“The Heart Asks Pleasure First”—Conceptualizing Psychiatric Diseases as MAINTENANCE Network Dysfunctions through Insights from slMFB DBS in Depression and Obsessive–Compulsive Disorder
Abstract
- The Heart asks Pleasure first,
- And then Excuse from Pain,
- And then those little Anodynes,
- That deaden suffering;
- And then to go to sleep
- And then, if it should be
- The will of its Inquisitor
- The liberty to die.
1. Introduction
2. Methods
3. Results
3.1. Literature Review
3.2. Anecdotal Observations from DBS Surgery
4. Discussion
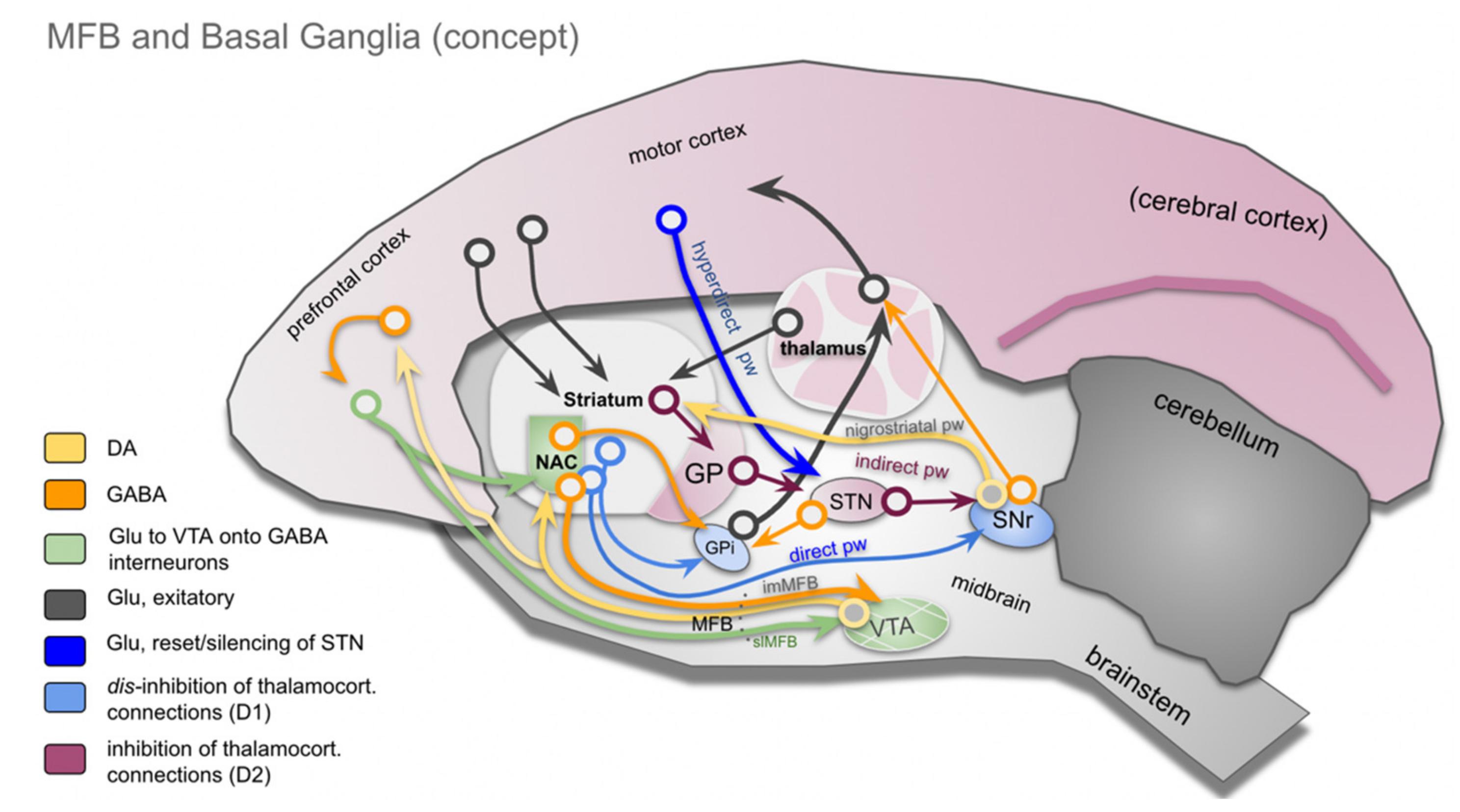
