Aftereffects to Prism Exposure without Adaptation: A Single Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Patient TMA
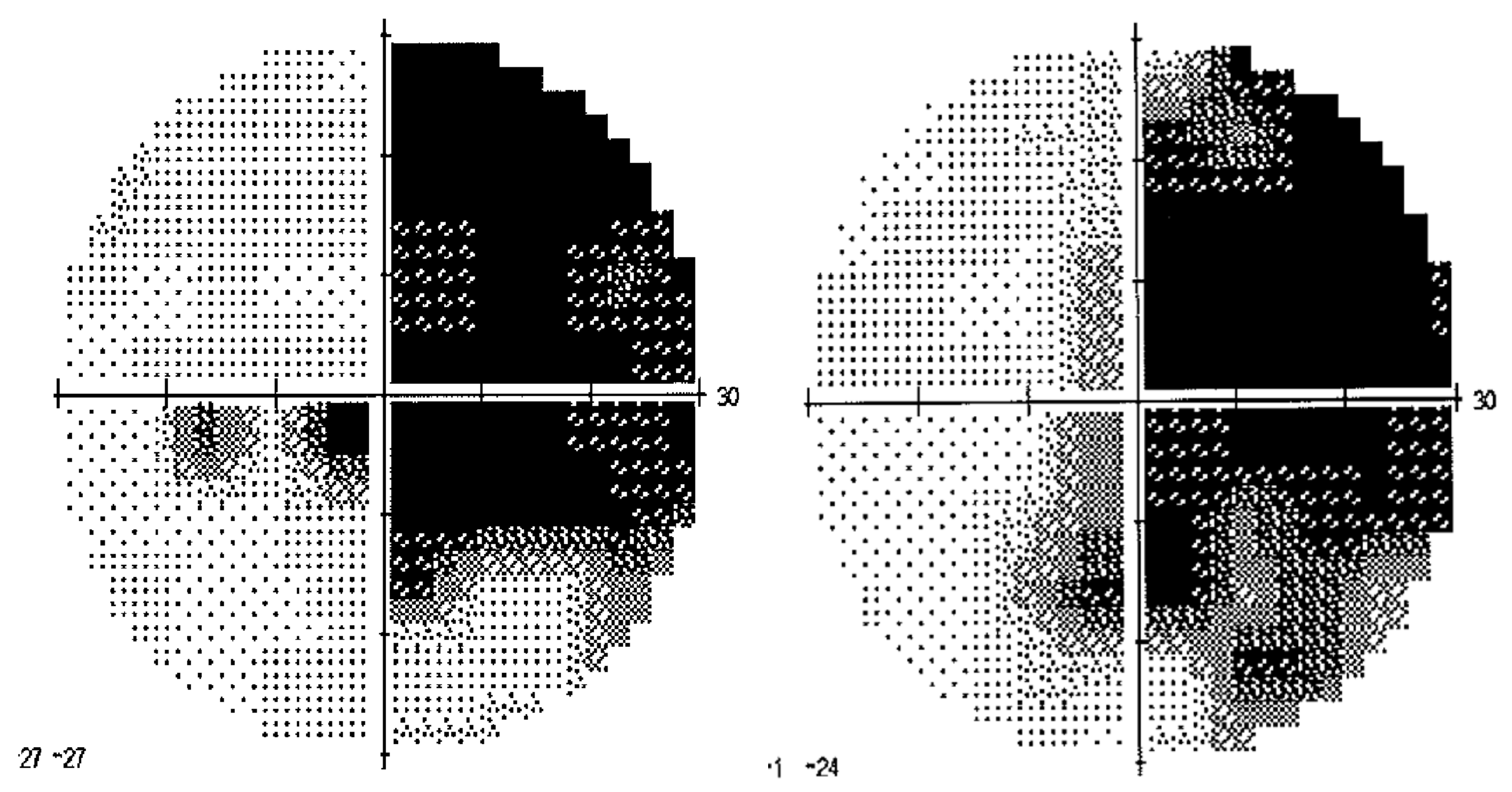
2.1.2. Control Participants
2.2. Procedure
2.2.1. Prism Adaptation EXPOSURE phase
2.2.2. Pre- and Post-Exposure Phases
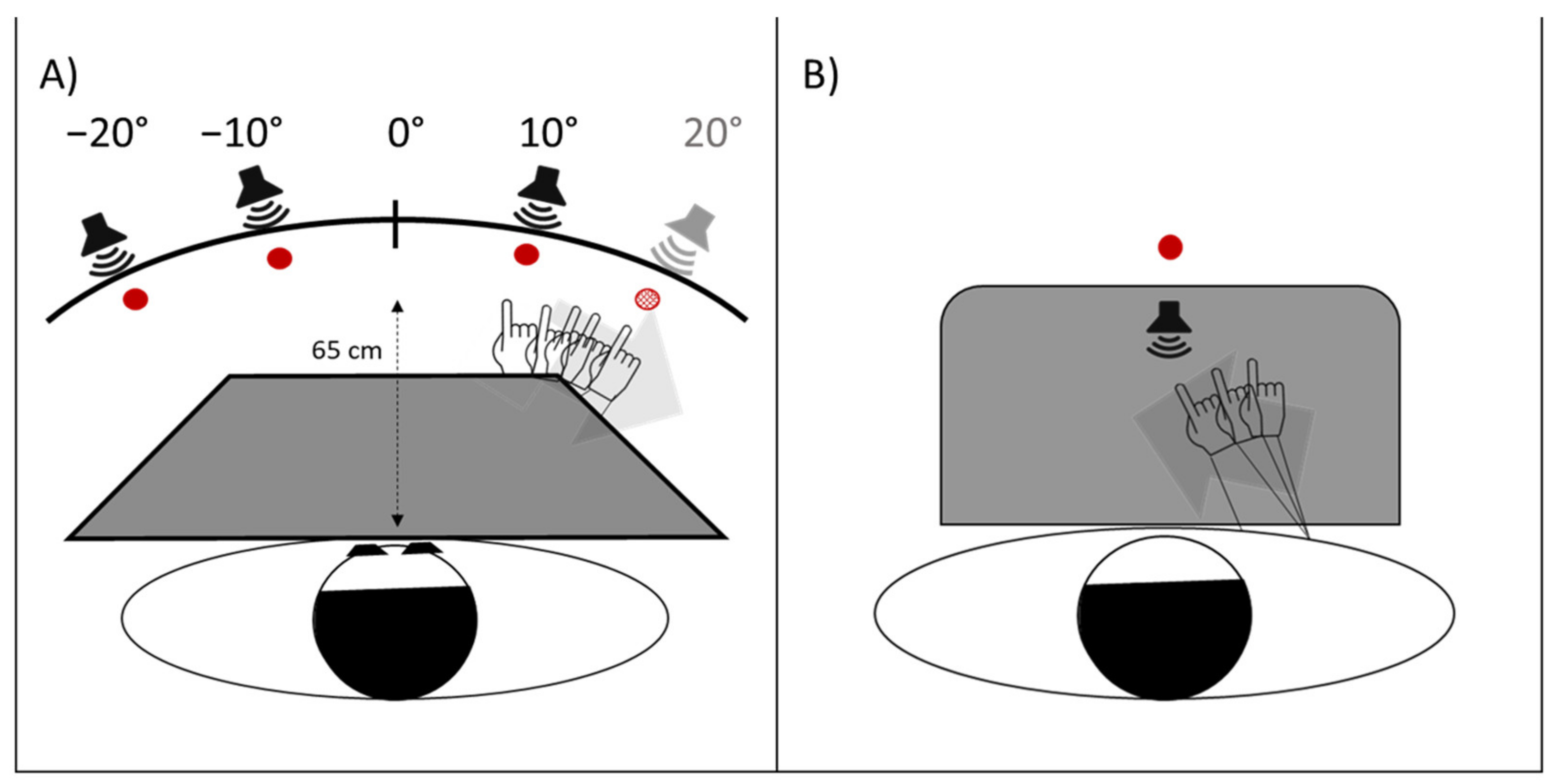
- Proprioceptive. Participants, seated in front of a table, with eyes closed, received instructions to point with the right index finger to the location on the table surface, perceived as the subjective straight-ahead (Figure 4B).
- Audio-proprioceptive. In darkness, participants received instructions to point with the right index finger to the location on the table surface subjectively perceived as the projection of a sound source. No information was given to participants about the location of the loudspeaker (Figure 4B).
- Visuo-proprioceptive. In darkness, participants received instructions to fixate a red LED placed in the straight-ahead position, at a 65 cm distance, and to point with the right index finger to the location on the table surface subjectively perceived as the projection of the light on the table. No information was given about the actual LED location, and a wooden box precluded participants from viewing the pointing movement, which then took place without any visual control (Figure 4B).
- Visual. In darkness, participants received instructions to stop verbally a red LED, moving horizontally just above eye level, at 65 cm from the participant’s mid-sagittal plane, when the light was subjectively perceived as straight-ahead. The 10 trials (five with the light moving from the right to the left visual periphery, five from the left to the right) were given in a random fixed order. For each test, 10 trials were given. Each participant performed the tests in the same order in the pre- and in the post-exposure phases. For each test, the mean deviation from the objective midline was calculated, both in the pre- and in the post- adaptation phase; positive values indicated a rightward deviation from the perceived body midline, negative values a leftward deviation [17].
