Pathogenesis of Olfactory Disorders in COVID-19
Abstract
:1. Introduction
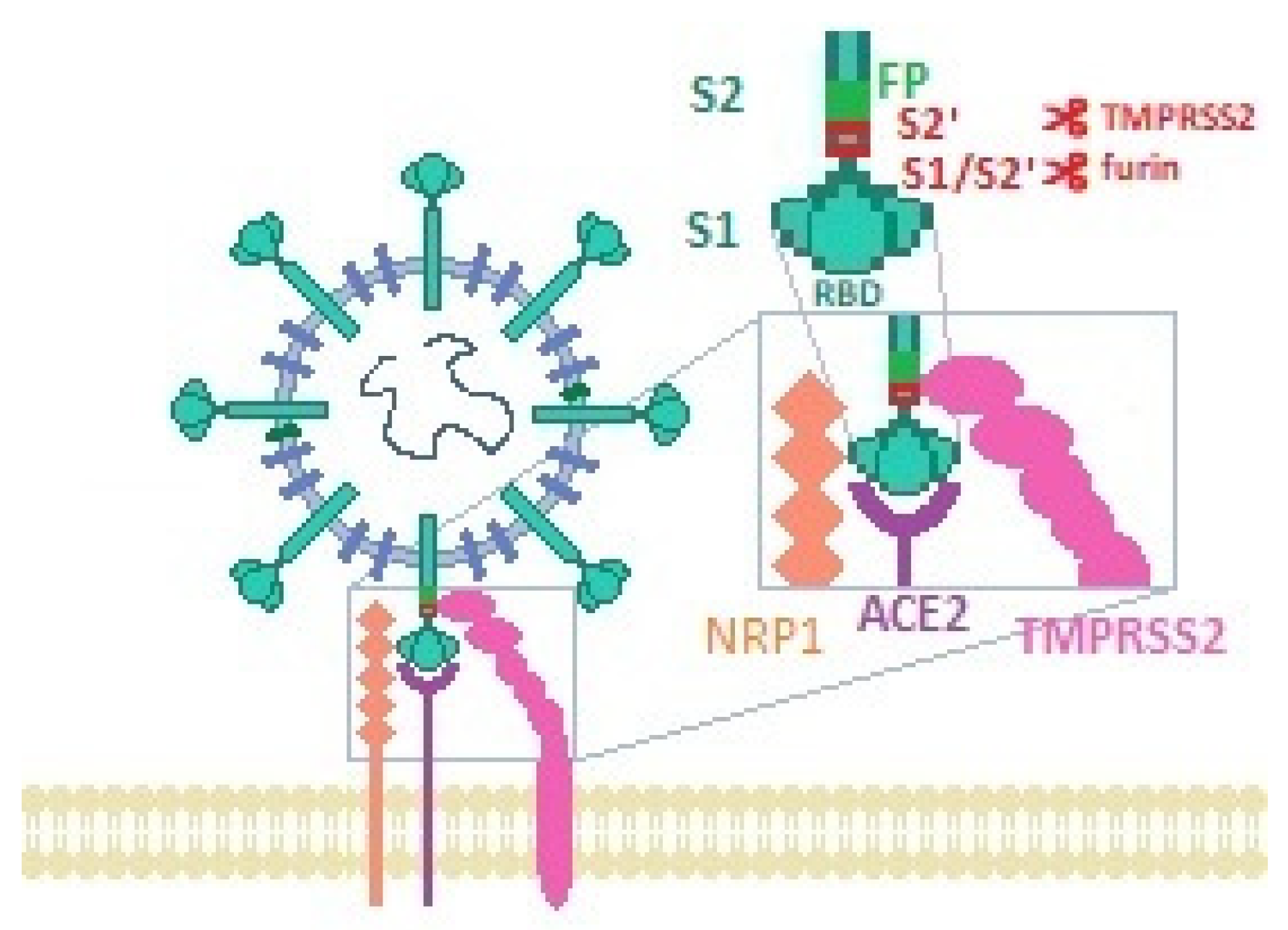
2. SARS-CoV-2 Cellular Entry Mechanism
3. The Conductive Pathomechanism of COVID-19-Related Anosmia
4. Olfactory Cleft Edema
5. Infection of Higher Olfactory Pathways
5.1. The Neuroinvasive Potential of SARS-CoV-2
5.2. The Transneuronal Route of CNS Involvement
6. Damage to the Olfactory Neuroepithelium
7. Genetic Link to the Pathomechanism of OD
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 944–950. [Google Scholar] [CrossRef]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopfenstein, T.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.-Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of anosmia in COVID-19. Méd. Mal. Infect. 2020, 50, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Place, S.; Van Laethem, Y.; Cabaraux, P.; Mat, Q.; Huet, K.; Plzak, J.; Horoi, M.; Hans, S.; et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020, 288, 335–344. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.-W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef]
- Paderno, A.; Schreiber, A.; Grammatica, A.; Raffetti, E.; Tomasoni, M.; Gualtieri, T.; Taboni, S.; Zorzi, S.; Lombardi, D.; Deganello, A.; et al. Smell and taste alterations in COVID-19: A cross-sectional analysis of different cohorts. Int. Forum Allergy Rhinol. 2020, 10, 955–962. [Google Scholar] [CrossRef]
- Qiu, C.; Cui, C.; Hautefort, C.; Haehner, A.; Zhao, J.; Yao, Q.; Zeng, H.; Nisenbaum, E.J.; Liu, L.; Zhao, Y.; et al. Olfactory and Gustatory Dysfunction as an Early Identifier of COVID-19 in Adults and Children: An International Multicenter Study. Otolaryngol. Head. Neck Surg. 2020, 163, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA 2020, 323, 2089. [Google Scholar] [CrossRef] [Green Version]
- Vaira, L.A.; Salzano, G.; Deiana, G.; De Riu, G. Anosmia and Ageusia: Common Findings in COVID-19 Patients. Laryngoscope 2020, 130, 1787–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, C.; Lechien, J.R.; Saussez, S. More that ACE2? NRP1 may play a central role in the underlying pathophysiological mechanism of olfactory dysfunction in COVID-19 and its association with enhanced survival. Med. Hypotheses 2021, 146, 110406. [Google Scholar] [CrossRef]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19–Related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021, 13, eabf8396. [Google Scholar] [CrossRef] [PubMed]
