Distinct Effects of Stimulus Repetition on Various Temporal Stages of Subject’s Own Name Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
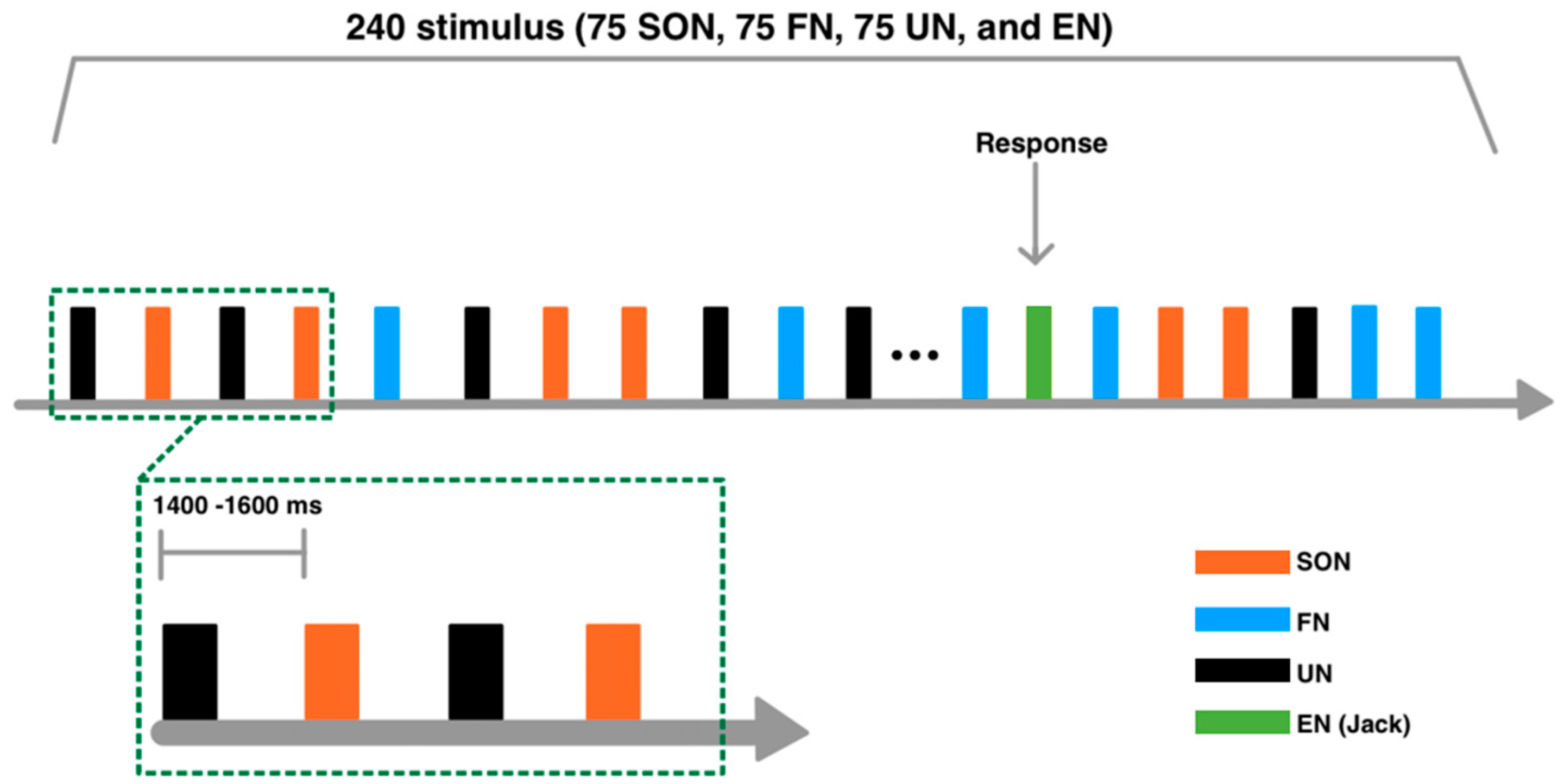
2.2. Stimuli
2.3. Experimental Procedure
2.4. EEG Recordings
2.5. Electroencephalogram Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bortolon, C.; Raffard, S. Self-face advantage over familiar and unfamiliar faces: A three-level meta-analytic approach. Psychon. Bull. Rev. 2018, 25, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, F.; Gu, N.; Gao, X.; Zhao, G. The cognitive advantage for one’s own name is not simply familiarity: An eye-tracking study. Psychon. Bull. Rev. 2013, 20, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Keyes, H.; Brady, N.; Reilly, R.B.; Foxe, J.J. My face or yours? Event-related potential correlates of self-face processing. Brain Cogn. 2010, 72, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Apps, M.A.; Tsakiris, M. The free-energy self: A predictive coding account of self-recognition. Neurosci. Biobehav. Rev. 2014, 41, 85–97. [Google Scholar] [CrossRef]
- Seth, A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013, 17, 565–573. [Google Scholar] [CrossRef]
- Eichenlaub, J.-B.; Ruby, P.; Morlet, D. What is the specificity of the response to the own first-name when presented as a novel in a passive oddball paradigm? An ERP study. Brain Res. 2012, 1447, 65–78. [Google Scholar] [CrossRef]
- Cavinato, M.; Volpato, C.; Silvoni, S.; Sacchetto, M.; Merico, A.; Piccione, F. Event-related brain potential modulation in patients with severe brain damage. Clin. Neurophysiol. 2011, 122, 719–724. [Google Scholar] [CrossRef]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.; Playford, E.D.; Leff, A. Patients with a severe prolonged Disorder of Consciousness can show classical EEG responses to their own name compared with others’ names. NeuroImage Clin. 2018, 19, 311–319. [Google Scholar] [CrossRef]
- Qin, P.; Di, H.; Yan, X.; Yu, S.; Yu, D.; Laureys, S.; Weng, X. Mismatch negativity to the patient’s own name in chronic disorders of consciousness. Neurosci. Lett. 2008, 448, 24–28. [Google Scholar] [CrossRef]
- Blume, C.; del Giudice, R.; Lechinger, J.; Wislowska, M.; Heib, D.P.; Hoedlmoser, K.; Schabus, M. Preferential processing of emotionally and self-relevant stimuli persists in unconscious N2 sleep. Brain Lang. 2017, 167, 72–82. [Google Scholar] [CrossRef]
- Tateuchi, T.; Itoh, K.; Nakada, T. Neural mechanisms underlying the orienting response to subject’s own name: An event-related potential study. Psychophysiology 2012, 49, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Tateuchi, T.; Itoh, K.; Nakada, T. Further characterization of “subject’s own name (SON) negativity”, an ERP component reflecting early preattentive detection of SON. BMC Res. Notes 2015, 8, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, R.P.; Wang, L.A.L.; Guthrie, W.; Cola, M.; Mccleery, J.P.; Pandey, J.; Schultz, R.T.; Miller, J.S. What’s in a name? A preliminary event-related potential study of response to name in preschool children with and without autism spectrum disorder. PLoS ONE 2019, 14, e0216051. [Google Scholar] [CrossRef] [PubMed]