4.1. Basal Ganglia, the (Human) MFB and the Role of DA
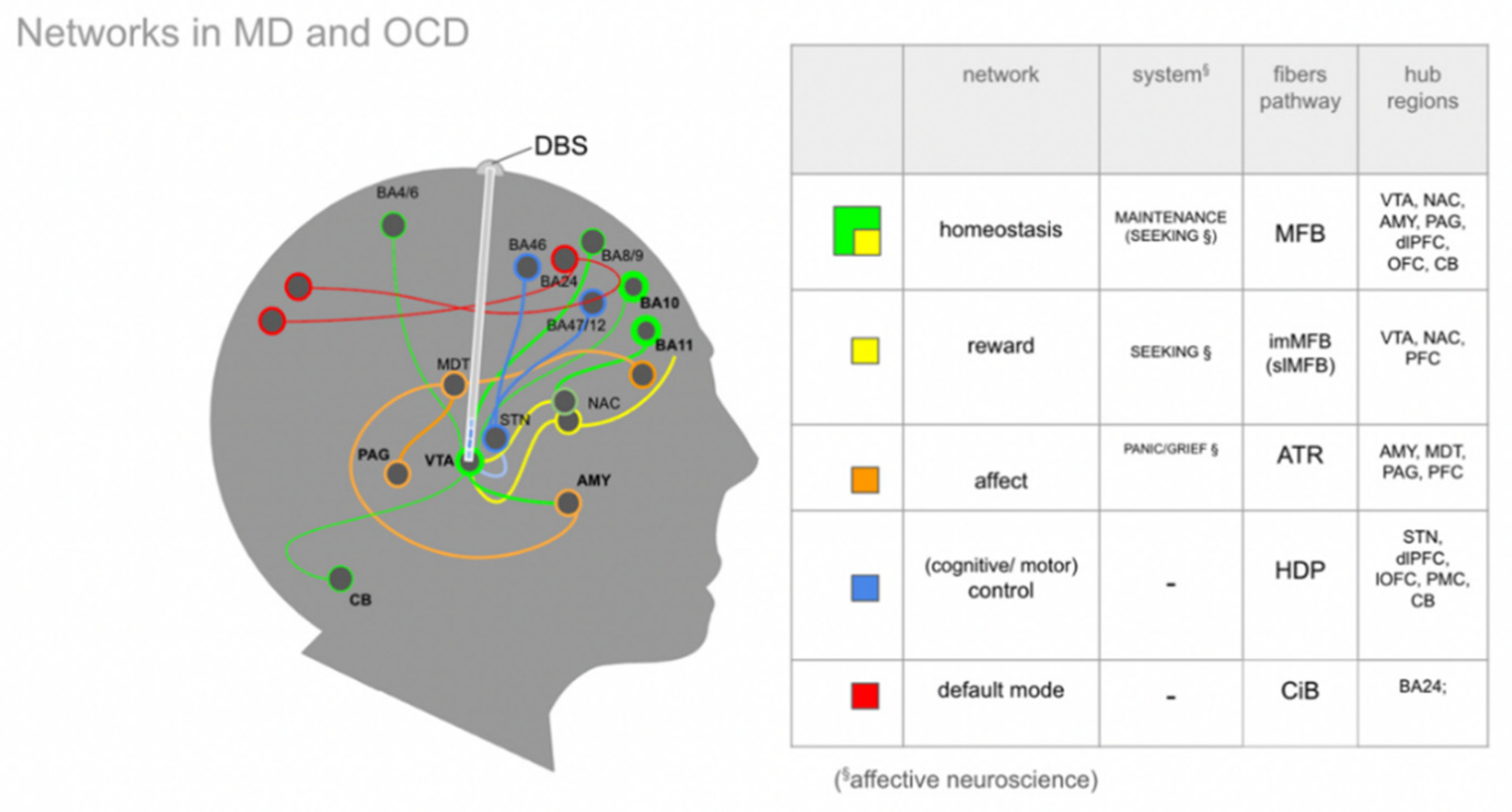
4.2. Brain Wide Networks Relevant for Depression and OCD
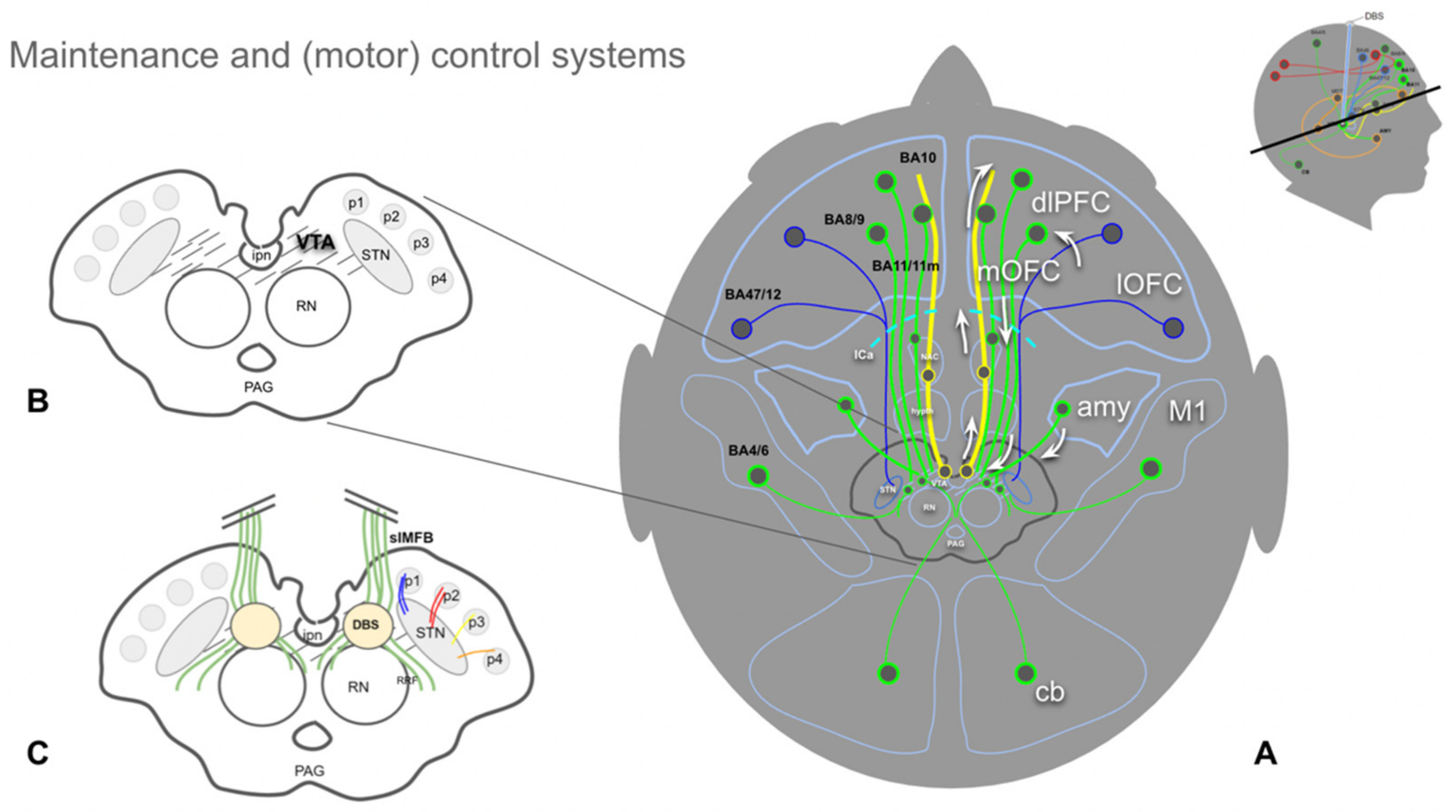
4.3. The Human Medial Forebrain Bundle (MFB)
4.4. Clinical Responses to Stimulation and Implantation (slMFB DBS) and the Role of Dopamine
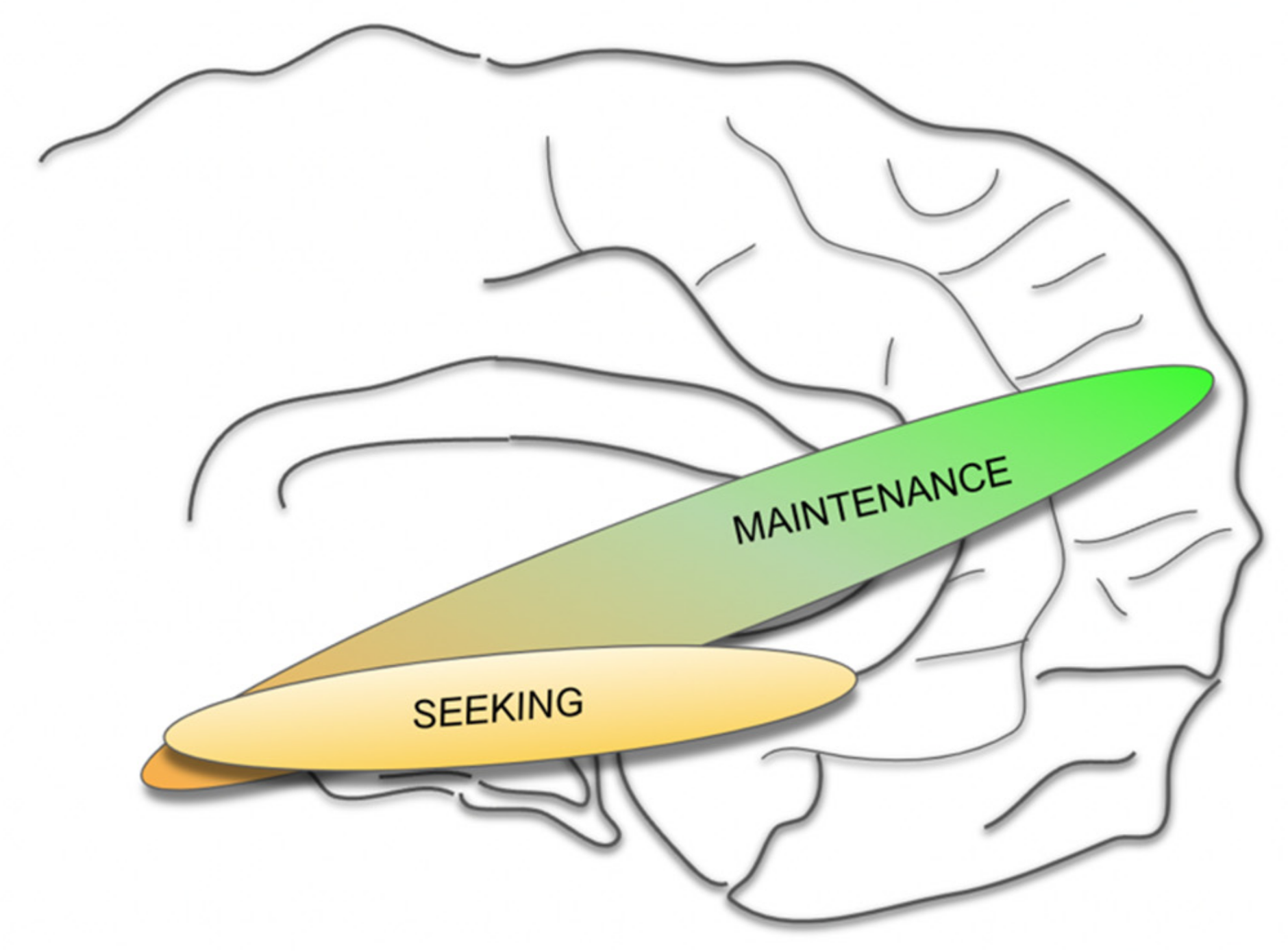
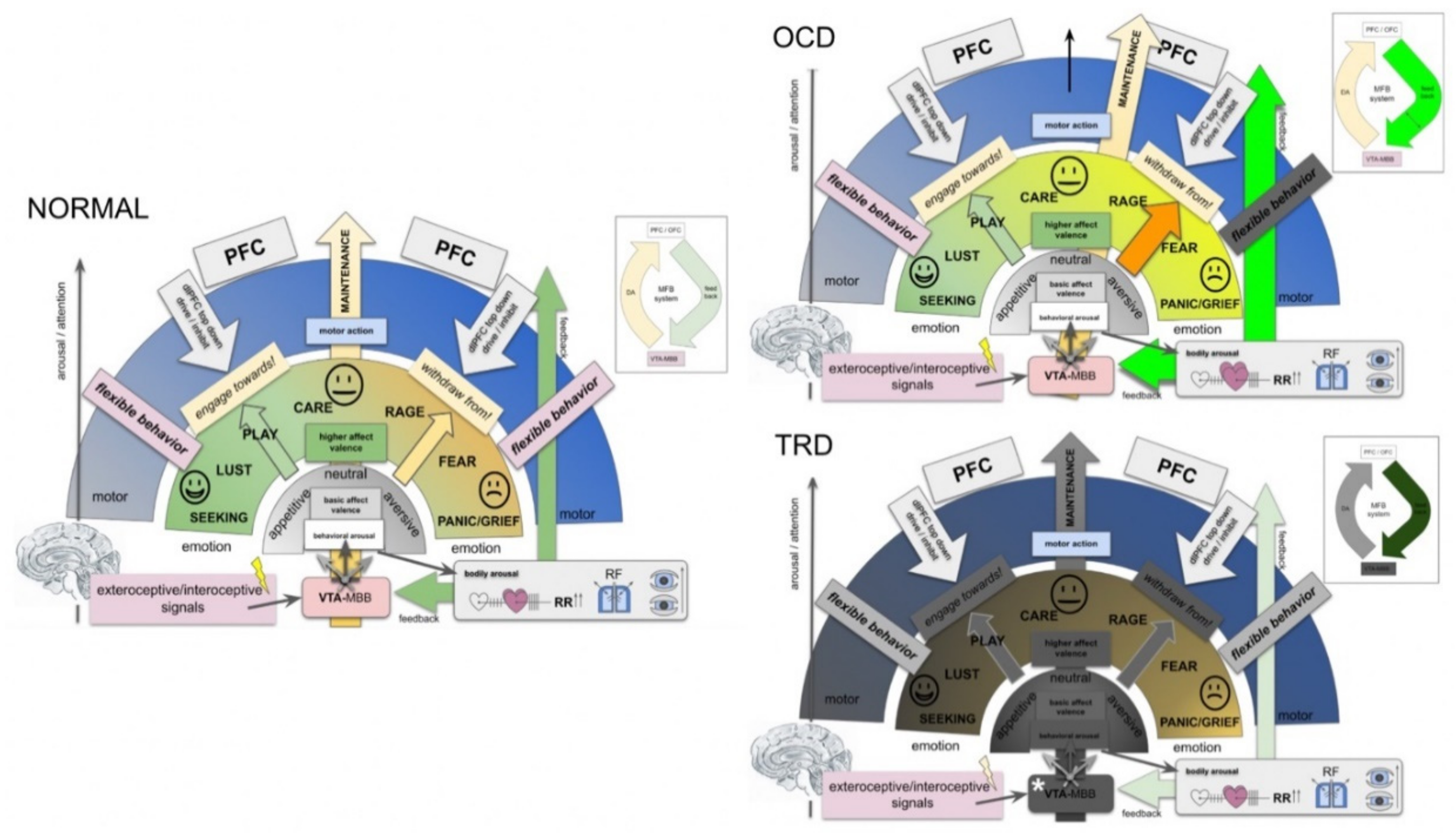
4.5. A MAINTENANCE System and Its Proposed Functions
- In the context of this work, emotions are interpreted as information classifiers, allowing the rapid interpretation of complex interoceptive and exteroceptive stimuli: affect valence.
- MAINTENANCE serves to interpret the most basic “affective modes”: appetence vs. aversiveness vs. neutrality (bluntness).
- As such, the greater MFB does not comprise a “module” but acts in different modes since it valences aversive vs. neutral vs. appetitive signaling [89].
- MAINTENANCE equally interprets appetitive and aversive interoceptive and exteroceptive signals (also with a projection of anticipated consequences in the future) and equips these signals with a very basal “emotional color” (do engage, do not engage).
