Substance Abuse in Emerging Adults: The Role of Neuromelanin and Ventral Striatal Response to Social and Monetary Rewards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Substance Use
2.3. Procedure
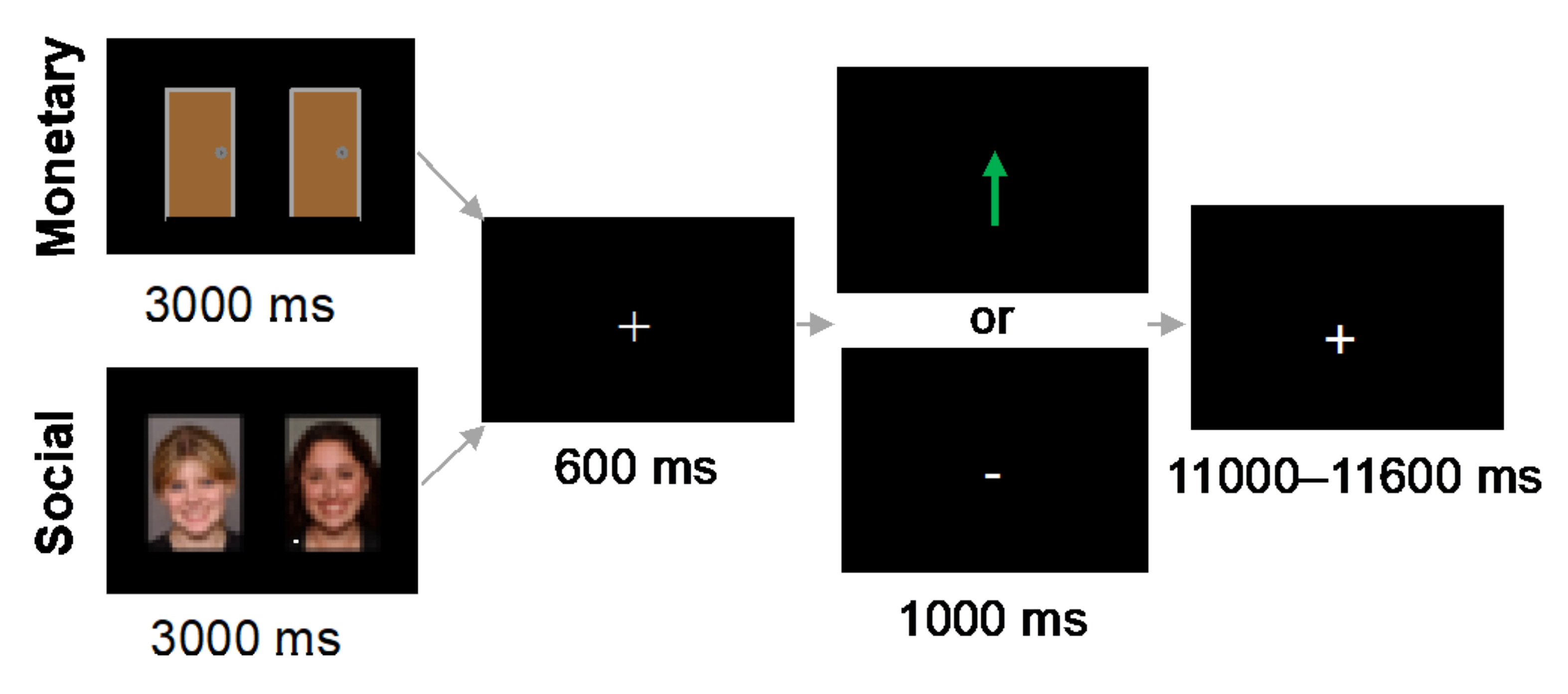
2.4. fMRI-Based Tasks
2.4.1. Monetary Reward Task
2.4.2. Social Reward Task
2.5. MRI Acquisition
2.5.1. Structural MRI
2.5.2. NM-MRI
2.5.3. fMRI
3. Data Analysis
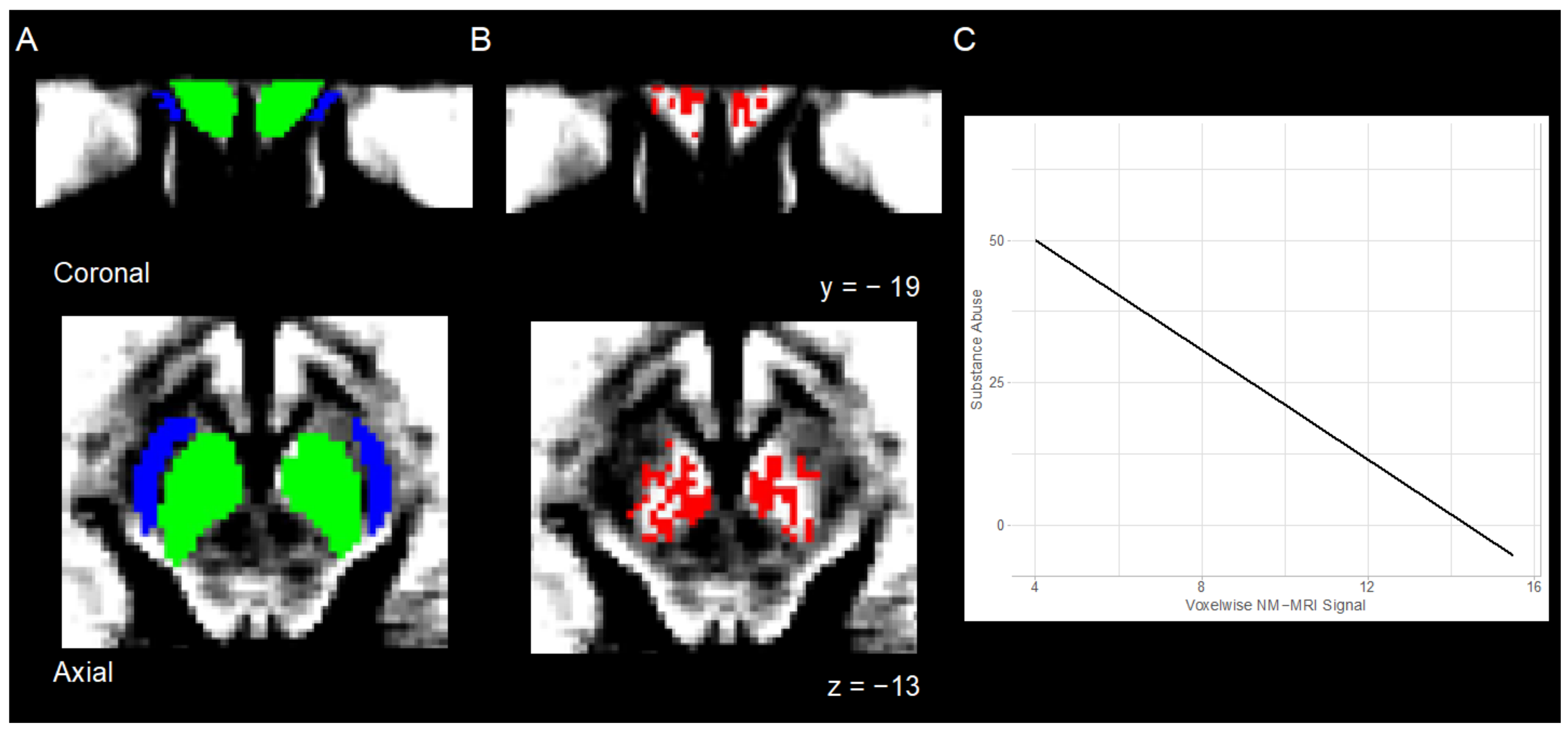