2.3. Statistical Analyses
3. Results
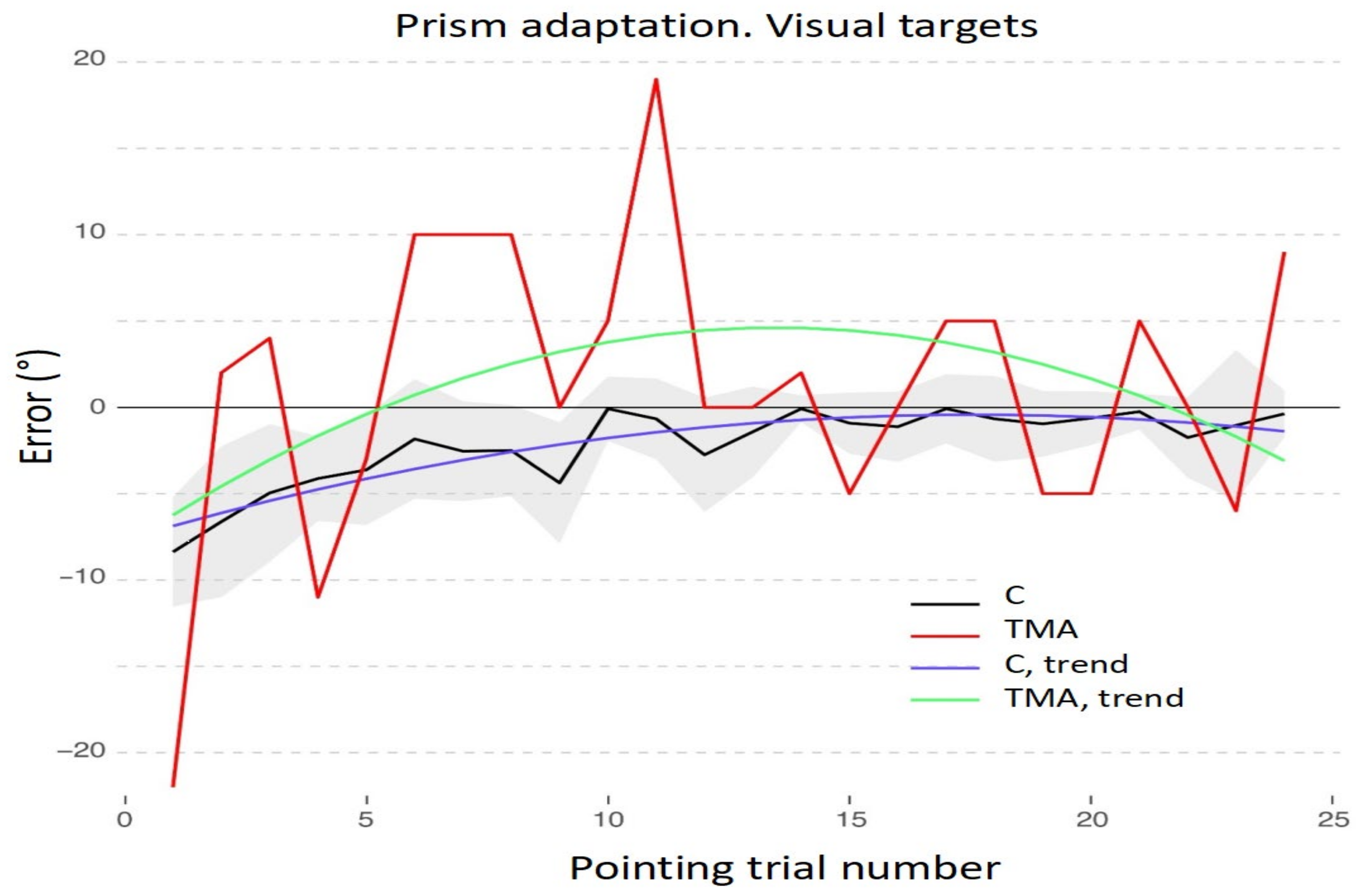
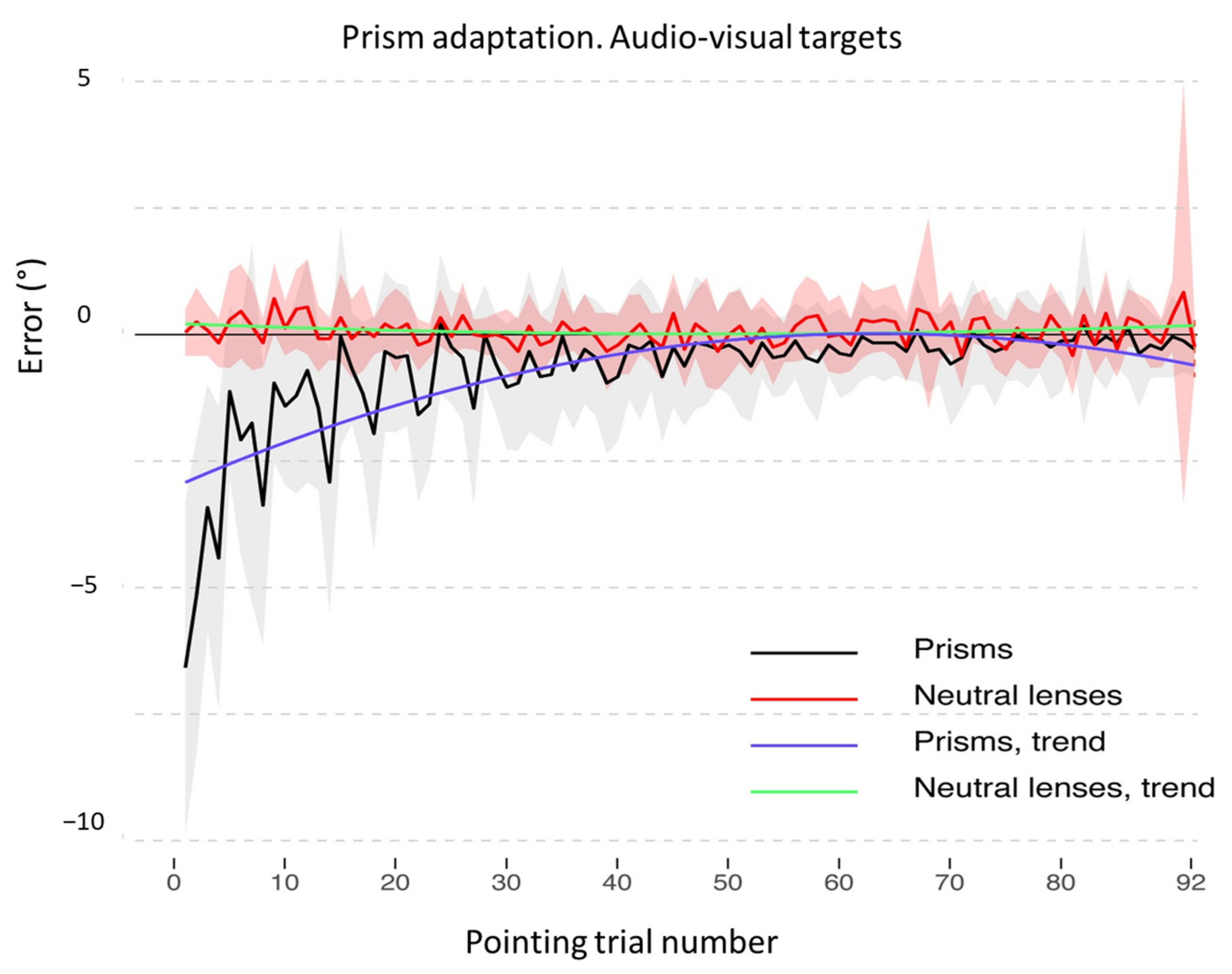
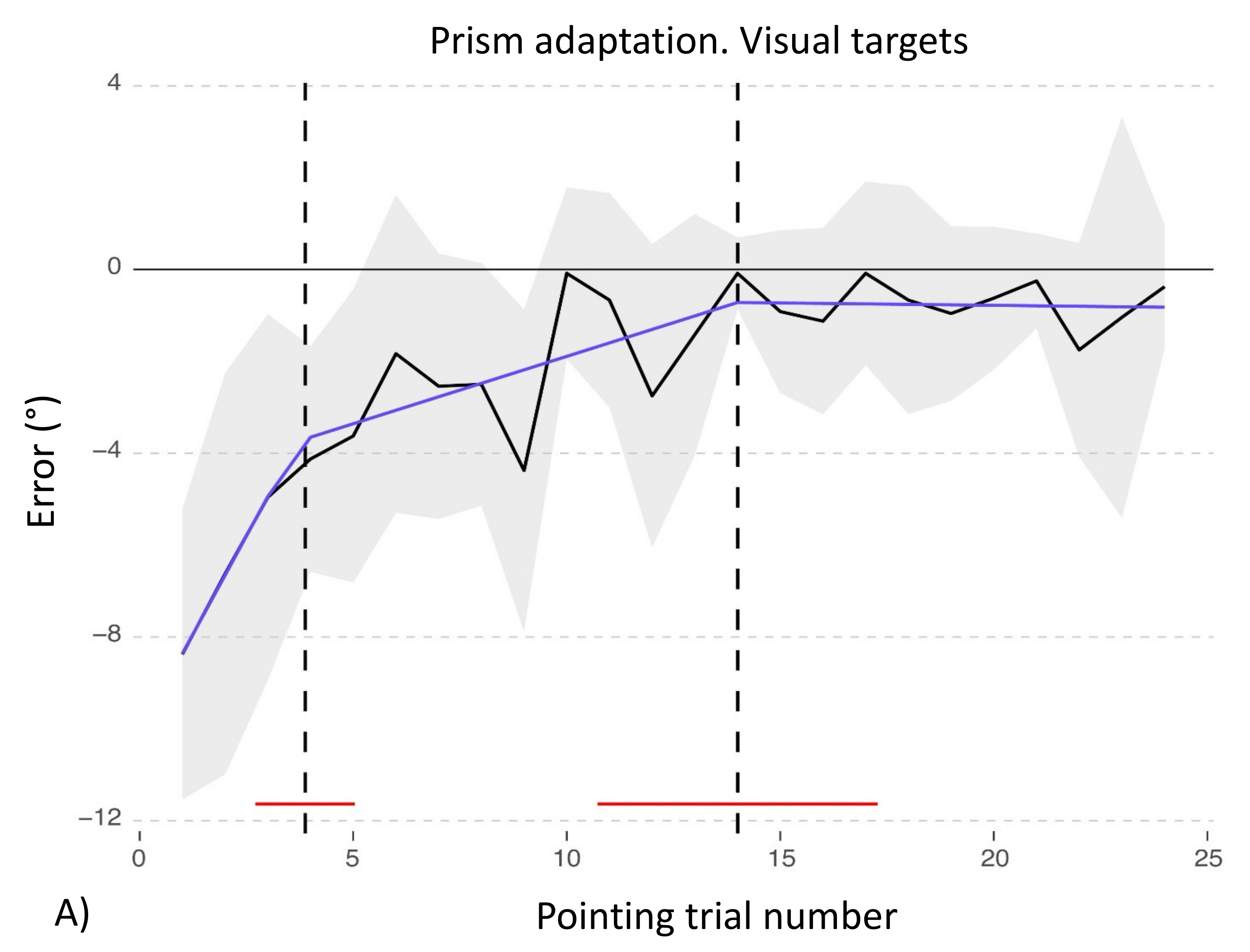
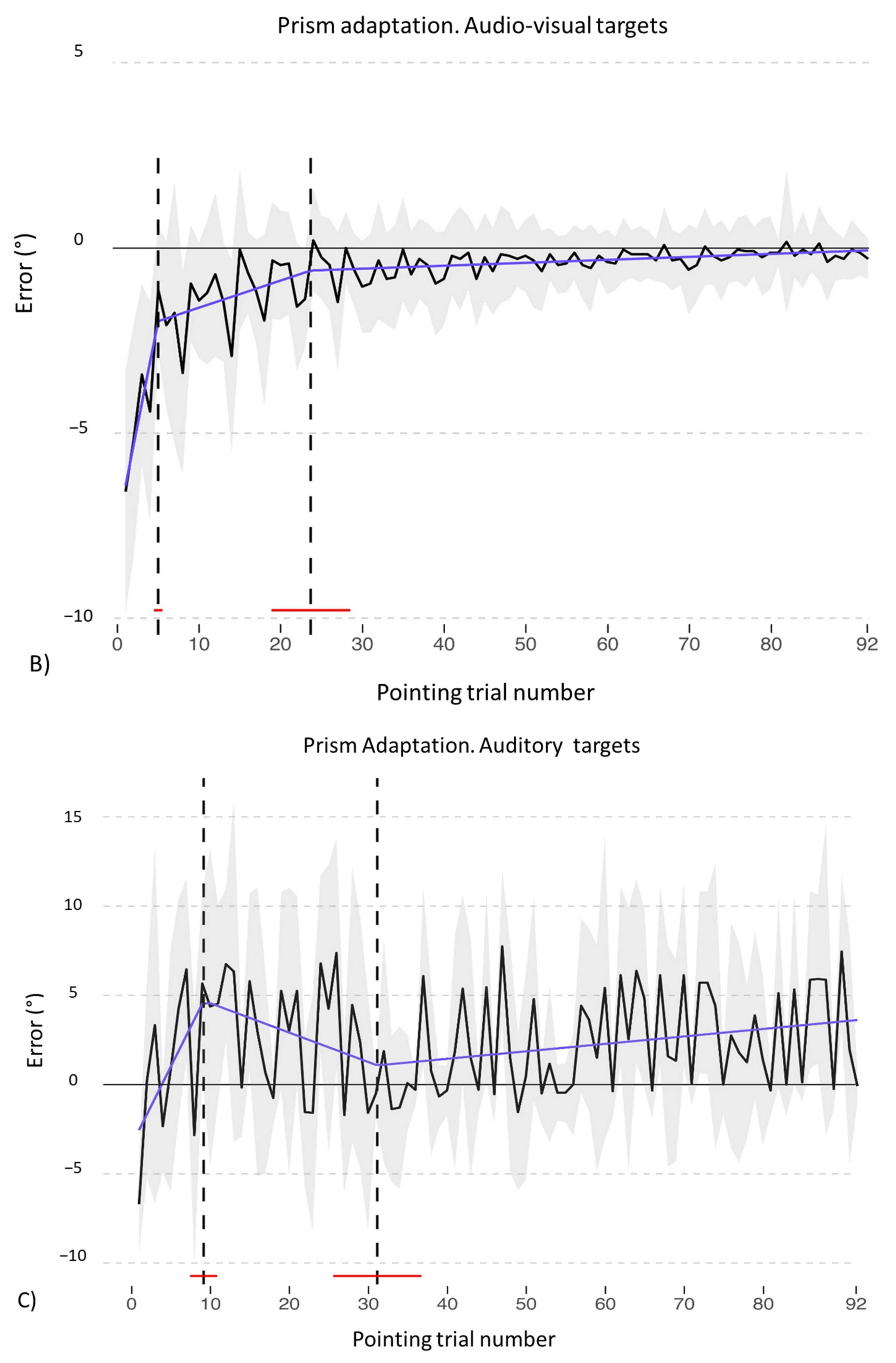
3.1. Adaptation during the Exposure Phase
3.2. Aftereffects
3.2.1. Control Participants
3.2.2. Patient TMA
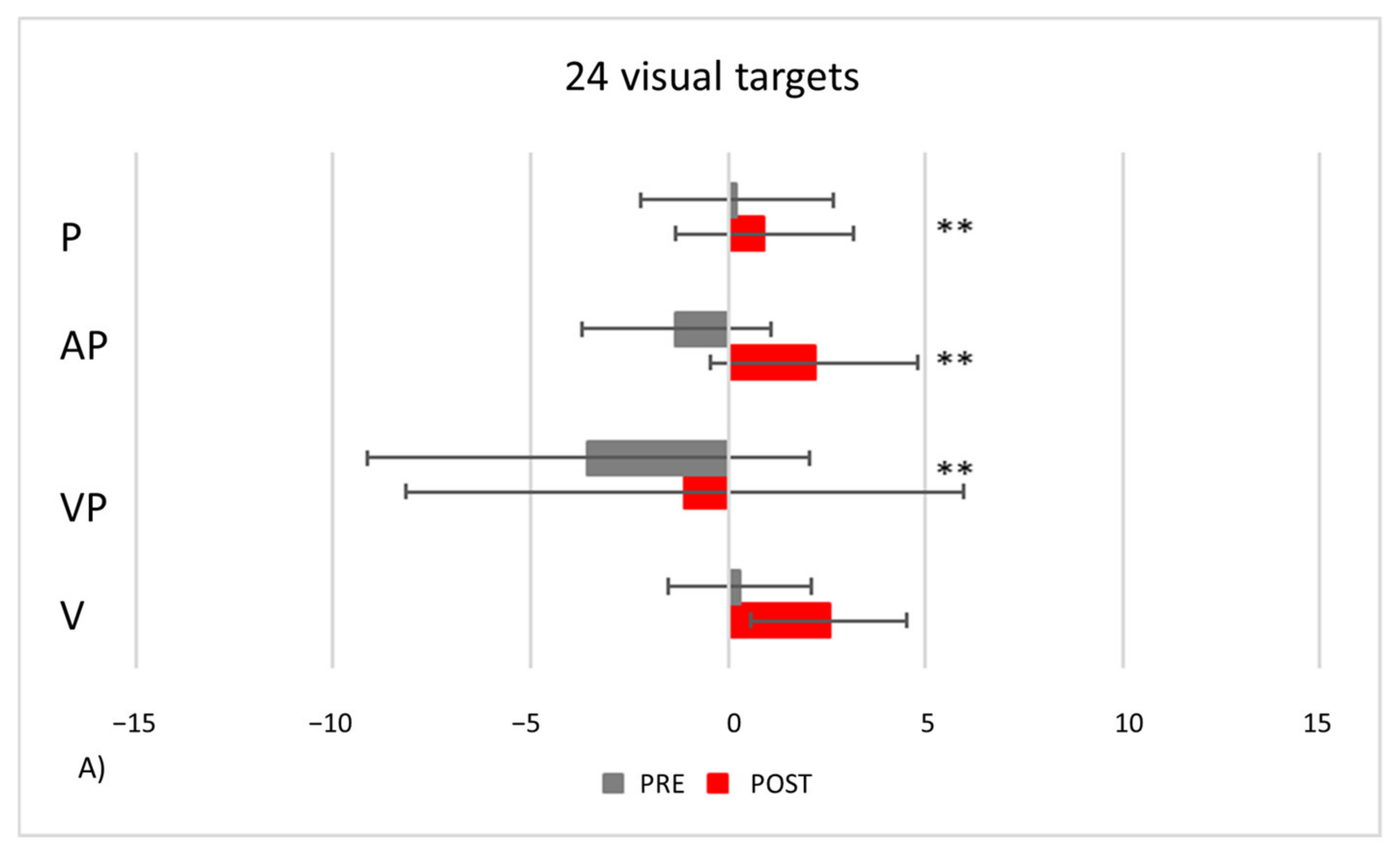
- PA condition: 24 visual targets. As compared to control participants, TMA shows AEs in the proprioceptive and audio-proprioceptive straight-ahead tests (Figure 13A). In the 24 visual target PA condition, Crawford-Howell t-tests for case-control comparisons show that the mean score of control participants (proprioceptive test: M = 2.25, SD = ±2.31; audio-proprioceptive test = 2.48 ± 3.34) does not differ from that of the patient in both the proprioceptive (5.05 ± 1.55, p = 0.24) and the