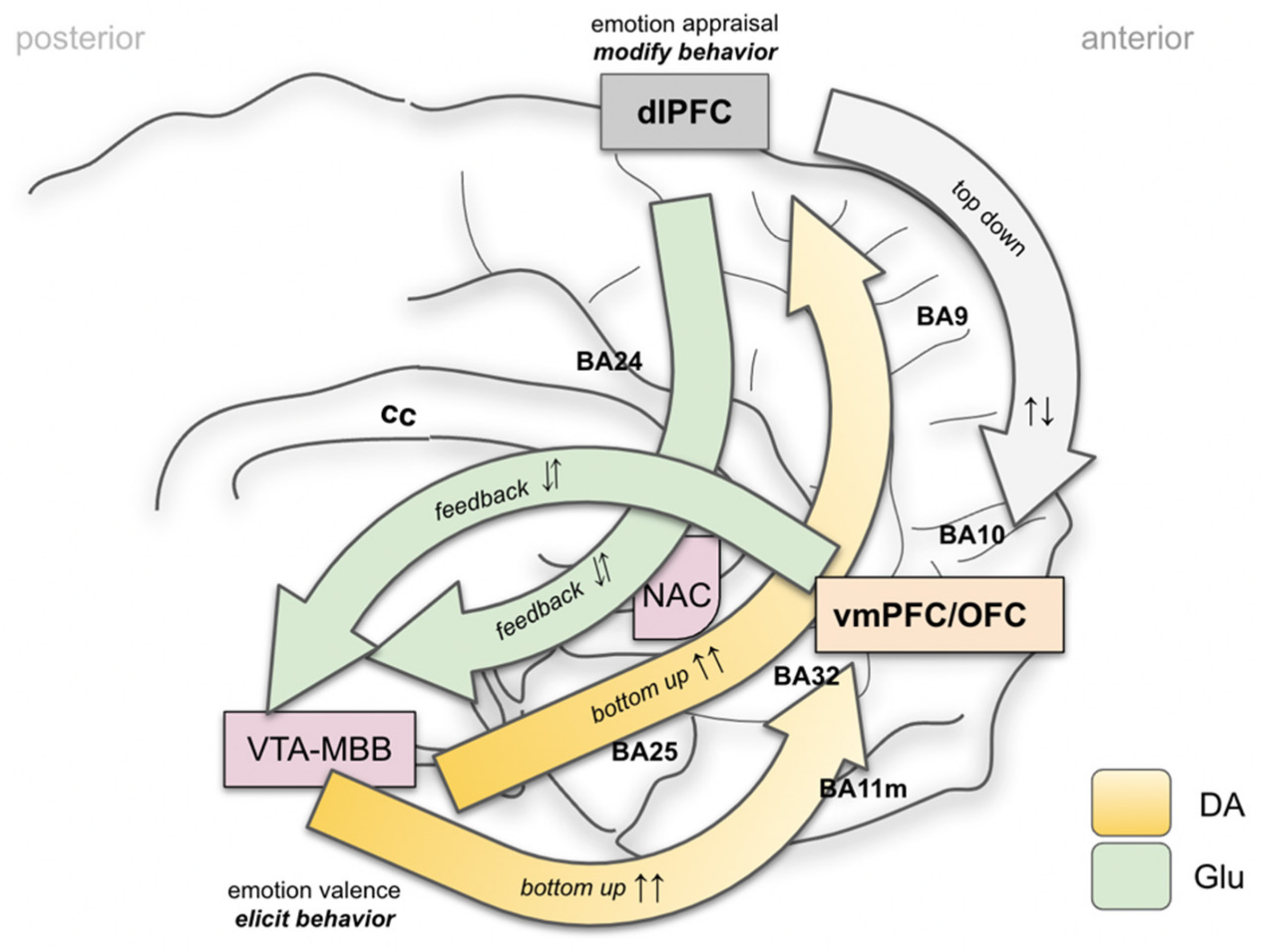
- In humans, and a highly developed prefrontal cortex, this interpretation needs to be reconciled with memorized experience, indicating an involvement of the Papez circuit and, therefore, of the mammillary bodies (VTA–MB complex) and interconnection at distinct evolutionary and anatomical levels (Figure 4 and Figure 7).
- Emotional signaling and the interpretation of this signal in the MAINTENANCE system leads to motor responses (engage ⇆ stop ⇆ withdraw) if the top-down emotion appraisal allows (Figure 6).
- The MAINTENANCE system induces an overall and behavioral arousal and
- sets autonomous bodily conditions to allow arousal accompanied by a motor response (heart rate, blood pressure, breathing). These settings are performed subconsciously and even before an emotional feeling is perceived. Price, in this respect, speaks from the “visceromotor network” [90].
- In a further higher emotional valence, distinct emotions are categorized (e.g., Panksepp’s seven emotional systems).
- Depending on the result of this higher valence, a specific motor program is executed.
- At each step of the evaluation, a top-down emotion appraisal (Figure 6) can be performed in cases where conscious focus is brought to the process.
- OCD: intrusive thoughts (from dlPFC) enter the MAINTENANCE system (VTA) and are attributed as aversive (ego-dystonic). A resulting motor program is then phenotypically expressed as (meaningless) compulsive action. High activity (Figure 7, OCD).
- Comorbid depression? The potential overload of the MAINTENANCE system, which only deals with the interpretation of obsessions, and therewith, blocks behavior which serves the organisms’ homeostasis.
- TRD: a dysfunction of the MAINTENANCE system with a loss of interoceptive and exteroceptive signal evaluation (Figure 7, TRD).
- Degradation.
- Potentially a (neurodegenerative?) loss of Dopamine transmission out of the VTA. As a result, no involuntary (bottom-up) motor program is formed, which leads to reduced overall activity.
- With increasing dysfunction in bottom-up emotion valence, decision making for motor programing has to be increasingly solved via the top-down system,
- which in turn occupies part of the cognitive reserve. Increased cognitive decision making thus is exhausting and might lead to decreased cognitive performance.
5. Future Perspective for slMFB DBS
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Groenewold, N.A.; Opmeer, E.M.; de Jonge, P.; Aleman, A.; Costafreda, S.G. Emotional Valence Modulates Brain Functional Abnormalities in Depression: Evidence from a Meta-Analysis of FMRI Studies. Neurosci. Biobehav. Rev. 2013, 37, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, T.E.; Bewernick, B.H.; Kayser, S.; Mädler, B.; Coenen, V.A. Rapid Effects of Deep Brain Stimulation for Treatment-Resistant Major Depression. Biol. Psychiatry 2013, 73, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, A.J.; Schulz, P.E.; Selvaraj, S.; Burrows, C.L.; Zunta-Soares, G.; Durkin, K.; Zanotti-Fregonara, P.; Quevedo, J.; Soares, J.C. A Longitudinal Study on Deep Brain Stimulation of the Medial Forebrain Bundle for Treatment-Resistant Depression. Trans. Psychiatry 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Coenen, V.A.; Schlaepfer, T.E.; Goll, P.; Reinacher, P.C.; Voderholzer, U.; Van Elst, L.T.; Urbach, H.; Freyer, T. The Medial Forebrain Bundle as a Target for Deep Brain Stimulation for Obsessive-Compulsive Disorder. CNS Spectr. 2016, 493, 282–289. [Google Scholar] [CrossRef]
- Coenen, V.A.; Honey, C.R.; Hurwitz, T.; Rahman, A.A.; McMaster, J.; Bürgel, U.; Mädler, B. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson′s disease. Neurosurgery 2009, 64, 1106–1115. [Google Scholar] [CrossRef]
- Coenen, V.A.; Schlaepfer, T.E.; Maedler, B.; Panksepp, J. Cross-Species Affective Functions of the Medial Forebrain Bundle-Implications for the Treatment of Affective Pain and Depression in Humans. Neurosci. Biobehav. Rev. 2011, 35, 1971–1981. [Google Scholar] [CrossRef]
- Alcaro, A.; Huber, R.; Panksepp, J. Behavioral Functions of the Mesolimbic Dopaminergic System: An Affective Neuroethological Perspective. Brain Res. Rev. 2007, 56, 283–321. [Google Scholar] [CrossRef]
- Alcaro, A.; Panksepp, J.; Witczak, J.; Hayes, D.J.; Northoff, G. Is Subcortical-Cortical Midline Activity in Depression Mediated by Glutamate and GABA? A Cross-Species Translational Approach. Neurosci. Biobehav. Rev. 2010, 34, 592–605. [Google Scholar] [CrossRef]
- Panksepp, J. The Basic Emotional Circuits of Mammalian Brains: Do Animals Have Affective Lives? Neurosci. Biobehav. Rev. 2011, 35, 1791–1804. [Google Scholar] [CrossRef]
- Coenen, V.A.; Döbrössy, M.D.; Teo, S.J.; Wessolleck, J.; Sajonz, B.E.A.; Reinacher, P.C.; Thierauf-Emberger, A.; Spittau, B.; Leupold, J.; von Elverfeldt, D.; et al. Diverging Prefrontal Cortex Fiber Connection Routes to the Subthalamic Nucleus and the Mesencephalic Ventral Tegmentum Investigated with Long Range (Normative) and Short Range (Ex-Vivo High Resolution) 7T DTI. Brain Struct. Funct. 2021, 227, 23–47. [Google Scholar] [CrossRef]
- Fenoy, A.J.; Schulz, P.; Selvaraj, S.; Burrows, C.; Spiker, D.; Cao, B.; Zunta-Soares, G.; Gajwani, P.; Quevedo, J.; Soares, J. Deep Brain Stimulation of the Medial Forebrain Bundle: Distinctive Responses in Resistant Depression. J. Affect. Disord. 2016, 203, 143–151. [Google Scholar] [CrossRef]