3.1. NM-MRI
3.1.1. Preprocessing
3.1.2. Individual Level Analysis
3.2. fMRI
3.2.1. Preprocessing
3.2.2. Individual Level Analysis
3.3. Group Level Analyses
3.3.1. Relation between NM-MRI Signal and Substance Abuse
3.3.2. Moderating Effect of NM-MRI Signal on the Relation between Ventral Striatal Function during Reward Processing and Substance Abuse
4. Results
4.1. Substance Abuse
4.2. Relation between NM-MRI Signal and Substance Abuse
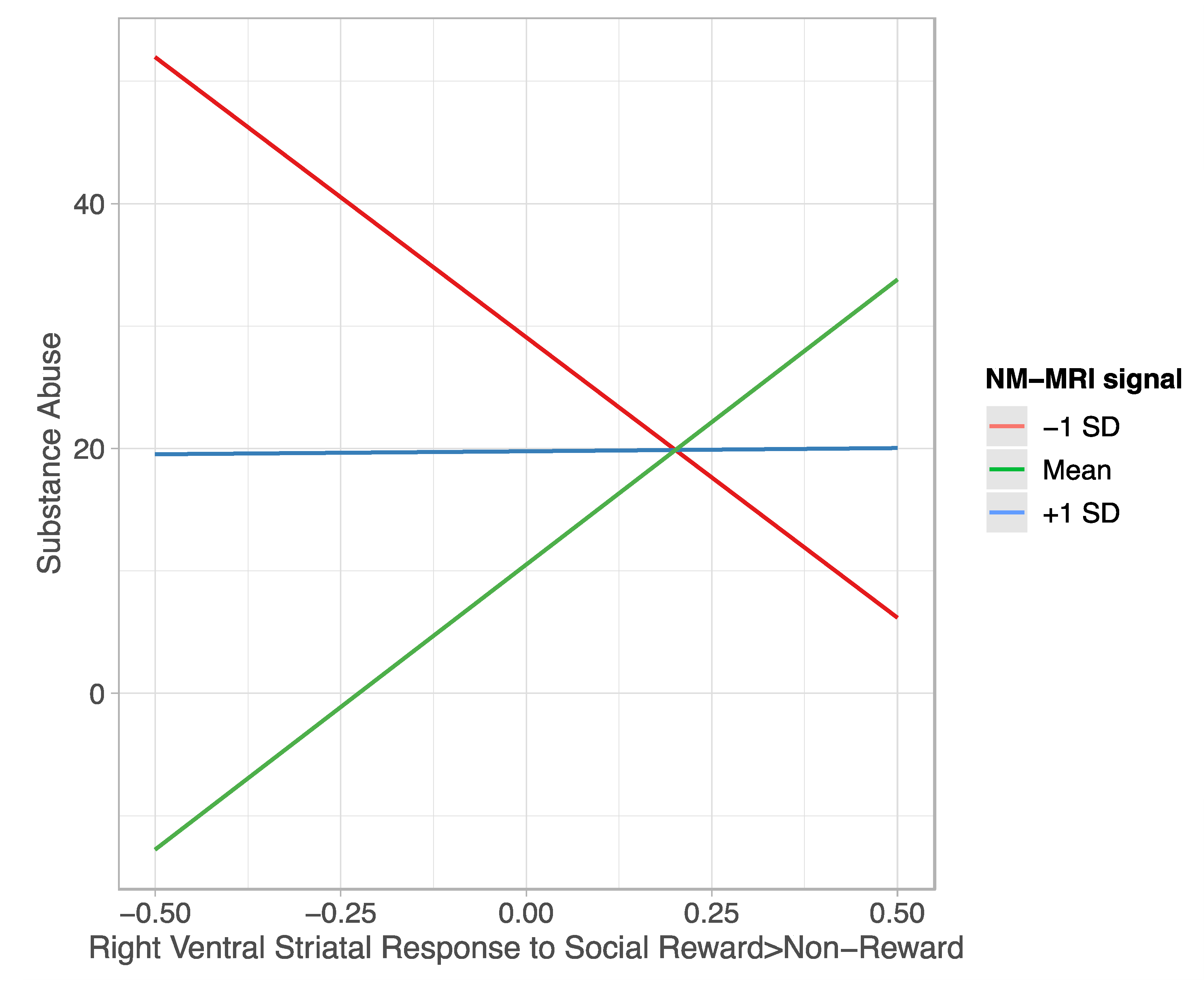
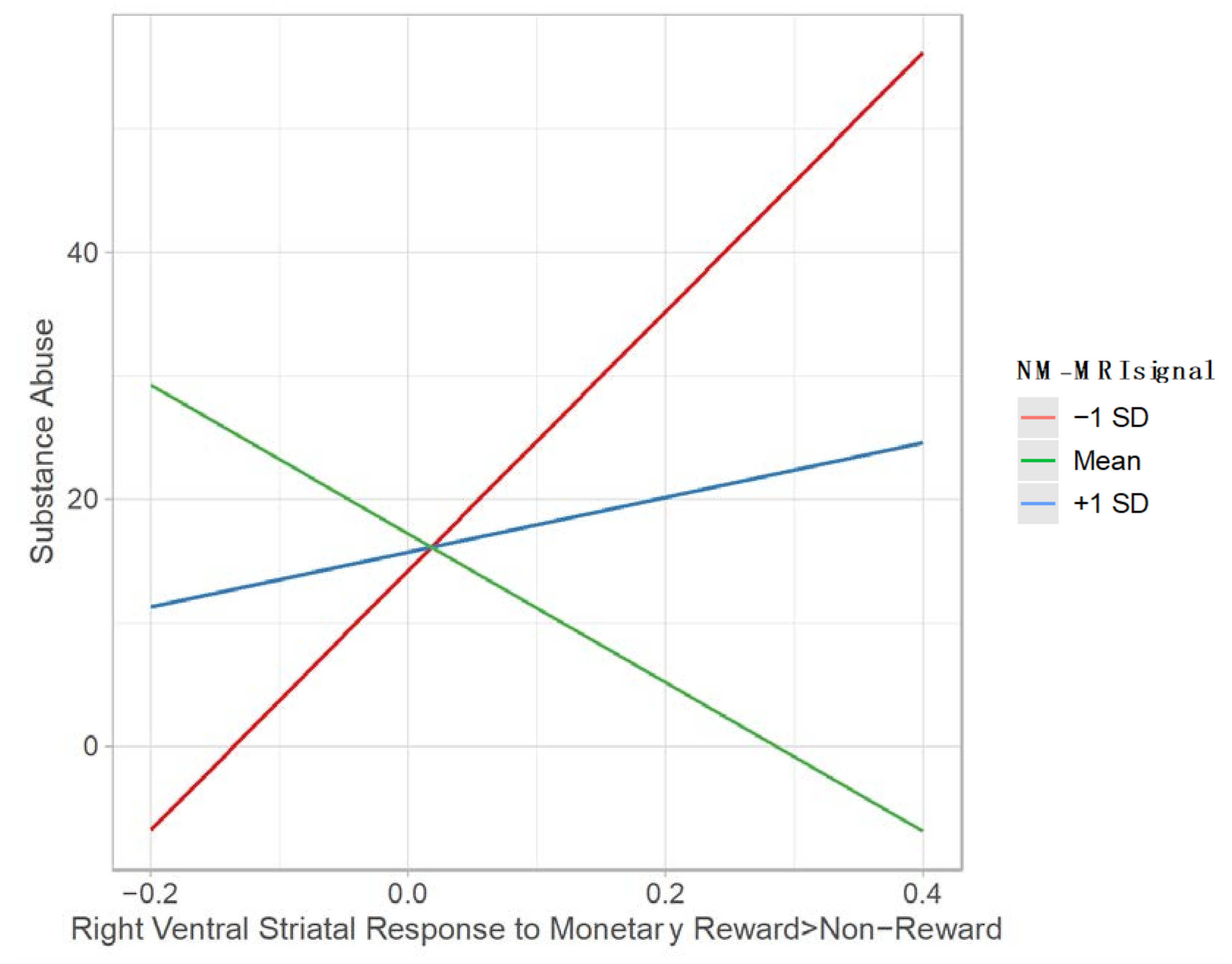
4.3. Moderating Effect of NM-MRI Signal on the Relation between Ventral Striatal Function during Reward Processing and Substance Abuse
4.3.1. Social Reward
4.3.2. Monetary Reward
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Results from the 2020 National Survey on Drug Use and Health: Detailed Tables; Center for Behavioral Health Statistics and Quality; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2021.
- Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration; Center for Behavioral Health Statistics and Quality: Rockville, MD, USA, 2020; p. 114.
- Teenage Drug Use Statistics: Data & Trends; National Center for Drug Abuse Statistics: Bethesda, MD, USA, 2021.
- Drug Abuse Statistics; National Center for Drug Abuse Statistics: Bethesda, MD, USA, 2019.
