Time Course of Motor Sleep Inertia Dissipation According to Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials and Procedure
2.3. Statistical Analyses
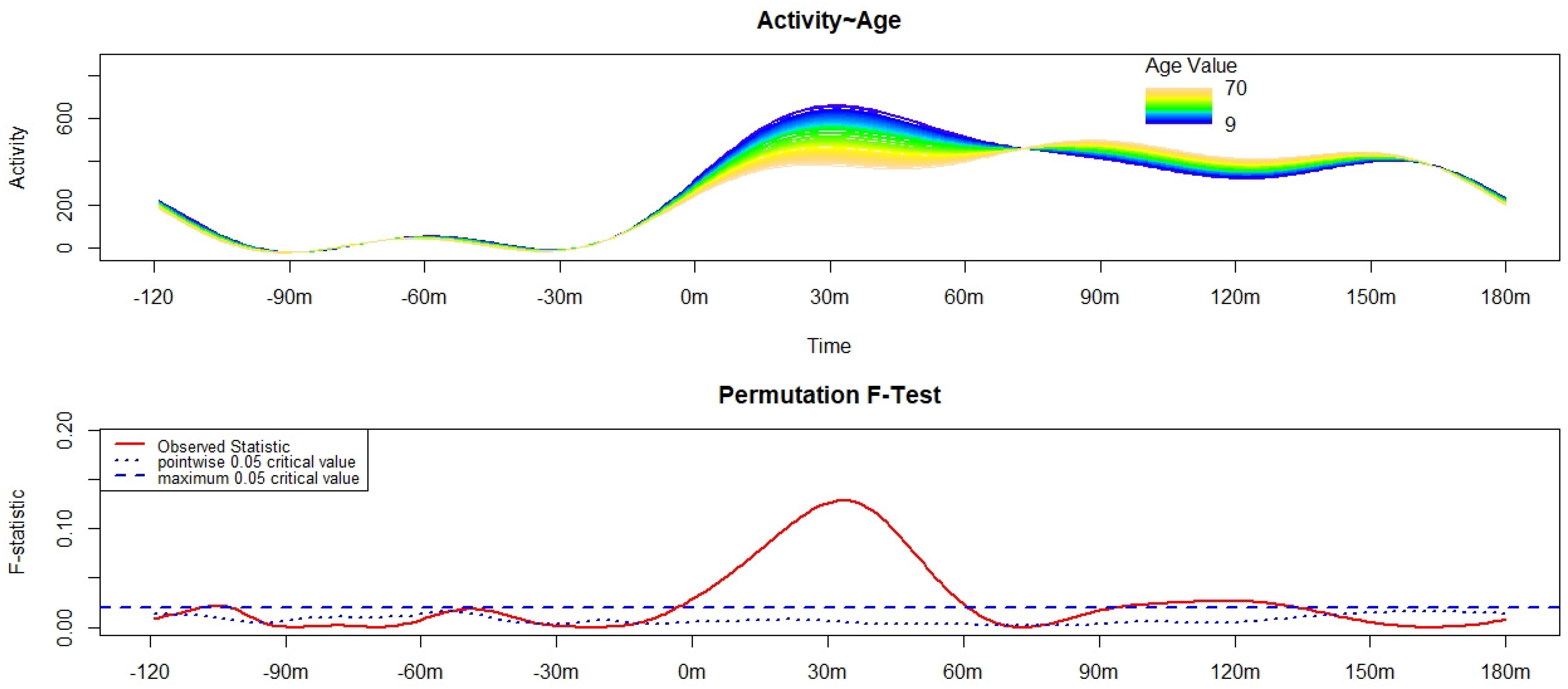
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tassi, P.; Muzet, A. Sleep Inertia. Sleep Med. Rev. 2000, 4, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Trotti, L.M. Waking up Is the Hardest Thing I Do All Day: Sleep Inertia and Sleep Drunkenness. Sleep Med. Rev. 2017, 35, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Mchill, A.; Hull, J.; Czeisler, C.; Klerman, E. The Effect of Chronic Sleep Restriction and Prior Sleep Duration on Sleep Inertia Measured Using Cognitive Performance. Sleep Med. 2017, 40, e163. [Google Scholar] [CrossRef]
- Marzano, C.; Ferrara, M.; Moroni, F.; De Gennaro, L. Electroencephalographic Sleep Inertia of the Awakening Brain. Neuroscience 2011, 176, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Occhionero, M.; Fabbri, M.; Tonetti, L.; Martoni, M.; Natale, V. Time Course of Sleep Inertia Dissipation in Memory Tasks. Appl. Sci. 2021, 11, 3354. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, X.-Y.; Wang, D.; Zhu, Z.; Niu, H.; Huang, S.; Zhou, X.; Yang, Z.; Fan, F. Who Is the Hardest to Wake up from Sleep? An Investigation of Self-Reported Sleep Inertia Using a Latent Profile Analysis. J. Sleep Res. 2022, e13552. [Google Scholar] [CrossRef]
- Wertz, A.T.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P. Effects of Sleep Inertia on Cognition. JAMA 2006, 295, 163–164. [Google Scholar] [CrossRef]
- Peter-Derex, L.; Magnin, M.; Bastuji, H. Heterogeneity of Arousals in Human Sleep: A Stereo-Electroencephalographic Study. NeuroImage 2015, 123, 229–244. [Google Scholar] [CrossRef]
- Hilditch, C.J.; McHill, A.W. Sleep Inertia: Current Insights. Nat. Sci. Sleep 2019, 11, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Akerstedt, T.; Folkard, S. Validation of the S and C Components of the Three-Process Model of Alertness Regulation. Sleep 1995, 18, 1–6. [Google Scholar] [CrossRef]
- Nehlig, A. Are we dependent upon cofee and cafeine? A review on human and animal data. Neurosci. Biobehav. Rev. 1999, 23, 563–576. [Google Scholar] [CrossRef]
- Van Dongen, H.P.; Price, N.J.; Mullington, J.M.; Szuba, M.P.; Kapoor, S.C.; Dinges, D.F. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep 2001, 24, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Dornbierer, D.A.; Yerlikaya, F.; Wespi, R.; Boxler, M.I.; Voegel, C.D.; Schnider, L.; Arslan, A.; Baur, D.M.; Baumgartner, M.R.; Binz, T.M.; et al. A novel bedtime pulsatile-release caffeine formula ameliorates sleep inertia symptoms immediately upon awakening. Sci. Rep. 2021, 11, 19734. [Google Scholar] [CrossRef] [PubMed]