- Coenen, V.A.; Bewernick, B.H.; Kayser, S.; Kilian, H.; Boström, J.; Greschus, S.; Hurlemann, R.; Klein, M.E.; Spanier, S.; Sajonz, B.; et al. Superolateral Medial Forebrain Bundle Deep Brain Stimulation in Major Depression: A Gateway Trial. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 26, 587. [Google Scholar] [CrossRef]
- Kisely, S.; Li, A.; Warren, N.; Siskind, D. A Systematic Review and Meta-Analysis of Deep Brain Stimulation for Depression. Depress. Anxiety 2018, 35, 468–480. [Google Scholar] [CrossRef]
- Dobrossy, M.D.; Ramanathan, C.; Vajari, D.A.A.; Tong, Y.; Schlaepfer, T.; Coenen, V.A. Neuromodulation in Psychiatric Disorders: Experimental and Clinical Evidence for Reward and Motivation Network Deep Brain Stimulation—Focus on the Medial Forebrain Bundle. Eur. J. Neurosci. 2020, 53, 89–113. [Google Scholar] [CrossRef]
- Fenoy, A.J.; Quevedo, J.; Soares, J.C. Deep Brain Stimulation of the “Medial Forebrain Bundle”: A Strategy to Modulate the Reward System and Manage Treatment-Resistant Depression. Mol. Psychiatr. 2021, 1–19. [Google Scholar] [CrossRef]
- Schlaepfer, T.E.; Bewernick, B.H.; Kayser, S.; Hurlemann, R.; Coenen, V.A. Deep Brain Stimulation of the Human Reward System for Major Depression—Rationale, Outcomes and Outlook. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014, 39, 1303–1314. [Google Scholar] [CrossRef]
- Döbrössy, M.D.; Furlanetti, L.L.; Coenen, V.A. Electrical Stimulation of the Medial Forebrain Bundle in Pre-Clinical Studies of Psychiatric Disorders. Neurosci. Biobehav. Rev. 2015, 49, 32–42. [Google Scholar] [CrossRef]
- Coenen, V.A.; Schumacher, L.V.; Kaller, C.; Schlaepfer, T.E.; Reinacher, P.C.; Egger, K.; Urbach, H.; Reisert, M. The Anatomy of the Human Medial Forebrain Bundle—Ventral Tegmental Area Connections to Reward-Associated Subcortical and Frontal Lobe Regions. NeuroImage Clin. 2018, 18, 770–783. [Google Scholar] [CrossRef]
- Coenen, V.A.; Schlaepfer, T.E.; Sajonz, B.; Döbrössy, M.; Kaller, C.P.; Urbach, H.; Reisert, M. Tractographic Description of Major Subcortical Projection Pathways Passing the Anterior Limb of the Internal Capsule. Corticopetal Organization of Networks Relevant for Psychiatric Disorders. NeuroImage Clin. 2020, 25, 102165. [Google Scholar] [CrossRef]
- Vajari, D.A.; Ramanathan, C.; Tong, Y.; Stieglitz, T.; Coenen, V.A.; Döbrössy, M.D. Medial Forebrain Bundle DBS Differentially Modulates Dopamine Release in the Nucleus Accumbens in a Rodent Model of Depression. Exp. Neurol. 2020, 327, 113224. [Google Scholar] [CrossRef]
- Ikemoto, S. Brain Reward Circuitry beyond the Mesolimbic Dopamine System: A Neurobiological Theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef]
- Coenen, V.A.; Panksepp, J.; Hurwitz, T.A.; Urbach, H.; Mädler, B. Human Medial Forebrain Bundle (MFB) and Anterior Thalamic Radiation (ATR): Imaging of Two Major Subcortical Pathways and the Dynamic Balance of Opposite Affects in Understanding Depression. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 223–236. [Google Scholar] [CrossRef]
- Dranias, M.R.; Grossberg, S.; Bullock, D. Dopaminergic and Non-Dopaminergic Value Systems in Conditioning and Outcome-Specific Revaluation. Brain Res. 2008, 1238, 239–287. [Google Scholar] [CrossRef]
- Hong, S. Dopamine System: Manager of Neural Pathways. Front. Hum. Neurosci. 2013, 7, 854. [Google Scholar] [CrossRef]
- Coenen, V.A.; Sajonz, B.; Reisert, M.; Bostroem, J.; Bewernick, B.; Urbach, H.; Jenkner, C.; Reinacher, P.C.; Schlaepfer, T.E.; Mädler, B. Tractography-Assisted Deep Brain Stimulation of the Superolateral Branch of the Medial Forebrain Bundle (SlMFB DBS) in Major Depression. NeuroImage Clin. 2018, 20, 580–593. [Google Scholar] [CrossRef]
- Kleef, G.A.V. How Emotions Regulate Social Life. Curr. Dir. Psychol. Sci. 2009, 18, 184–188. [Google Scholar] [CrossRef]
- Panksepp, J. Affective Neuroscience; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Davidson, B.; Giacobbe, P.; Mithani, K.; Levitt, A.; Rabin, J.S.; Lipsman, N.; Hamani, C. Lack of Clinical Response to Deep Brain Stimulation of the Medial Forebrain Bundle in Depression. Brain Stimul. 2020, 13, 1268–1270. [Google Scholar] [CrossRef]
- Oldani, L.; Benatti, B.; Macellaro, M.; Porta, M.; Servello, D.; Zekaj, E.; Dell′Osso, B. A Case of Treatment-Resistant Bipolar Depression and Comorbid OCD Treated with Deep Brain Stimulation of the Medial Forebrain Bundle: 5 Years Follow-up Results. J. Clin. Neurosci. 2021, 89, 103–105. [Google Scholar] [CrossRef]
- Bewernick, B.H.; Kayser, S.; Gippert, S.M. Acute Antidepressant Effects of Deep Brain Stimulation–Review and Data from SlMFB-Stimulation. Med. Psychiatry 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Bewernick, B.H.; Kayser, S.; Gippert, S.M.; Switala, C.; Coenen, V.A.; Schlaepfer, T.E. Deep Brain Stimulation to the Medial Forebrain Bundle for Depression- Long-Term Outcomes and a Novel Data Analysis Strategy. Brain Stimul. 2017, 10, 664–671. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Striatal Plasticity and Basal Ganglia Circuit Function. Neuron 2008, 60, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Picconi, B.; Tozzi, A.; Ghiglieri, V.; Filippo, M.D. Direct and Indirect Pathways of Basal Ganglia: A Critical Reappraisal. Nat. Neurosci. 2014, 17, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Borgognon, S.; Cottet, J.; Badoud, S.; Bloch, J.; Brunet, J.-F.; Rouiller, E.M. Cortical Projection from the Premotor or Primary Motor Cortex to the Subthalamic Nucleus in Intact and Parkinsonian Adult Macaque Monkeys: A Pilot Tracing Study. Front. Neural Circuits 2020, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef]