- Gray, K.M.; Squeglia, L.M. Research Review: What have we learned about adolescent substance use? J. Child Psychol. Psychiatry 2017, 59, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Barnett, N.P.; Clark, M. Attendance at alcohol-free and alcohol-service parties and alcohol consumption among college students. Addict. Behav. 2010, 35, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.A.; Tildesley, E.; Hops, H.; Li, F. The influence of peers on young adult substance use. Heal. Psychol. 2002, 21, 349–357. [Google Scholar] [CrossRef]
- Goncy, E.A.; Mrug, S. Where and When Adolescents Use Tobacco, Alcohol, and Marijuana: Comparisons by Age, Gender, and Race. J. Stud. Alcohol Drugs 2013, 74, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Ramo, D.E.; Brown, S.A. Classes of substance abuse relapse situations: A comparison of adolescents and adults. Psychol. Addict. Behav. 2008, 22, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.M.; Zucca, F.A.; Girgis, R.R.; Baker, S.C.; Weinstein, J.J.; Sharp, M.E.; Bellei, C.; Valmadre, A.; Vanegas, N.; Kegeles, L.S.; et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc. Natl. Acad. Sci. USA 2019, 116, 5108–5117. [Google Scholar] [CrossRef] [Green Version]
- Ashok, A.H.; Mizuno, Y.; Volkow, N.D.; Howes, O.D. Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine. JAMA Psychiatry 2017, 74, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Spanagel, R. Alcoholism: A Systems Approach From Molecular Physiology to Addictive Behavior. Physiol. Rev. 2009, 89, 649–705. [Google Scholar] [CrossRef] [Green Version]
- Sulzer, D.; Cassidy, C.; Horga, G.; Kang, U.; Fahn, S.; Casella, L.; Pezzoli, G.; Langley, J.; Hu, X.P.; Zucca, F.A.; et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. npj Park. Dis. 2018, 4, 11. [Google Scholar] [CrossRef]
- Zecca, L.; Fariello, R.; Riederer, P.; Sulzer, D.; Gatti, A.; Tampellini, D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2001, 510, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Tanaka, H.; Tsukabe, A.; Kunitomi, Y.; Nishizawa, M.; Hashimoto, R.; Yamamori, H.; Fujimoto, M.; Fukunaga, M.; Tomiyama, N. Neuromelanin Magnetic Resonance Imaging Reveals Increased Dopaminergic Neuron Activity in the Substantia Nigra of Patients with Schizophrenia. PLoS ONE 2014, 9, e104619. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Carpenter, K.M.; Konova, A.B.; Cheung, V.; Grassetti, A.; Zecca, L.; Abi-Dargham, A.; Martinez, D.; Horga, G. Evidence for Dopamine Abnormalities in the Substantia Nigra in Cocaine Addiction Revealed by Neuromelanin-Sensitive MRI. Am. J. Psychiatry 2020, 177, 1038–1047. [Google Scholar] [CrossRef]
- Heitzeg, M.M.; Cope, L.M.; Martz, M.E.; Hardee, J.E. Neuroimaging Risk Markers for Substance Abuse: Recent Findings on Inhibitory Control and Reward System Functioning. Curr. Addict. Rep. 2015, 2, 91–103. [Google Scholar] [CrossRef]
- Leyton, M.; Vezina, P. Dopamine ups and downs in vulnerability to addictions: A neurodevelopmental model. Trends Pharmacol. Sci. 2014, 35, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Doremus-Fitzwater, T.L.; Varlinskaya, E.; Spear, L.P. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010, 72, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Gardner, E.; Berman, M.O.; Gold, M. “Liking” and “Wanting” Linked to Reward Deficiency Syndrome (RDS): Hypothesizing Differential Responsivity in Brain Reward Circuitry. Curr. Pharm. Des. 2012, 18, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Spear, L. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Blakemore, S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008, 9, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.; Heller, A.S.; Gee, D.; Cohen, A.O. Development of the emotional brain. Neurosci. Lett. 2019, 693, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.E.; Leibenluft, E.; McClure, E.B.; Pine, D.S. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005, 35, 163–174. [Google Scholar] [CrossRef]