- Holeckova, I.; Fischer, C.; Giard, M.-H.; Delpuech, C.; Morlet, D. Brain responses to a subject’s own name uttered by a familiar voice. Brain Res. 2006, 1082, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Holeckova, I.; Fischer, C.; Morlet, D.; Delpuech, C.; Costes, N.; Mauguière, F. Subject’s own name as a novel in a MMN design: A combined ERP and PET study. Brain Res. 2008, 1189, 152–165. [Google Scholar] [CrossRef]
- Li, R.; Song, W.; Du, J.; Huo, S.; Shan, G. Electrophysiological correlates of processing subject’s own name. NeuroReport 2015, 26, 937–944. [Google Scholar] [CrossRef]
- Fan, W.; Chen, J.; Wang, X.-Y.; Cai, R.; Tan, Q.; Chen, Y.; Yang, Q.; Zhang, S.; Wu, Y.; Yang, Z.; et al. Electrophysiological Correlation of the Degree of Self-Reference Effect. PLoS ONE 2013, 8, e80289. [Google Scholar] [CrossRef]
- Fan, X.; Han, S. Neural responses to one’s own name under mortality threat. Neuropsychologia 2018, 108, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Wu, H.-Y.; Lu, H.-T.; Huang, T.-T.; Zhang, H.; Zhang, T. Assessment of mismatch negativity and P300 response in patients with disorders of consciousness. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4896–4906. [Google Scholar]
- Niu, G.; Yao, L.; Kong, F.; Luo, Y.; Duan, C.; Sun, X.; Zhou, Z. Behavioural and ERP evidence of the self-advantage of online self-relevant information. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Doradzińska, Łucja; Wójcik, M.J.; Paź, M.; Nowicka, M.M.; Nowicka, A.; Bola, M. Unconscious perception of one’s own name modulates amplitude of the P3B ERP component. Neuropsychologia 2020, 147, 107564. [Google Scholar] [CrossRef]
- Tamura, K.; Mizuba, T.; Iramina, K. Hearing subject’s own name induces the late positive component of event-related potential and beta power suppression. Brain Res. 2016, 1635, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, V.; Bradley, M.M.; Codispoti, M.; Lang, P.J. Massed and distributed repetition of natural scenes: Brain potentials and oscillatory activity. Psychophysiology 2015, 52, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, V.; Mastria, S.; Codispoti, M. The interplay between attention and long-term memory in affective habituation. Psychophysiology 2020, 57, e13572. [Google Scholar] [CrossRef] [PubMed]
- Jäncke, L.; Kühnis, J.; Rogenmoser, L.; Elmer, S. Time course of EEG oscillations during repeated listening of a well-known aria. Front. Hum. Neurosci. 2015, 9, 401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engell, A.; McCarthy, G. Repetition suppression of face-selective evoked and induced EEG recorded from human cortex. Hum. Brain Mapp. 2014, 35, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.D.; Dhar, M.; Goris, J.; Brass, M.; Wiersema, J.R. Atypical neural responding to hearing one’s own name in adults with ASD. J. Abnorm. Psychol. 2018, 127, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Martinez, A.; Qu, Z.; Hillyard, S.A. Earliest stages of visual cortical processing are not modified by attentional load. Hum. Brain Mapp. 2013, 35, 3008–3024. [Google Scholar] [CrossRef]