- Mega, M.S.; Cummings, J.L. Frontal-Subcortical Circuits and Neuropsychiatric Disorders. J. Neuropsychiatry Clin. Neurosci. 1994, 6, 358–370. [Google Scholar] [CrossRef]
- Macpherson, T.; Hikida, T. Role of Basal Ganglia Neurocircuitry in the Pathology of Psychiatric Disorders. Psychiatry Clin. Neurosci. 2019, 73, 289–301. [Google Scholar] [CrossRef]
- Temel, Y.; Blokland, A.; Steinbusch, H.W.M.; Visser-Vandewalle, V. The Functional Role of the Subthalamic Nucleus in Cognitive and Limbic Circuits. Prog. Neurobiol. 2005, 76, 393–413. [Google Scholar] [CrossRef]
- Nambu, A.; Tokuno, H.; Takada, M. Functional Significance of the Cortico-Subthalamo-Pallidal “hyperdirect” Pathway. Neurosci. Res. 2002, 43, 111–117. [Google Scholar] [CrossRef]
- Cox, J.; Witten, I.B. Striatal Circuits for Reward Learning and Decision-Making. Nat. Rev. Neurosci. 2019, 20, 482–494. [Google Scholar] [CrossRef]
- Von Monakow, K.H.; Akert, K.; Künzle, H. Projections of precentral and premotor cortex to the red nucleus and other midbrain areas in Macaca fascicularis. Exp. Brain Res. 1979, 34, 91–105. [Google Scholar] [CrossRef]
- Monakow, K.H.; Akert, K.; Künzle, H. Projections of the Precentral Motor Cortex and Other Cortical Areas of the Frontal Lobe to the Subthalamic Nucleus in the Monkey. Exp. Brain Res. 1978, 33, 395–403. [Google Scholar] [CrossRef]
- Nambu, A.; Tokuno, H.; Inase, M.; Takada, M. Corticosubthalamic Input Zones from Forelimb Representations of the Dorsal and Ventral Divisions of the Premotor Cortex in the Macaque Monkey: Comparison with the Input Zones from the Primary Motor Cortex and the Supplementary Motor Area. Neurosci. Lett. 1997, 239, 13–16. [Google Scholar] [CrossRef]
- Nambu, A.; Takada, M.; Inase, M.; Tokuno, H. Dual Somatotopical Representations in the Primate Subthalamic Nucleus: Evidence for Ordered but Reversed Body-Map Transformations from the Primary Motor Cortex and the Supplementary Motor Area. J. Neurosci. 1996, 16, 2671–2683. [Google Scholar] [CrossRef]
- Nambu, A.; Tokuno, H.; Hamada, I.; Kita, H.; Imanishi, M.; Akazawa, T.; Ikeuchi, Y.; Hasegawa, N. Excitatory Cortical Inputs to Pallidal Neurons Via the Subthalamic Nucleus in the Monkey. J. Neurophysiol. 2000, 84, 289–300. [Google Scholar] [CrossRef]
- Nambu, A. A New Dynamic Model of the Cortico-Basal Ganglia Loop. Prog. Brain Res. 2004, 143, 461–466. [Google Scholar] [CrossRef]
- Polyakova, Z.; Chiken, S.; Hatanaka, N.; Nambu, A. Cortical Control of Subthalamic Neuronal Activity through the Hyperdirect and Indirect Pathways in Monkeys. J. Neurosci. 2020, 40, 7451–7463. [Google Scholar] [CrossRef]
- Frank, M.J.; Samanta, J.; Moustafa, A.A.; Sherman, S.J. Hold Your Horses: Impulsivity, Deep Brain Stimulation, and Medication in Parkinsonism. Science 2007, 318, 1309–1312. [Google Scholar] [CrossRef]
- Downar, J. Orbitofrontal Cortex: A ‘Non-Rewarding’ New Treatment Target in Depression? Curr. Biol. 2019, 29, R59–R62. [Google Scholar] [CrossRef]
- Wallis, J.D.; Dias, R.; Robbins, T.W.; Roberts, A.C. Dissociable Contributions of the Orbitofrontal and Lateral Prefrontal Cortex of the Marmoset to Performance on a Detour Reaching Task. Eur. J. Neurosci. 2001, 13, 1797–1808. [Google Scholar] [CrossRef]
- Sesack, S.R.; Grace, A.A. Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology 2010, 35, 27–47. [Google Scholar] [CrossRef]
- MacLean, P.D. The Triune Brain in Evolution—Role in Paleocerebral Functions; Plenum: New York, NY, USA, 1990; ISBN 9780306431685. [Google Scholar]
- Li, B.J.; Friston, K.; Mody, M.; Wang, H.N.; Lu, H.B.; Hu, D.W. A Brain Network Model for Depression: From Symptom Understanding to Disease Intervention. Wil. Online Libr. 2018, 24, 1004–1019. [Google Scholar] [CrossRef]
- Brodmann, K. Vergleichende Lokalisationslehre der Grosshirnrinde in Ihren Prinzipien Dargestellt Aufgrund des Zellenbaues; von Grimme & Troemel: Leipzig, Germany, 1909; ISBN 310463543. [Google Scholar]
- Bracht, T.; Tüscher, O.; Schnell, S.; Kreher, B.; Rüsch, N.; Glauche, V.; Lieb, K.; Ebert, D.; Il′yasov, K.A.; Hennig, J.; et al. Extraction of Prefronto-Amygdalar Pathways by Combining Probability Maps. Psychiatry Res. Neuroimag. 2009, 174, 217–222. [Google Scholar] [CrossRef]
- Oades, R.D.; Halliday, M.G. Ventral Tegmental (A10) System: Neurobiology. 1. Anatomy and Connectivity. Brain Res. Rev. 1987, 12, 117–165. [Google Scholar] [CrossRef]
- Soravia, L.M.; Denier, N.; Moggi, F.; Grieder, M.; Federspiel, A.; Tschuemperlin, R.M.; Batschelet, H.M.; Vollstädt-Klein, S.; Wiest, R.; Stein, M.; et al. Reduced Structural Connectivity of the Amygdala Is Associated with Childhood Trauma in Adult Patients with Alcohol Use Disorder. Addict. Biol. 2022, 27, e13164. [Google Scholar] [CrossRef]
- Mantovani, A.; Pavlicova, M.; Avery, D.; Nahas, Z.; McDonald, W.M.; Wajdik, C.D.; Holtzheimer, P.E.; George, M.S.; Sackeim, H.A.; Lisanby, S.H. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatmnt-resistant depression. Depress. Anxiety 2012, 29, 883–890. [Google Scholar] [CrossRef]
- Trevizol, A.P.; Shiozawa, P.; Cook, I.A.; Sato, I.A.; Kaku, C.B.; Guimarães, F.B.S.; Sachdev, P.; Sarkhel, S.; Cordeiro, Q. Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder. J. Ect. 2016, 32, 262–266. [Google Scholar] [CrossRef]