- Henkin, R.I. How does Covid-19 infection affect smell? Am. J. Otolaryngol. 2021, 42, 102912. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Lechien, J.R.; Hopkins, C. Anosmia: An evolution of our understanding of its importance in COVID-19 and what questions remain to be answered. Eur. Arch. Otorhinolaryngol. 2021, 278, 2187–2191. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhin 2017, 54, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef] [PubMed]
- Liang, F. Sustentacular Cell Enwrapment of Olfactory Receptor Neuronal Dendrites: An Update. Genes 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kachramanoglou, C.; Li, D.; Andrews, P.; Choi, D. Anatomy and Cellular Constituents of the Human Olfactory Mucosa: A Review. J. Neurol. Surg. B 2014, 75, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Glezer, I.; Bruni-Cardoso, A.; Schechtman, D.; Malnic, B. Viral infection and smell loss: The case of COVID-19. J. Neurochem. 2021, 157, 930–943. [Google Scholar] [CrossRef]
- Bilinska, K.; Butowt, R. Anosmia in COVID-19: A Bumpy Road to Establishing a Cellular Mechanism. ACS Chem. Neurosci. 2020, 11, 2152–2155. [Google Scholar] [CrossRef] [PubMed]
- Ueha, R.; Kondo, K.; Kagoya, R.; Shichino, S.; Ueha, S.; Yamasoba, T. ACE2, TMPRSS2, and Furin expression in the nose and olfactory bulb in mice and human. Rhin 2020, 59, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Saniasiaya, J.; Abdul Gani, N. Olfactory and Gustatory Dysfunctions as a Clinical Manifestation of Coronavirus Disease 2019 in a Malaysian Tertiary Center. Ann. Otol. Rhinol. Laryngol. 2021, 130, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Saito, K.; Min, W.-P.; Vladau, C.; Toida, K.; Itoh, H.; Murakami, S. Identification of Viruses in Patients With Postviral Olfactory Dysfunction. Laryngoscope 2007, 117, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, C.; Kumar, N. Loss of Sense of Smell Asmarker of COVID-19 Infection: Joint Statement from the British Rhinological Society and ENT-UK. Published March 21. 2020. Available online: https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf (accessed on 20 April 2020).