- Krauchi, K.; Cajochen, C.; Wirz-Justice, A. Waking up properly: Is there a role of thermoregulation in sleep inertia? J. Sleep Res. 2004, 13, 121–127. [Google Scholar] [CrossRef]
- Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [Green Version]
- Mignot, E. Why We Sleep: The Temporal Organization of Recovery. PLoS Biol. 2008, 6, e106. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hsu, C.-F.; Xu, D.; Yu, J.; Lei, X. Loss of Frontal Regulator of Vigilance during Sleep Inertia: A Simultaneous EEG-FMRI Study. Hum. Brain Mapp. 2020, 41, 4288–4298. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; Chen, S.C.J.; Hsu, C.Y.; Wu, C.W.; Wu, Y.C.; Hung, C.S.; Yang, A.C.; Liu, P.Y.; Biswal, B.; Lin, C.P. Local Awakening: Regional Reorganizations of Brain Oscillations after Sleep. NeuroImage 2014, 102, 894–903. [Google Scholar] [CrossRef]
- Elder, G.J.; Wetherell, M.A.; Barclay, N.L.; Ellis, J.G. The Cortisol Awakening Response—Applications and Implications for Sleep Medicine. Sleep Med. Rev. 2014, 18, 215–224. [Google Scholar] [CrossRef]
- Lemola, S.; Perkinson-Gloor, N.; Arx, P.H.; Brand, S.; Holsboer-trachsler, E.; Grob, A.; Weber, P. Morning Cortisol Secretion in School-Age Children Is Related to the Sleep Pattern of the Preceding Night. Psychoneuroendocrinology 2015, 52, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, F.; van Heemst, D.; Iranmanesh, A.; Takahashi, P.; Yang, R.; Veldhuis, J.D. Impact of Age, Sex and Body Mass Index on Cortisol Secretion in 143 Healthy Adults. Endocr. Connect. 2017, 6, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martoni, M.; Carissimi, A.; Fabbri, M.; Filardi, M.; Tonetti, L.; Natale, V. 24-h Actigraphic Monitoring of Motor Activity, Sleeping and Eating Behaviors in Underweight, Normal Weight, Overweight and Obese Children. Eat. Weight Disord. EWD 2016, 21, 669–677. [Google Scholar] [CrossRef]
- Natale, V.; Lehnkering, H.; Siegmund, R. Handedness and Circadian Motor Asymmetries in Humans: Preliminary Findings. Physiol. Behav. 2010, 100, 322–326. [Google Scholar] [CrossRef]
- Natale, V.; Léger, D.; Martoni, M.; Bayon, V.; Erbacci, A. The Role of Actigraphy in the Assessment of Primary Insomnia: A Retrospective Study. Sleep Med. 2014, 15, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, L. Validity of the Morningness-Eveningness Questionnaire for Adolescents (MEQ-A). Sleep Hypn. 2007, 9, 47–51. [Google Scholar]