- Tacikowski, P.; Jednoróg, K.; Marchewka, A.; Nowicka, A. How multiple repetitions influence the processing of self-, famous and unknown names and faces: An ERP study. Int. J. Psychophysiol. 2011, 79, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.C.; Macrae, C.N.; Mitchell, J.P. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc. Natl. Acad. Sci. USA 2008, 105, 4507–4512. [Google Scholar] [CrossRef]
- Tomé, D.; Barbosa, F.; Nowak, K.; Marques-Teixeira, J. The development of the N1 and N2 components in auditory oddball paradigms: A systematic review with narrative analysis and suggested normative values. J. Neural Transm. 2014, 122, 375–391. [Google Scholar] [CrossRef]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2007, 45, 152–170. [Google Scholar] [CrossRef]
- Berchicci, M.; Spinelli, D.; Di Russo, F. New insights into old waves. Matching stimulus- and response-locked ERPs on the same time-window. Biol. Psychol. 2016, 117, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.M.; Casanova, M.F.; Casanova, E.; Lamina, E.; Kelly, D.P.; Khachidze, I. Event-related Potentials (ERP) in Cognitive Neuroscience Research and Applications. NeuroRegulation 2017, 4, 14–27. [Google Scholar] [CrossRef]
- Conley, E. The N100 auditory cortical evoked potential indexes scanning of auditory short-term memory. Clin. Neurophysiol. 1999, 110, 2086–2093. [Google Scholar] [CrossRef]
- Qin, P.; Wang, M.; Northoff, G. Linking bodily, environmental and mental states in the self—A three-level model based on a meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 77–95. [Google Scholar] [CrossRef]
- Summerfield, C.; Trittschuh, E.H.; Monti, J.M.; Mesulam, M.-M.; Egner, T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci. 2008, 11, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Key, A.P.; Jones, D.; Peters, S.U. Response to own name in children: ERP study of auditory social information processing. Biol. Psychol. 2016, 119, 210–215. [Google Scholar] [CrossRef]
- Hirata, S.; Matsuda, G.; Ueno, A.; Fuwa, K.; Sugama, K.; Kusunoki, K.; Fukushima, H.; Hiraki, K.; Tomonaga, M.; Hasegawa, T. Event-Related Potentials in Response to Subjects’ Own Names: A Comparison between Humans and a Chimpanzee. Commun. Integr. Biol. 2011, 4, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Long, Q.; Li, X.; Yang, J.; Li, H.; Yuan, J.; Long, Q. Self-relevant processing of stranger’s name in Chinese society: Surname matters. Neurosci. Lett. 2018, 668, 126–132. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xie, M.; Wang, Y.; Qin, P. Distinct Effects of Stimulus Repetition on Various Temporal Stages of Subject’s Own Name Processing. Brain Sci. 2022, 12, 411. https://doi.org/10.3390/brainsci12030411
Zhang Y, Xie M, Wang Y, Qin P. Distinct Effects of Stimulus Repetition on Various Temporal Stages of Subject’s Own Name Processing. Brain Sciences. 2022; 12(3):411. https://doi.org/10.3390/brainsci12030411
Chicago/Turabian StyleZhang, Yihui, Musi Xie, Yuzhi Wang, and Pengmin Qin. 2022. "Distinct Effects of Stimulus Repetition on Various Temporal Stages of Subject’s Own Name Processing" Brain Sciences 12, no. 3: 411. https://doi.org/10.3390/brainsci12030411
APA StyleZhang, Y., Xie, M., Wang, Y., & Qin, P. (2022). Distinct Effects of Stimulus Repetition on Various Temporal Stages of Subject’s Own Name Processing. Brain Sciences, 12(3), 411. https://doi.org/10.3390/brainsci12030411