- Welge-Lüssen, A.; Wolfensberger, M. Olfactory Disorders following Upper Respiratory Tract Infections. In Advances in Oto-Rhino-Laryngology; Hummel, T., Welge-Lüssen, A., Eds.; KARGER: Basel, Switzerland, 2006; Volume 63, pp. 125–132. ISBN 978-3-8055-8123-3. [Google Scholar]
- Pellegrino, R.; Cooper, K.W.; Di Pizio, A.; Joseph, P.V.; Bhutani, S.; Parma, V. Coronaviruses and the Chemical Senses: Past, Present, and Future. Chem. Senses 2020, 45, 415–422. [Google Scholar] [CrossRef]
- Bryche, B.; St Albin, A.; Murri, S.; Lacôte, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.W.; Brann, D.H.; Farruggia, M.C.; Bhutani, S.; Pellegrino, R.; Tsukahara, T.; Weinreb, C.; Joseph, P.V.; Larson, E.D.; Parma, V.; et al. COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron 2020, 107, 219–233. [Google Scholar] [CrossRef] [PubMed]
- van Riel, D.; Verdijk, R.; Kuiken, T. The olfactory nerve: A shortcut for influenza and other viral diseases into the central nervous system: The olfactory nerve: A shortcut for viruses into the CNS. J. Pathol. 2015, 235, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Gorzkowski, V.; Bevilacqua, S.; Charmillon, A.; Jankowski, R.; Gallet, P.; Rumeau, C.; Nguyen, D.T. Evolution of Olfactory Disorders in COVID -19 Patients. Laryngoscope 2020, 130, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadarrama-Ortiz, P.; Choreño-Parra, J.A.; Sánchez-Martínez, C.M.; Pacheco-Sánchez, F.J.; Rodríguez-Nava, A.I.; García-Quintero, G. Neurological Aspects of SARS-CoV-2 Infection: Mechanisms and Manifestations. Front. Neurol. 2020, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Gengler, I.; Wang, J.C.; Speth, M.M.; Sedaghat, A.R. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: A systematic review of the current evidence. Laryngoscope Investig. Otolaryngol. 2020, 5, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Randeva, H.; Chatha, K.; Hall, M.; Spandidos, D.; Karteris, E.; Kyrou, I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol. Med. Rep. 2020, 22, 4221–4226. [Google Scholar] [CrossRef] [PubMed]
- Fodoulian, L.; Tuberosa, J.; Rossier, D.; Boillat, M.; Kan, C.; Pauli, V.; Egervari, K.; Lobrinus, J.A.; Landis, B.N.; Carleton, A.; et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. iScience 2020, 23, 101839. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2021, 11, 573095. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, W.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, M.H.; Porvari, K.S.; Kellokumpu, S.; Kyllönen, A.P.; Vihko, P.T. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J. Pathol. 2001, 193, 134–140. [Google Scholar] [CrossRef]
- Bougakov, D.; Podell, K.; Goldberg, E. Multiple Neuroinvasive Pathways in COVID-19. Mol. Neurobiol. 2021, 58, 564–575. [Google Scholar] [CrossRef]
- Butowt, R.; Bilinska, K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem. Neurosci. 2020, 11, 1200–1203. [Google Scholar] [CrossRef] [Green Version]
- Vaira, L.A.; Hopkins, C.; Salzano, G.; Petrocelli, M.; Melis, A.; Cucurullo, M.; Ferrari, M.; Gagliardini, L.; Pipolo, C.; Deiana, G.; et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020, 42, 1560–1569. [Google Scholar] [CrossRef]
- Chung, T.W.-H.; Sridhar, S.; Zhang, A.J.; Chan, K.-H.; Li, H.-L.; Wong, F.K.-C.; Ng, M.-Y.; Tsang, R.K.-Y.; Lee, A.C.-Y.; Fan, Z.; et al. Olfactory Dysfunction in Coronavirus Disease 2019 Patients: Observational Cohort Study and Systematic Review. Open Forum Infect. Dis. 2020, 7, ofaa199. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef]