- Nepon, T.; Pepler, D.J.; Craig, W.M.; Connolly, J.; Flett, G.L. A Longitudinal Analysis of Peer Victimization, Self-Esteem, and Rejection Sensitivity in Mental Health and Substance Use Among Adolescents. Int. J. Ment. Heal. Addict. 2020, 19, 1135–1148. [Google Scholar] [CrossRef]
- Morgan, D.; Grant, K.A.; Gage, H.D.; Mach, R.H.; Kaplan, J.R.; Prioleau, O.; Nader, S.H.; Buchheimer, N.; Ehrenkaufer, R.L.; Nader, M.A. Social dominance in monkeys: Dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 2002, 5, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, T.; Walsh, N.D.; Mobbs, D.; Schweizer, S.; van Harmelen, A.-L.; Dunn, B.; Dunn, V.; Goodyer, I.; Stretton, J. Social pain and social gain in the adolescent brain: A common neural circuitry underlying both positive and negative social evaluation. Sci. Rep. 2017, 7, 42010. [Google Scholar] [CrossRef]
- Rudolph, K.D.; Davis, M.M.; Skymba, H.V.; Modi, H.H.; Telzer, E.H. Social experience calibrates neural sensitivity to social feedback during adolescence: A functional connectivity approach. Dev. Cogn. Neurosci. 2020, 47, 100903. [Google Scholar] [CrossRef] [PubMed]
- Chein, J.; Albert, D.; O’Brien, L.; Uckert, K.; Steinberg, L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2010, 14, F1–F10. [Google Scholar] [CrossRef] [Green Version]
- Gilman, J.M.; Lee, S.; Kuster, J.K.; Lee, M.J.; Kim, B.W.; van der Kouwe, A.; Blood, A.J.; Breiter, H.C. Variable activation in striatal subregions across components of a social influence task in young adult cannabis users. Brain Behav. 2016, 6, e00459. [Google Scholar] [CrossRef]
- Gilman, J.M.; Schuster, R.M.; Curran, M.; Calderon, V.; Van Der Kouwe, A.; Evins, A.E. Neural mechanisms of sensitivity to peer information in young adult cannabis users. Cogn. Affect. Behav. Neurosci. 2016, 16, 646–661. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.; Kendrick, K.M.; Scheele, D.; Dau, W.; Banger, M.; Maier, W.; Weber, B.; Ma, Y.; Hurlemann, R.; Becker, B. Altered striatal reward processing in abstinent dependent cannabis users: Social context matters. Eur. Neuropsychopharmacol. 2019, 29, 356–364. [Google Scholar] [CrossRef]
- Garrison, K.A.; Yip, S.W.; Balodis, I.M.; Carroll, K.M.; Potenza, M.N.; Krishnan-Sarin, S. Reward-related frontostriatal activity and smoking behavior among adolescents in treatment for smoking cessation. Drug Alcohol Depend. 2017, 177, 268–276. [Google Scholar] [CrossRef]
- Peters, J.; Bromberg, U.; Schneider, S.; Brassen, S.; Menz, M.; Banaschewski, T.; Conrod, P.J.; Flor, H.; Gallinat, J.; Garavan, H.; et al. Lower Ventral Striatal Activation During Reward Anticipation in Adolescent Smokers. Am. J. Psychiatry 2011, 168, 540–549. [Google Scholar] [CrossRef]
- Schneider, S.; Peters, J.; Bromberg, U.; Brassen, S.; Miedl, S.F.; Banaschewski, T.; Barker, G.J.; Conrod, P.; Flor, H.; Garavan, H.; et al. Risk Taking and the Adolescent Reward System: A Potential Common Link to Substance Abuse. Am. J. Psychiatry 2012, 169, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Aloi, J.; Blair, K.S.; Crum, K.I.; Bashford-Largo, J.; Zhang, R.; Lukoff, J.; Carollo, E.; White, S.F.; Hwang, S.; Filbey, F.M.; et al. Alcohol Use Disorder, But Not Cannabis Use Disorder, Symptomatology in Adolescents Is Associated With Reduced Differential Responsiveness to Reward Versus Punishment Feedback During Instrumental Learning. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2020, 5, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Aloi, J.; Crum, K.I.; Blair, K.S.; Zhang, R.; Bashford-Largo, J.; Bajaj, S.; Schwartz, A.; Carollo, E.; Hwang, S.; Leiker, E.; et al. Individual associations of adolescent alcohol use disorder versus cannabis use disorder symptoms in neural prediction error signaling and the response to novelty. Dev. Cogn. Neurosci. 2021, 48, 100944. [Google Scholar] [CrossRef]