- Lusicic, A.; Schruers, K.R.; Pallanti, S.; Castle, D.J. Transcranial Magnetic Stimulation in the Treatment of Obsessive–Compulsive Disorder: Current Perspectives. Neuropsychiatr. Dis. Treat. 2018, 14, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories Are Not Written in Stone: Re-Writing Fear Memories by Means of Non-Invasive Brain Stimulation and Optogenetic Manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don′t Hurt Me No More: State-Dependent Transcranial Magnetic Stimulation for the Treatment of Specific Phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S. Neurobiological Advances of Learned Fear in Humans. Adv. Clin. Exp. Med. 2022, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R.; Geeraedts, L.M.G.; Veening, J.G. The medial forebrain bundle of the rat. I. General introduction. J. Comp. Neurol. 1982, 206, 49–81. [Google Scholar] [CrossRef]
- Morgane, P.J.; Panksepp, J. Handbook of the Hypothalamus: Behavioral Studies of the Hypothalamus. Part B; Marcel Dekker Inc.: New York, NY, USA, 1981; Volume 2, ISBN 9780824768348. [Google Scholar]
- Veening, J.G.; Swanson, L.W.; Cowan, W.M.; Nieuwenhuys, R.; Geeraedts, L.M.G. The medial forebrain bundle of the rat. II. An autoradiographic study of the topography of the major descending and ascending components. J. Comp. Neurol. 1982, 206, 82–108. [Google Scholar] [CrossRef]
- Geeraedts, L.; Nieuwenhuys, R.; Veening, J.G. Medial Forebrain Bundle of the Rat: III. Cytoarchitecture of the Rostral (Telencephalic) Part of the Medial Forebrain Bundle Bed Nucleus. Wil. Online Libr. 1990, 294, 507–536. [Google Scholar] [CrossRef]
- Geeraedts, L.M.; Nieuwenhuys, R.; Veening, J.G. Medial Forebrain Bundle of the Rat: IV. Cytoarchitecture of the Caudal (Lateral Hypothalamic) Part of the Medial Forebrain Bundle Bed Nucleus. J. Comp. Neurol. 1990, 294, 537–568. [Google Scholar] [CrossRef]
- Hosp, J.A.; Coenen, V.A.; Rijntjes, M.; Egger, K.; Urbach, H.; Weiller, C.; Reisert, M. Ventral Tegmental Area Connections to Motor and Sensory Cortical Fields in Humans. Brain Struct. and Funct. 2019, 44, 125. [Google Scholar] [CrossRef]
- Nauta, W.J. Neural Associations of the Frontal Cortex. Acta Neurobiol. Exp. 1972, 32, 125–140. [Google Scholar]
- Price, J.L. Chapter 3: Connections of Orbital Cortex. In The Orbitofrontal Cortex; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- MacNiven, K.H.; Leong, J.K.; Knutson, B. Medial Forebrain Bundle Structure Is Linked to Human Impulsivity. Sci. Adv. 2020, 6, eaba4788. [Google Scholar] [CrossRef]
- Williams, S.M.; Goldman-Rakic, P.S. Characterization of the Dopaminergic In Nervation of the Primate Frontal Cortex Using a Dopamine-Specific Antibody. Cereb. Cortex 1993, 3, 199–222. [Google Scholar] [CrossRef]
- Dandekar, M.P.; Luse, D.; Hoffmann, C.; Cotton, P.; Peery, T.; Ruiz, C.; Hussey, C.; Giridharan, V.V.; Soares, J.C.; Quevedo, J.; et al. Increased Dopamine Receptor Expression and Anti-Depressant Response Following Deep Brain Stimulation of the Medial Forebrain Bundle. J. Affect. Disord. 2017, 217, 80–88. [Google Scholar] [CrossRef]
- Thiele, S.; Furlanetti, L.; Pfeiffer, L.-M.; Coenen, V.A.; Döbrössy, M.D. The Effects of Bilateral, Continuous, and Chronic Deep Brain Stimulation of the Medial Forebrain Bundle in a Rodent Model of Depression. Exp. Neurol. 2018, 303, 153–161. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K.; Olson, L.; Ungerstedt, U. Ascending Systems of Catecholamine Neurons from the Lower Brain Stem. Acta Physiol. Scand. 1964, 62, 485–486. [Google Scholar] [CrossRef]
- Ungerstedt, U. Stereotaxic Mapping of the Monoamine Pathways in the Rat Brain. Acta Physiol. Scand. 1971, 82, 1–48. [Google Scholar] [CrossRef]
- Jones, B.E. Arousal Systems. Front Biosci 2003, 8, 438–451. [Google Scholar] [CrossRef]
- Price, J.L.; Carmichael, S.T.; Drevets, W.C. Chapter 31 Networks Related to the Orbital and Medial Prefrontal Cortex; a Substrate for Emotional Behavior? Prog. Brain Res. 1996, 107, 523–536. [Google Scholar] [CrossRef]
- Jones, B.E.; Bobillier, P.; Pin, C.; Jouvet, M. The Effect of Lesions of Catecholamine-Containing Neurons upon Monoamine Content of the Brain and EEG and Behavioral Waking in the Cat. Brain Res. 1973, 58, 157–177. [Google Scholar] [CrossRef]
- Moal, M.L.; Simon, H. Mesocorticolimbic Dopaminergic Network: Functional and Regulatory Roles. Physiol. Rev. 1991, 71, 155–234. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The Brain Reward Circuitry in Mood Disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.-C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid Regulation of Depression-Related Behaviours by Control of Midbrain Dopamine Neurons. Nature 2012, 493, 532–536. [Google Scholar] [CrossRef]
- Papez, J.W. A Proposed mechanism of emotion. Arch. Neurol. Psychiatry 1937, 38, 725–743. [Google Scholar] [CrossRef]
- OLDS, J. Self-Stimulation of the Brain; Its Use to Study Local Effects of Hunger, Sex, and Drugs. Science 1958, 127, 315–324. [Google Scholar] [CrossRef]
- Olds, J. Satiation Effects in Self-Stimulation of the Brain. J. Comp. Physiol. Psych. 1958, 51, 675–678. [Google Scholar] [CrossRef]
- Wise, R.A.; Bozarth, M.A. Brain Reward Circuitry: Four Circuit Elements “Wired” in Apparent Series. Brain Res. Bull. 1984, 12, 203–208. [Google Scholar] [CrossRef]
- Ota, C.; Nakano, T. Self-Face Activates the Dopamine Reward Pathway without Awareness. Cereb. Cortex 2021, 31, 4420–4426. [Google Scholar] [CrossRef]