- Lechien, J.R.; Cabaraux, P.; Chiesa-Estomba, C.M.; Khalife, M.; Hans, S.; Calvo-Henriquez, C.; Martiny, D.; Journe, F.; Sowerby, L.; Saussez, S. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 2020, 42, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Draf, J.; Dräger, S.; de With, K.; Hummel, T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. ORL 2020, 82, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, M.; Hautefort, C.; Hamel, A.-L.; Verillaud, B.; Herman, P.; Houdart, E.; Eloit, C. Sudden and Complete Olfactory Loss of Function as a Possible Symptom of COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altundag, A.; Yıldırım, D.; Tekcan Sanli, D.E.; Cayonu, M.; Kandemirli, S.G.; Sanli, A.N.; Arici Duz, O.; Saatci, O. Olfactory Cleft Measurements and COVID-19–Related Anosmia. Otolaryngol. Head Neck Surg. 2021, 164, 1337–1344. [Google Scholar] [CrossRef]
- Kandemirli, S.G.; Altundag, A.; Yildirim, D.; Tekcan Sanli, D.E.; Saatci, O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 2021, 28, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Lantos, J.E.; Strauss, S.B.; Phillips, C.D.; Campion, T.R.; Navi, B.B.; Parikh, N.S.; Merkler, A.E.; Mir, S.; Zhang, C.; et al. Brain Imaging of Patients with COVID-19: Findings at an Academic Institution during the Height of the Outbreak in New York City. AJNR Am. J. Neuroradiol. 2020, 41, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Naeini, A.S.; Karimi-Galougahi, M.; Raad, N.; Ghorbani, J.; Taraghi, A.; Haseli, S.; Mehrparvar, G.; Bakhshayeshkaram, M. Paranasal sinuses computed tomography findings in anosmia of COVID-19. Am. J. Otolaryngol. 2020, 41, 102636. [Google Scholar] [CrossRef]
- Lechien, J.R.; Michel, J.; Radulesco, T.; Chiesa-Estomba, C.M.; Vaira, L.A.; De Riu, G.; Sowerby, L.; Hopkins, C.; Saussez, S. Clinical and Radiological Evaluations of COVID -19 Patients With Anosmia: Preliminary Report. Laryngoscope 2020, 130, 2526–2531. [Google Scholar] [CrossRef] [PubMed]
- Han, A.Y.; Mukdad, L.; Long, J.L.; Lopez, I.A. Anosmia in COVID-19: Mechanisms and Significance. Chem. Senses 2020, 45, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Salzano, G.; Fois, A.G.; Piombino, P.; De Riu, G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum Allergy Rhinol. 2020, 10, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Rathore, F.A. Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 2020, 77, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sánchez, C.M.; Díaz-Maroto, I.; Fernández-Díaz, E.; Sánchez-Larsen, Á.; Layos-Romero, A.; García-García, J.; González, E.; Redondo-Peñas, I.; Perona-Moratalla, A.B.; Del Valle-Pérez, J.A.; et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020, 95, e1060–e1070. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.S.; Joshi, S.V.; Naik, S.; Sangle, S.; Abraham, N.M. Quantitative assessment of olfactory dysfunction accurately detects asymptomatic COVID-19 carriers. EClinicalMedicine 2020, 28, 100575. [Google Scholar] [CrossRef]
- Azim, D.; Nasim, S.; Kumar, S.; Hussain, A.; Patel, S. Neurological Consequences of 2019-nCoV Infection: A Comprehensive Literature Review. Cureus 2020, 12, e8790. [Google Scholar] [CrossRef]