- Pabon, E.; Crane, N.A.; Radoman, M.; Weafer, J.; Langenecker, S.A.; Phan, K.L.; de Wit, H. Neural activation during anticipation of monetary gain or loss does not associate with positive subjective response to alcohol in binge drinkers. Drug Alcohol Depend. 2020, 218, 108432. [Google Scholar] [CrossRef] [PubMed]
- Jager, G.; Block, R.I.; Luijten, M.; Ramsey, N. Tentative Evidence for Striatal Hyperactivity in Adolescent Cannabis-Using Boys: A Cross-Sectional Multicenter fMRI Study. J. Psychoact. Drugs 2013, 45, 156–167. [Google Scholar] [CrossRef]
- Tapert, S.F.; Cheung, E.H.; Brown, G.G.; Frank, L.R.; Paulus, M.P.; Schweinsburg, A.D.; Meloy, M.J.; Brown, S.A. Neural Response to Alcohol Stimuli in Adolescents With Alcohol Use Disorder. Arch. Gen. Psychiatry 2003, 60, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Hasler, B.P.; Sitnick, S.L.; Shaw, D.S.; Forbes, E. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. Neuroimaging 2013, 214, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Radoman, M.; Crane, N.A.; Gorka, S.M.; Weafer, J.; Langenecker, S.A.; De Wit, H.; Phan, K.L. Striatal activation to monetary reward is associated with alcohol reward sensitivity. Neuropsychopharmacology 2020, 46, 343–350. [Google Scholar] [CrossRef]
- Morales, A.; Jones, S.A.; Ehlers, A.; LaVine, J.B.; Nagel, B.J. Ventral striatal response during decision making involving risk and reward is associated with future binge drinking in adolescents. Neuropsychopharmacology 2018, 43, 1884–1890. [Google Scholar] [CrossRef] [Green Version]
- Weiland, B.J.; Zucker, R.A.; Zubieta, J.-K.; Heitzeg, M.M. Striatal dopaminergic reward response relates to age of first drunkenness and feedback response in at-risk youth. Addict. Biol. 2016, 22, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Distefano, A.; Jackson, F.; Levinson, A.R.; Infantolino, Z.P.; Jarcho, J.M.; Nelson, B.D. A comparison of the electrocortical response to monetary and social reward. Soc. Cogn. Affect. Neurosci. 2018, 13, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Sabharwal, A.; Infantolino, Z.P.; Jarcho, J.M.; Nelson, B.D. Time-Frequency Delta Activity to Social Feedback Demonstrates Differential Associations With Depression and Social Anxiety Symptoms. Front. Behav. Neurosci. 2019, 13, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, B.D.; Jarcho, J.M. Neural response to monetary and social feedback demonstrates differential associations with depression and social anxiety. Soc. Cogn. Affect. Neurosci. 2021, 16, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Quarmley, M.E.; Nelson, B.D.; Clarkson, T.; White, L.K.; Jarcho, J.M. I Knew You Weren’t Going to Like Me! Neural Response to Accurately Predicting Rejection Is Associated With Anxiety and Depression. Front. Behav. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Patrick, C.J.; Kramer, M.D.; Krueger, R.F.; Markon, K.E. Optimizing efficiency of psychopathology assessment through quantitative modeling: Development of a brief form of the Externalizing Spectrum Inventory. Psychol. Assess. 2013, 25, 1332–1348. [Google Scholar] [CrossRef]
- Egger, H.L.; Pine, D.S.; Nelson, E.; Leibenluft, E.; Ernst, M.; Towbin, K.E.; Angold, A. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): A new set of children’s facial emotion stimuli. Int. J. Methods Psychiatr. Res. 2011, 20, 145–156. [Google Scholar] [CrossRef]
- Frazier, J.A.; Chiu, S.; Breeze, J.L.; Makris, N.; Lange, N.; Kennedy, D.N.; Herbert, M.R.; Bent, E.K.; Koneru, V.K.; Dieterich, M.E.; et al. Structural Brain Magnetic Resonance Imaging of Limbic and Thalamic Volumes in Pediatric Bipolar Disorder. Am. J. Psychiatry 2005, 162, 1256–1265. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Seidman, L.J.; Makris, N.; Ahern, T.; O’Brien, L.M.; Caviness, V.S.; Kennedy, D.N.; Faraone, S.V.; Tsuang, M.T. Hypothalamic Abnormalities in Schizophrenia: Sex Effects and Genetic Vulnerability. Biol. Psychiatry 2007, 61, 935–945. [Google Scholar] [CrossRef]
