An Equivocal SCC Lesion—Antiepileptic-Induced CLOCC
Abstract
:1. Introduction
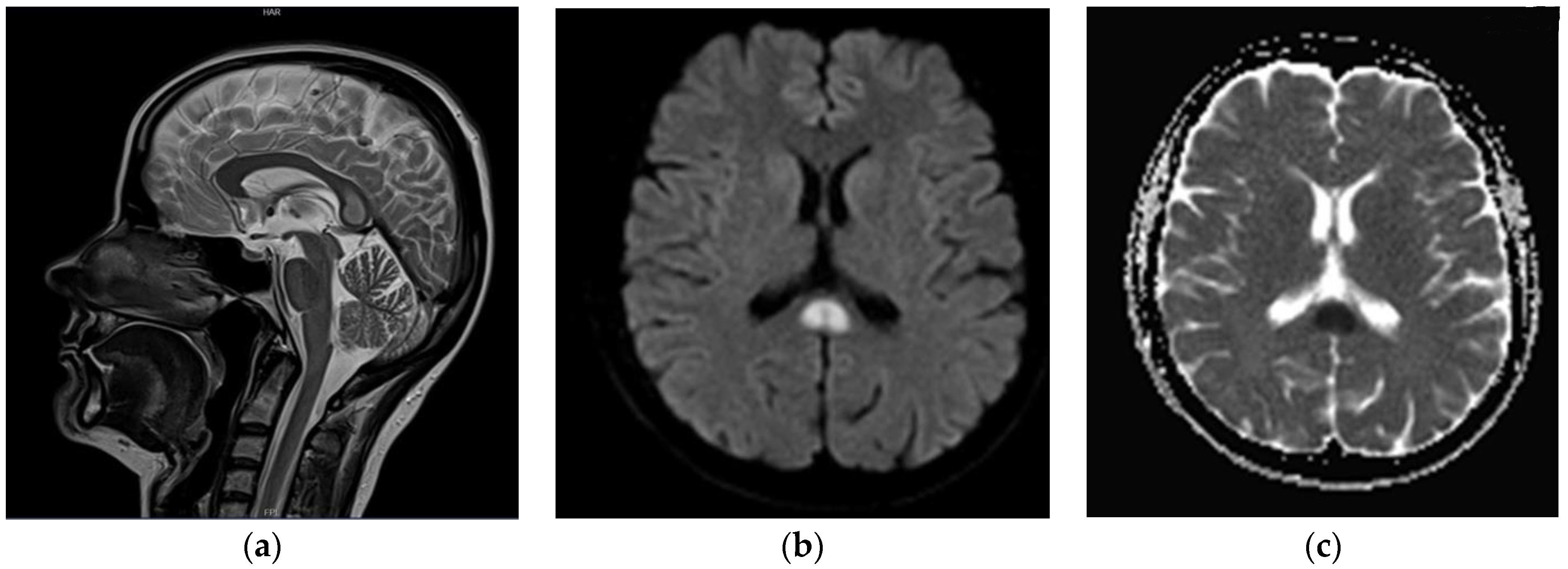
2. Case Presentation
3. Discussion
- −
- demyelinating lesions in the course of multiple sclerosis (MS);
- −
- Marchiavafa-Bignami syndrome—pathomechanism: toxic effect of alcohol, electrolyte and osmotic disturbances, malnutrition and vitamin deficiencies;
- −
- inflammatory involvement;
- −
- neoplastic tumors (including lymphomas);
- −
- Susac syndrome (autoimmune process initiating inflammatory changes and obstruction of cerebral capillaries) [5].
4. Conclusions
- −
- Pathologies involving the corpus callosum include congenital, demyelination, infection, neoplasm, trauma and vascular changes.
- −
- Isolated, non-specific lesions of the SCC usually indicate multiple sclerosis; however, other pathologies such as CLOCC should be considered.
- −
- Anti-epileptic drugs may be the cause of cytotoxic lesions of the corpus callosum.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldstein, A.; Covington, B.P.; Mahabadi, N.; Mesfin, F.B. Neuroanatomy, Corpus Callosum; StatPearls: Columbia, SC, USA, 2021. [Google Scholar]
- Park, S.E.; Choi, D.S.; Shin, H.S.; Baek, H.J.; Choi, H.C.; Kim, J.E.; Choi, H.Y.; Park, M.J. Splenial Lesions of the Corpus Callosum: Disease Spectrum and MRI Findings. Korean J. Radiol. 2017, 18, 710–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, D.; Yang, B.; Yan, J.; Pu, Y.; Zhang, J.; Wen, M.; Yang, Z.; Liu, L. Reversible splenial lesion syndrome (RESLES) coinciding with cerebral venous thrombosis: A report of two cases. Ther. Adv. Neurol. Disord. 2017, 10, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-L.; Hodes, J.F.; Zheng, X.; Hu, X.-Y. Reversible Splenial Lesion Syndrome with Some Novel Causes and Clinical Manifestations. Intern. Med. 2020, 59, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Delev, D.; Cipriani, D.; Neidert, N.; Kellner, E.; Masalha, W.; Mercas, B.; Mader, I.; Reinacher, P.; Weyerbrock, A.; et al. Surgery for IDH1/2 wild-type glioma invading the corpus callosum. Acta Neurochir. 2021, 163, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Yamout, B.; Sahraian, M.; Bohlega, S.; Al-Jumah, M.; Goueider, R.; Dahdaleh, M.; Inshasi, J.; Hashem, S.; Alsharoqi, I.; Khoury, S.; et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult. Scler. Relat. Disord. 2020, 37, 101459. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, D.; Fillard, P.; Paradot, G.; Tadié, M.; Lasjaunias, P.; Ducreux, D. Diffusion Tensor Imaging Characteristics of the Corpus Callosum in Mild, Moderate, and Severe Traumatic Brain Injury. Am. J. Neuroradiol. 2008, 29, 1730–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staszewski, J.; Macek, K.; Stepień, A. Reversible demyelinisation of corpus callosum in the course of Marchiafava-Bignami disease. Neurol. Neurochir. Polska 2006, 40, 156–161. [Google Scholar]
- Fitsiori, A.; Nguyen, D.; Karentzos, A.; Delavelle, J.; I Vargas, M. The corpus callosum: White matter or terra incognita. Br. J. Radiol. 2011, 84, 5–18. [Google Scholar] [CrossRef]