- Berridge, K.C. Affective Valence in the Brain: Modules or Modes? Nat. Rev. Neurosci. 2019, 20, 225–234. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain Structural and Functional Abnormalities in Mood Disorders: Implications for Neurocircuitry Models of Depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Furlanetti, L.L.; Coenen, V.A.; Aranda, I.A.; Döbrössy, M.D. Chronic Deep Brain Stimulation of the Medial Forebrain Bundle Reverses Depressive-like Behavior in a Hemiparkinsonian Rodent Model. Exp. Brain Res. 2015, 233, 3073–3085. [Google Scholar] [CrossRef][Green Version]
- Thiele, S.; Sörensen, A.; Weis, J.; Braun, F.; Meyer, P.T.; Coenen, V.A.; Döbrössy, M.D. Deep Brain Stimulation of the Medial Forebrain Bundle in a Rodent Model of Depression: Exploring Dopaminergic Mechanisms with Raclopride and Micro-PET. Stereotact. Funct. Neurosurg. 2020, 98, 8–20. [Google Scholar] [CrossRef]
- Furlanetti, L.L.; Döbrössy, M.D.; Aranda, I.A.; Coenen, V.A. Feasibility and Safety of Continuous and Chronic Bilateral Deep Brain Stimulation of the Medial Forebrain Bundle in the Naïve Sprague-Dawley Rat. Behav. Neurol. 2015, 2015, 256196. [Google Scholar] [CrossRef]
- Tong, Y.; Pfeiffer, L.; Serchov, T.; Coenen, V.A.; Döbrössy, M.D. Optogenetic Stimulation of Ventral Tegmental Area Dopaminergic Neurons in a Female Rodent Model of Depression: The Effect of Different Stimulation Patterns. J. Neurosci. Res. 2022, 100, 897–911. [Google Scholar] [CrossRef]
- Coenen, V.A.; Sajonz, B.E.; Hurwitz, T.A.; Böck, M.; Hosp, J.A.; Reinacher, P.C.; Urbach, H.; Blazhenets, G.; Meyer, P.T.; Reisert, M. A Neuroanatomy of Positive Affect Display—Subcortical Fiber Pathways Relevant for Initiation and Modulation of Smiling and Laughing. Front. Behav. Neurosci. 2022, 94. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Graat, I.; Denys, D. Why Has Deep Brain Stimulation Had So Little Impact in Psychiatry? Front. Neurol. 2021, 12, 757142. [Google Scholar] [CrossRef]
- Wu, H.; Hariz, M.; Visser-Vandewalle, V.; Zrinzo, L.; Coenen, V.A.; Sheth, S.A.; Bervoets, C.; Naesström, M.; Blomstedt, P.; Coyne, T.; et al. Deep Brain Stimulation for Refractory Obsessive-Compulsive Disorder (OCD): Emerging or Established Therapy? Mol. Psychiatry 2021, 26, 60–65. [Google Scholar] [CrossRef]
- Grant, R.A.; Halpern, C.H.; Baltuch, G.H.; O′Reardon, J.P.; Caplan, A. Ethical Considerations in Deep Brain Stimulation for Psychiatric Illness. J. Clin. Neurosci. 2014, 21, 1–5. [Google Scholar] [CrossRef]
- Khan, A.; Mar, K.F.; Brown, W.A. The Conundrum of Depression Clinical Trials. Int. Clin. Psychopharmacol. 2018, 33, 239–248. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; Husain, M.M.; Lisanby, S.H.; Taylor, S.F.; Whitworth, L.A.; McClintock, S.; Slavin, K.V.; Berman, J.; McKhann, G.M.; Patil, P.G.; et al. Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression: A Multisite, Randomised, Sham-Controlled Trial. Lancet Psychiatry 2017, 4, 839–849. [Google Scholar] [CrossRef]
- Dougherty, D.D.; Rezai, A.R.; Carpenter, L.L.; Howland, R.H.; Bhati, M.T.; O’Reardon, J.P.; Eskandar, E.N.; Baltuch, G.H.; Machado, A.D.; Kondziolka, D.; et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biol. Psychiatry 2015, 78, 240–248. [Google Scholar] [CrossRef]
- Wise, R.A. Dopamine, Learning and Motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef]
- Wise, R.A. Forebrain Substrates of Reward and Motivation. J. Comp. Neurol. 2005, 493, 115–121. [Google Scholar] [CrossRef]
- Wise, R.A.; McDevitt, R.A. Drive and Reinforcement Circuitry in the Brain: Origins, Neurotransmitters, and Projection Fields. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 43, 680–689. [Google Scholar] [CrossRef]
- Mirenowicz, J.; Schultz, W. Preferential Activation of Midbrain Dopamine Neurons by Appetitive Rather than Aversive Stimuli. Nature 1996, 379, 449–451. [Google Scholar] [CrossRef]
- Furlanetti, L.L.; Coenen, V.A.; Dobrossy, M.D. Ventral Tegmental Area Dopaminergic Lesion-Induced Depressive Phenotype in the Rat Is Reversed by Deep Brain Stimulation of the Medial Forebrain Bundle. Behav. Brain Res. 2016, 299, 132–140. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coenen, V.A.; Schlaepfer, T.E.; Sajonz, B.E.A.; Reinacher, P.C.; Döbrössy, M.D.; Reisert, M. “The Heart Asks Pleasure First”—Conceptualizing Psychiatric Diseases as MAINTENANCE Network Dysfunctions through Insights from slMFB DBS in Depression and Obsessive–Compulsive Disorder. Brain Sci. 2022, 12, 438. https://doi.org/10.3390/brainsci12040438
Coenen VA, Schlaepfer TE, Sajonz BEA, Reinacher PC, Döbrössy MD, Reisert M. “The Heart Asks Pleasure First”—Conceptualizing Psychiatric Diseases as MAINTENANCE Network Dysfunctions through Insights from slMFB DBS in Depression and Obsessive–Compulsive Disorder. Brain Sciences. 2022; 12(4):438. https://doi.org/10.3390/brainsci12040438
Chicago/Turabian StyleCoenen, Volker A., Thomas E. Schlaepfer, Bastian E. A. Sajonz, Peter C. Reinacher, Máté D. Döbrössy, and Marco Reisert. 2022. "“The Heart Asks Pleasure First”—Conceptualizing Psychiatric Diseases as MAINTENANCE Network Dysfunctions through Insights from slMFB DBS in Depression and Obsessive–Compulsive Disorder" Brain Sciences 12, no. 4: 438. https://doi.org/10.3390/brainsci12040438
APA StyleCoenen, V. A., Schlaepfer, T. E., Sajonz, B. E. A., Reinacher, P. C., Döbrössy, M. D., & Reisert, M. (2022). “The Heart Asks Pleasure First”—Conceptualizing Psychiatric Diseases as MAINTENANCE Network Dysfunctions through Insights from slMFB DBS in Depression and Obsessive–Compulsive Disorder. Brain Sciences, 12(4), 438. https://doi.org/10.3390/brainsci12040438