- Makris, N.; Goldstein, J.M.; Kennedy, D.; Hodge, S.M.; Caviness, V.S.; Faraone, S.; Tsuang, M.T.; Seidman, L.J. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res. 2006, 83, 155–171. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Methodology in the Social Sciences, 3rd ed.; The Guilford Press: New York, NY, USA, 2022; ISBN 978-1-4625-4903-0. [Google Scholar]
- Soe-Agnie, S.E.; Paap, M.C.S.; Nijman, H.L.I.; De Jong, C.A.J. Psychometric Properties of the Externalizing Spectrum Inventory: Replication and Extension across Clinical and Non-Clinical Samples. J. Personal. Assess. 2020, 103, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Sapuan, A.; Dineen, R.; Auer, D.P. Life span pigmentation changes of the substantia nigra detected by neuromelanin-sensitive MRI. Mov. Disord. 2018, 33, 1792–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telzer, E.H. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Dev. Cogn. Neurosci. 2015, 17, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Pine, D.S.; Brotman, M.A.; Smith, A.R.; Cox, R.W.; Haller, S.P. Trial and error: A hierarchical modeling approach to test-retest reliability. NeuroImage 2021, 245, 118647. [Google Scholar] [CrossRef] [PubMed]
- Heitzeg, M.M.; E Hardee, J.; Beltz, A.M. Sex differences in the developmental neuroscience of adolescent substance use risk. Curr. Opin. Behav. Sci. 2018, 23, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.T.; Sanford, B.J.; Meyers, K.K.; Love, T.; E Hazlett, K.; Wang, H.; Ni, L.; Walker, S.J.; Mickey, B.J.; Korycinski, S.T.; et al. Response of the μ-opioid system to social rejection and acceptance. Mol. Psychiatry 2013, 18, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.T.; Sanford, B.J.; Meyers, K.K.; Love, T.; E Hazlett, K.; Walker, S.J.; Mickey, B.J.; A Koeppe, R.; Langenecker, S.; Zubieta, J.-K. It still hurts: Altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol. Psychiatry 2015, 20, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankar, A.; Yttredahl, A.A.; Fourcade, E.W.; Mickey, B.J.; Love, T.M.; Langenecker, S.A.; Hsu, D.T. Dissociable Neural Responses to Monetary and Social Gain and Loss in Women With Major Depressive Disorder. Front. Behav. Neurosci. 2019, 13, 149. [Google Scholar] [CrossRef] [Green Version]
- Yttredahl, A.; McRobert, E.; Sheler, B.; Mickey, B.J.; Love, T.M.; Langenecker, S.; Zubieta, J.-K.; Hsu, D.T. Abnormal emotional and neural responses to romantic rejection and acceptance in depressed women. J. Affect. Disord. 2018, 234, 231–238. [Google Scholar] [CrossRef]
- Sazhin, D.; Frazier, A.M.; Haynes, C.R.; Johnston, C.R.; Chat, I.K.-Y.; Dennison, J.B.; Bart, C.P.; McCloskey, M.E.; Chein, J.M.; Fareri, D.S.; et al. The Role of Social Reward and Corticostriatal Connectivity in Substance Use. J. Psychiatry Brain Sci. 2020, 4, e200024. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarcho, J.M.; Wyngaarden, J.B.; Johnston, C.R.; Quarmley, M.; Smith, D.V.; Cassidy, C.M. Substance Abuse in Emerging Adults: The Role of Neuromelanin and Ventral Striatal Response to Social and Monetary Rewards. Brain Sci. 2022, 12, 352. https://doi.org/10.3390/brainsci12030352
Jarcho JM, Wyngaarden JB, Johnston CR, Quarmley M, Smith DV, Cassidy CM. Substance Abuse in Emerging Adults: The Role of Neuromelanin and Ventral Striatal Response to Social and Monetary Rewards. Brain Sciences. 2022; 12(3):352. https://doi.org/10.3390/brainsci12030352
Chicago/Turabian StyleJarcho, Johanna M., James B. Wyngaarden, Camille R. Johnston, Megan Quarmley, David V. Smith, and Clifford M. Cassidy. 2022. "Substance Abuse in Emerging Adults: The Role of Neuromelanin and Ventral Striatal Response to Social and Monetary Rewards" Brain Sciences 12, no. 3: 352. https://doi.org/10.3390/brainsci12030352