audio-proprioceptive straight-ahead tests (−4.60 ± 15.55, p = 0.05). However, the mean score of control participants (visuo-proprioceptive test = 3.49 ± 1.62) differs from that of the patient in the visuo-proprioceptive straight-ahead test (−1.65 ± 7.43, p = 0.004): as compared with the control group, TMA’s mean straight-ahead post-adaptation deviation is not shifted rightwards.
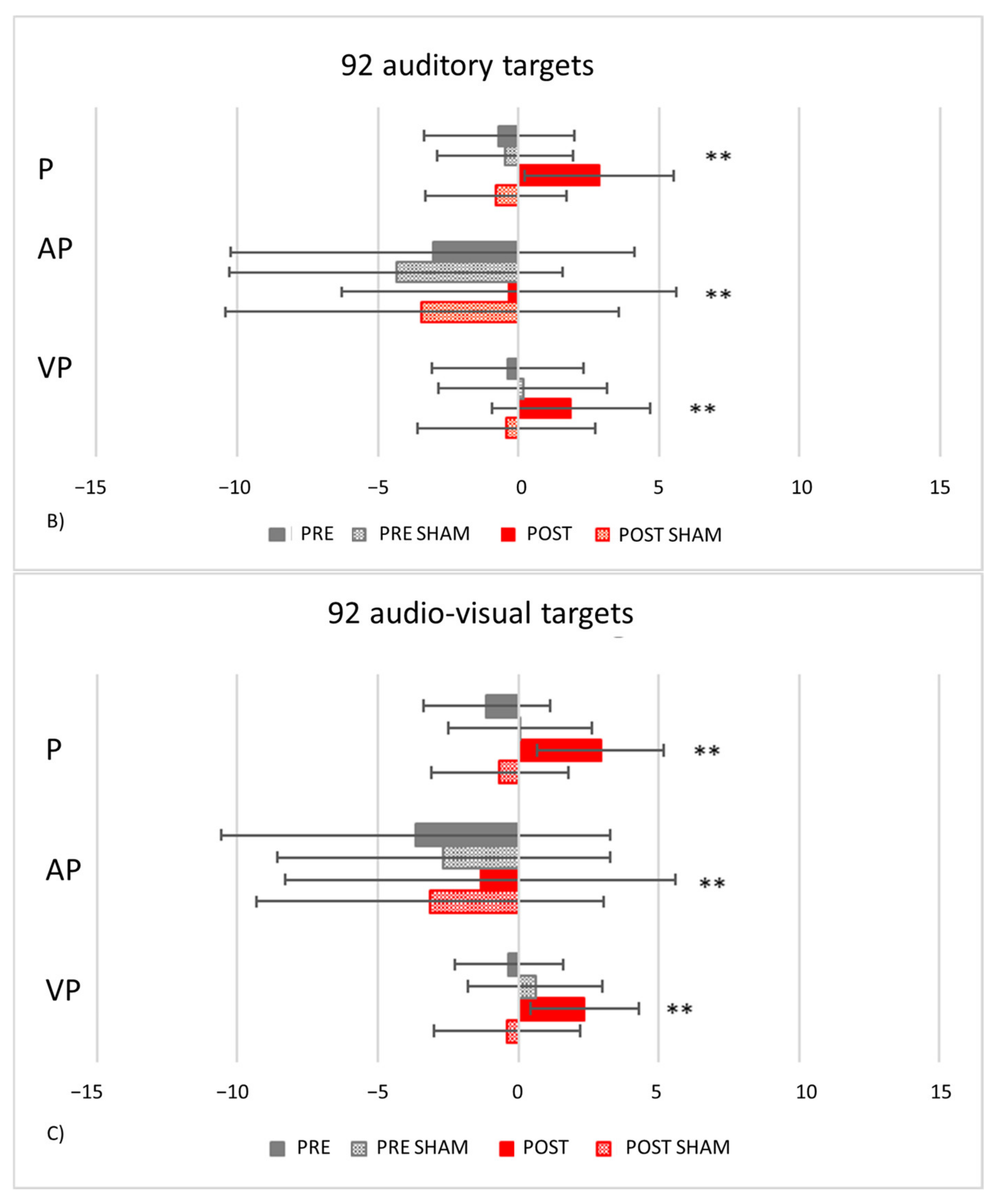
- PA condition: 92 auditory targets. AEs in the visuo-proprioceptive straight-ahead tests are present (Figure 13B). A Crawford t-test shows that the mean score of control participants in the visuo-proprioceptive straight-ahead test (3.54 ± 1.85) differs from the patient’s score (14.5 ± 5.75, p < 0.001), namely: TMA shows larger AEs than control participants. The mean score of control participants (2.235 ± 1.55) differs