- Tonetti, L.; Erbacci, A.; Fabbri, M.; Martoni, M.; Natale, V. Effects of Transitions into and out of Daylight Saving Time on the Quality of the Sleep/Wake Cycle: An Actigraphic Study in Healthy University Students. Chronobiol. Int. 2013, 30, 1218–1222. [Google Scholar] [CrossRef]
- Tonetti, L.; Fabbri, M.; Erbacci, A.; Filardi, M.; Martoni, M.; Natale, V. Effects of Dawn Simulation on Attentional Performance in Adolescents. Eur. J. Appl. Physiol. 2015, 115, 579–587. [Google Scholar] [CrossRef]
- Occhionero, M.; Tonetti, L.; Fabbri, M.; Boreggiani, M.; Martoni, M.; Giovagnoli, S.; Natale, V. Prospective Memory, Sleep, and Age. Brain Sci. 2020, 10, 422. [Google Scholar] [CrossRef]
- Natale, V.; Plazzi, G.; Martoni, M. Actigraphy in the Assessment of Insomnia: A Quantitative Approach. Sleep 2009, 32, 767–771. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xian, H.; Licis, A.; Deych, E.; Ding, J.; McLeland, J.; Toedebusch, C.; Li, T.; Duntley, S.; Shannon, W. Measuring the Impact of Apnea and Obesity on Circadian Activity Patterns Using Functional Linear Modeling of Actigraphy Data. J. Circadian Rhythms 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-Analysis of Quantitative Sleep Parameters from Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values across the Human Lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social Jetlag and Obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliandro, R.; Streng, A.A.; van Kerkhof, L.W.M.; van der Horst, G.T.J.; Chaves, I. Social Jetlag and Related Risks for Human Health: A Timely Review. Nutrients 2021, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- Kolomeichuk, S.N.; Randler, C.; Morozov, A.V.; Gubin, D.G.; Drake, C.L. Social Jetlag and Excessive Daytime Sleepiness from a Sample of Russian Children and Adolescents. Nat. Sci. Sleep 2021, 13, 729–737. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonetti, L.; Fabbri, M.; Giovagnoli, S.; Martoni, M.; Occhionero, M.; Natale, V. Time Course of Motor Sleep Inertia Dissipation According to Age. Brain Sci. 2022, 12, 424. https://doi.org/10.3390/brainsci12040424
Tonetti L, Fabbri M, Giovagnoli S, Martoni M, Occhionero M, Natale V. Time Course of Motor Sleep Inertia Dissipation According to Age. Brain Sciences. 2022; 12(4):424. https://doi.org/10.3390/brainsci12040424
Chicago/Turabian StyleTonetti, Lorenzo, Marco Fabbri, Sara Giovagnoli, Monica Martoni, Miranda Occhionero, and Vincenzo Natale. 2022. "Time Course of Motor Sleep Inertia Dissipation According to Age" Brain Sciences 12, no. 4: 424. https://doi.org/10.3390/brainsci12040424








