Approach. Brain Sciences, 12(3), 315. https://doi.org/10.3390/brainsci12030315