from the patient’s score in the proprioceptive straight-ahead test (−1.20 ± 1.87, p = 0.040). Similarly, the mean score of control participants (2.17 ± 2.69 in the audio-proprioceptive straight-ahead test) differs from the patient’s score (−5.5 ± 2.91, p = 0.006). However, in these tests TMA’s mean straight-ahead post-adaptation deviation is not shifted rightwards, as expected after adaptation to prisms displacing the visual scene leftward.
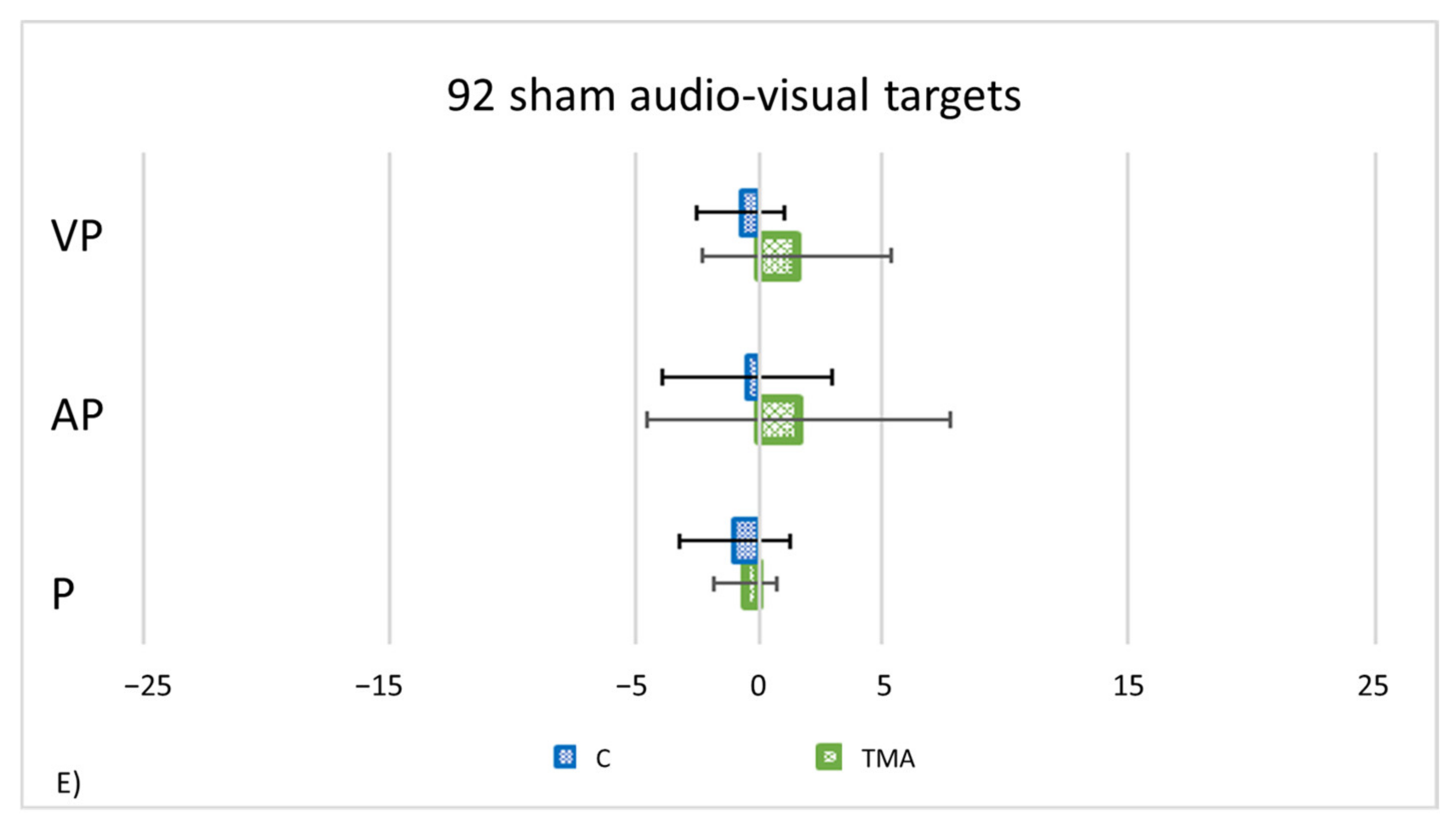
- PA condition: 92 audio-visual targets. AEs in all straight-ahead tests are present (Figure 13C); t-tests for case-control comparisons show that the mean scores of control participants (proprioceptive test: 2.70 ± 2.22; audio-proprioceptive test: 2.30 ± 2.73; visuo-proprioceptive test: 4.05 ± 1.75) do not differ from those of TMA (proprioceptive