- Moonis, G.; Filippi, C.G.; Kirsch, C.F.E.; Mohan, S.; Stein, E.G.; Hirsch, J.A.; Mahajan, A. The Spectrum of Neuroimaging Findings on CT and MRI in Adults With COVID-19. Am. J. Roentgenol. 2021, 217, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla, A.P.; Lovero, R.; Lo Muzio, L.; Testa, N.F.; Schirinzi, A.; Palmieri, G.; Pozzessere, P.; Procacci, V.; Di Comite, M.; Ciavarella, D.; et al. Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS Chem. Neurosci. 2020, 11, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Sanli, D.E.T.; Altundag, A.; Kandemirli, S.G.; Yildirim, D.; Sanli, A.N.; Saatci, O.; Kirisoglu, C.E.; Dikensoy, O.; Murrja, E.; Yesil, A.; et al. Relationship between disease severity and serum IL-6 levels in COVID-19 anosmia. Am. J. Otolaryngol. 2021, 42, 102796. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Pouga, L. Encephalitic syndrome and anosmia in COVID-19: Do these clinical presentations really reflect SARS-CoV-2 neurotropism? A theory based on the review of 25 COVID-19 cases. J. Med. Virol. 2021, 93, 550–558. [Google Scholar] [CrossRef]
- Lima, M.; Siokas, V.; Aloizou, A.-M.; Liampas, I.; Mentis, A.-F.A.; Tsouris, Z.; Papadimitriou, A.; Mitsias, P.D.; Tsatsakis, A.; Bogdanos, D.P.; et al. Unraveling the Possible Routes of SARS-COV-2 Invasion into the Central Nervous System. Curr. Treat. Options Neurol. 2020, 22, 37. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Brison, É.; Meessen-Pinard, M.; Talbot, P.J. Neuroinvasive and Neurotropic Human Respiratory Coronaviruses: Potential Neurovirulent Agents in Humans. In Infectious Diseases and Nanomedicine I; Adhikari, R., Thapa, S., Eds.; Advances in Experimental Medicine and Biology; Springer India: New Delhi, India, 2014; Volume 807, pp. 75–96. ISBN 978-81-322-1776-3. [Google Scholar]
- Jacob, F.; Pather, S.R.; Huang, W.-K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 2020, 27, 937–950.e9. [Google Scholar] [CrossRef]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961.e5. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gori, A.; Leone, F.; Loffredo, L.; Cinicola, B.L.; Brindisi, G.; De Castro, G.; Spalice, A.; Duse, M.; Zicari, A.M. COVID-19-Related Anosmia: The Olfactory Pathway Hypothesis and Early Intervention. Front. Neurol. 2020, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, G.; Chiumello, D.; Canevini, M.P.; Priori, A.; Mazzanti, M.; Centanni, S.; Felisati, G. First ultrastructural autoptic findings of SARS-CoV-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020, 86, S0375–S9393. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Politi, L.S.; Salsano, E.; Grimaldi, M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020, 77, 1028. [Google Scholar] [CrossRef]
- Laurendon, T.; Radulesco, T.; Mugnier, J.; Gérault, M.; Chagnaud, C.; El Ahmadi, A.-A.; Varoquaux, A. Bilateral transient olfactory bulb edema during COVID-19–related anosmia. Neurology 2020, 95, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Fragkou, P.C.; Lachanis, S.; Palaiodimou, L.; Lambadiari, V.; Papathanasiou, M.; Sfikakis, P.P.; Voumvourakis, K.I.; Tsiodras, S. Olfactory bulb and mucosa abnormalities in persistent COVID-19-induced anosmia: A magnetic resonance imaging study. Eur. J. Neurol. 2021, 28, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, H.; Kizilog˘lu, A.; Dilek, O.; Belibag˘li, C.; Kaya, Ö.; Yılmaz, C.; Gülek, B. Evaluation of the olfactory bulb volume and morphology in patients with coronavirus disease 2019: Can differences create predisposition to anosmia? Rev. Assoc. Med. Bras. 2021, 67, 1491–1497. [Google Scholar] [CrossRef]
- Esposito, F.; Cirillo, M.; De Micco, R.; Caiazzo, G.; Siciliano, M.; Russo, A.G.; Monari, C.; Coppola, N.; Tedeschi, G.; Tessitore, A. Olfactory loss and brain connectivity after COVID-19. Hum. Brain Mapp. 2022, 43, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus Infection Causes Neuronal Death in the Absence of Encephalitis in Mice Transgenic for Human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [Green Version]