- Zacharia, T.T.; Law, M.; Naidich, T.P.; Leeds, N.E. Central Nervous System Lymphoma Characterization by Diffusion-Weighted Imaging and MR Spectroscopy. J. Neuroimaging 2008, 18, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Galnares-Olalde, J.; Vázquez-Mézquita, A.; Gómez-Garza, G.; Reyes-Vázquez, D.; Higuera-Ortiz, V.; Alegría-Loyola, M.; Mendez-Dominguez, A. Cytotoxic Lesions of the Corpus Callosum Caused by Thermogenic Dietary Supplements. Am. J. Neuroradiol. 2019, 40, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Starkey, J.; Kobayashi, N.; Numaguchi, Y.; Moritani, T. Cytotoxic Lesions of the Corpus Callosum That Show Restricted Diffusion: Mechanisms, Causes, and Manifestations. Radiographics 2017, 37, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Prilipko, O.; Delavelle, J.; Lazeyras, F.; Seeck, M. Reversible Cytotoxic Edema in the Splenium of the Corpus Callosum Related to Antiepileptic Treatment: Report of Two Cases and Literature Review. Epilepsia 2005, 46, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Moreau, A.; Ego, A.; Vandergheynst, F.; Taccone, F.S.; Sadeghi, N.; Montesinos, I.; Gaspard, N.; Gorham, J. Cytotoxic lesions of the corpus callosum (CLOCCs) associated with SARS-CoV-2 infection. J. Neurol. 2021, 268, 1592–1594. [Google Scholar] [CrossRef] [PubMed]


| Value | Normal Range | Units | |
|---|---|---|---|
| Creatinine | 0.6 | 0.6–1.3 | mg/dL |
| eGFR | >=90 | >60 | mL/min/1.73 m2 |
| Folic acid | 18.56 | 1.80–9.00 | ng/mL |
| Borreliosis—IgM a/b | 8.5 (negative) | <18.0 | AU/mL |
| Borreliosis—IgG a/b | <5.0 (negative) | <5.0 | AU/mL |
| Vitamin B12 | 272 | 211–911 | pg/mL |
| D-dimers (G29) | 164 | <500 | ng/mL |
| INR | 1.0 | 0.8–1.2 | - |
| Prothrombin index | 95.6 | 70.0–130.0 | % |
| Prothrombin time | 11.4 | 12.0–16.0 | s |
| Kaolin clotting time | 29.4 | 26.0–40.0 | s |
| Glucose (venous blood, serum) | 95 | 70–99 | mg/dL |
| Serum sodium | 141 | 135–145 | mmol/L |
| Serum potassium | 4.0 | 3.5–5.0 | mmol/L |
| C-reactive protein (CRP)—quantitative | 2.950 | <5.000 | mg/L |
| Serum urea | 23.60 | 15.00–40.00 | mg/dL |
| Leukocytes (WBC) | 6.47 | 3.50–9.00 | ×109/L |
| Erythrocytes (RBC) | 4.19 | 4.20–5.40 | ×1012/L |
| Hemoglobin (HGB) | 12.9 | 11.5–16.0 | g/dL |
| Hematocrit (HCT) | 37.6 | 37.0–47.0 | % |
| Platelets (PLT) | 158 | 130–450 | ×109/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczyńska, M.; Zbroja, M.; Cyranka, W.; Halczuk, I.; Kopyto, E.; Halczuk, I.; Drelich-Zbroja, A. An Equivocal SCC Lesion—Antiepileptic-Induced CLOCC. Brain Sci. 2022, 12, 384. https://doi.org/10.3390/brainsci12030384
Kuczyńska M, Zbroja M, Cyranka W, Halczuk I, Kopyto E, Halczuk I, Drelich-Zbroja A. An Equivocal SCC Lesion—Antiepileptic-Induced CLOCC. Brain Sciences. 2022; 12(3):384. https://doi.org/10.3390/brainsci12030384
Chicago/Turabian StyleKuczyńska, Maryla, Monika Zbroja, Weronika Cyranka, Izabela Halczuk, Ewa Kopyto, Iwona Halczuk, and Anna Drelich-Zbroja. 2022. "An Equivocal SCC Lesion—Antiepileptic-Induced CLOCC" Brain Sciences 12, no. 3: 384. https://doi.org/10.3390/brainsci12030384
APA StyleKuczyńska, M., Zbroja, M., Cyranka, W., Halczuk, I., Kopyto, E., Halczuk, I., & Drelich-Zbroja, A. (2022). An Equivocal SCC Lesion—Antiepileptic-Induced CLOCC. Brain Sciences, 12(3), 384. https://doi.org/10.3390/brainsci12030384