- Dubé, M.; Le Coupanec, A.; Wong, A.H.M.; Rini, J.M.; Desforges, M.; Talbot, P.J. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J. Virol 2018, 92, e00404-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.-H.; Chen, Q.; Gu, H.-J.; Yang, G.; Wang, Y.-X.; Huang, X.-Y.; Liu, S.-S.; Zhang, N.-N.; Li, X.-F.; Xiong, R.; et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe 2020, 28, 124–133.e4. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Yang, Y.; Yu, W.; Zhao, Y.; Long, H.; Gao, J.; Ding, K.; Ma, C.; Li, J.; Zhao, S.; et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Sig. Transduct. Target. 2021, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef]
- Vaira, L.A.; Hopkins, C.; Sandison, A.; Manca, A.; Machouchas, N.; Turilli, D.; Lechien, J.R.; Barillari, M.R.; Salzano, G.; Cossu, A.; et al. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J. Laryngol. Otol. 2020, 134, 1123–1127. [Google Scholar] [CrossRef]
- Rodriguez, S.; Cao, L.; Rickenbacher, G.T.; Benz, E.G.; Magdamo, C.; Gomez, L.R.; Holbrook, E.H.; Albers, A.D.; Gallagher, R.; Westover, M.B.; et al. Innate immune signaling in the olfactory epithelium reduces odorant receptor levels: Modeling transient smell loss in COVID-19 patients. medRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.-M.; Chin, A.W.H.; Fung, K.; Choy, K.-T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.A.P.M.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, A.D.; et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 2022, in press. [CrossRef]
- Torabi, A.; Mohammadbagheri, E.; Akbari Dilmaghani, N.; Bayat, A.-H.; Fathi, M.; Vakili, K.; Alizadeh, R.; Rezaeimirghaed, O.; Hajiesmaeili, M.; Ramezani, M.; et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. ACS Chem. Neurosci. 2020, 11, 1909–1913. [Google Scholar] [CrossRef]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More Than Smell—COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef]
- Hopkins, C.; Surda, P.; Vaira, L.A.; Lechien, J.R.; Safarian, M.; Saussez, S.; Kumar, N. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinology 2020, 59, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; D’Ascanio, L.; La Mantia, I.; Ralli, M.; Brenner, M.J. Parosmia after COVID-19: Olfactory training, neuroinflammation and distortions of smell. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.F.; Shastri, A.J.; Fletez-Brant, K.; The 23andMe COVID-19 Team; Auton, A.; Chubb, A.; Fitch, A.; Kung, A.; Altman, A.; Kill, A.; et al. The UGT2A1/UGT2A2 locus is associated with COVID-19-related loss of smell or taste. Nat. Genet. 2022, 54, 121–124. [Google Scholar] [CrossRef]
- Khan, M.; Yoo, S.-J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949.e15. [Google Scholar] [CrossRef] [PubMed]
- Lazard, D.; Zupko, K.; Poria, Y.; Net, P.; Lazarovits, J.; Horn, S.; Khen, M.; Lancet, D. Odorant signal termination by olfactory UDP glucuronosyl transferase. Nature 1991, 349, 790–793. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziuzia-Januszewska, L.; Januszewski, M. Pathogenesis of Olfactory Disorders in COVID-19. Brain Sci. 2022, 12, 449. https://doi.org/10.3390/brainsci12040449
Ziuzia-Januszewska L, Januszewski M. Pathogenesis of Olfactory Disorders in COVID-19. Brain Sciences. 2022; 12(4):449. https://doi.org/10.3390/brainsci12040449
Chicago/Turabian StyleZiuzia-Januszewska, Laura, and Marcin Januszewski. 2022. "Pathogenesis of Olfactory Disorders in COVID-19" Brain Sciences 12, no. 4: 449. https://doi.org/10.3390/brainsci12040449
APA StyleZiuzia-Januszewska, L., & Januszewski, M. (2022). Pathogenesis of Olfactory Disorders in COVID-19. Brain Sciences, 12(4), 449. https://doi.org/10.3390/brainsci12040449