test: 4.85 ± 1.61, p = 0.35; audio-proprioceptive test: 7.45 ± 3.19, p = 0.07; visuo-proprioceptive test: 4.05 ± 2.86, p = 1).PA condition: sham. Crawford t-tests show no significant differences between TMA’s mean straight-ahead post-PA deviations and those of control participants in all conditions (Figure 13D,E).
3.3. Summary of Statistical Results
4. Discussion
4.1. Aftereffects (AEs) without Error Reduction
4.2. Recalibration and Spatial Realignment as Indipendent Mechanisms of PA
4.3. Anatomo-Functional Neural Correlates Involved in PA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| LANGUAGE | ||||
|---|---|---|---|---|
| Raw Score | Adjusted Score | Equivalent Score | Cut Off | |
| Phonemic Fluency Test [46] | 37 | 33 | 4 | ≤16 |
| Semantic Fluency Test [46] | 43 | 43 | 4 | ≤24 |
| E.N.P.A Reading [47] | ||||
| Words | n. p./10 | - | - | 6.4 |
| Non-words | n. p./5 | - | - | 4.0 |
| E.N.P.A writing [47] | ||||
| Words | 10/10 | 10 | - | 6.3 |
| Non-words | 2/5 * | 1.1 | - | 1.4 |
| E.N.P.A Naming [47] | ||||
| Colors | 5/5 | 5 | - | - |
| Token Test [48] | 11/36 * | 9 | - | <29 |
| Boston Naming Test [49] | 13/60 * | - | - | <43 |
| MEMORY | ||||
| Raw Score | Adjusted Score | Equivalent Score | Cut Off | |
| Auditory Digit Span [51] | 6/9 | 5.5 | 4 | ≤3.50 |
| Corsi’s Visuo-Spatial Span [51] | 5/9 | 5 | 4 | ≤3.25 |
| Recall of an auditory Short Story | [84] | |||
| Immediate Recall | 6.9/8 | 7.1 | 4 | <3.09 |
| Delayed Recall | 6.9/8 | 6.9 | 4 | <2.38 |
| Oblivion | 0 | 0 | 4 | >2.33 |
| Visuo-Spatial Supra-Span Learning [52] | 24.86/29.16 | 25.11 | 4 | ≤4.50 |
| VISUAL-SPATIAL AND CONSTRUCTIONAL ABILITIES | ||||
| Raw Score | Adjusted Score | Equivalent Score | Cut Off | |
| Line cancellation [53] | 19/21 * (Omissions: L = 0; R = 3) | - | - | ≥1 |
| Letter cancellation [55] | n. p. | - | - | ≥4 |
| Copy of geometric drawings [85] | 3/14 * | 2 | 0 | ≤7.75 |
| Drawings from memory | ||||
| Daisy | 2/2 | - | - | - |
| Butterfly | 2/2 | - | - | - |
| VISUAL PERCEPTION | ||||
| Raw Score | Adjusted Score | Equivalent Score | Cut Off | |
| V.O.S.P [43] | ||||
| Screening Test | 0/20 * | - | - | 15 |
| Line Orientation Test | 5/30 * | 8 | 0 | <17 |
| B.O.R.B. [45] | ||||
| Test 6: Overlapping Figure—Single letters: | ||||
| Response accuracy | 0/36 | - | - | - |
| Response time | 122.45 | - | - | - |
Appendix B. Statistical Results
| (a) | χ2 | df | Pr (>χ2) | ||
|---|---|---|---|---|---|
| Pointing number, quadratic trend | 514.02 | 2 | <0.001 | ||
| Lens type | 29.76 | 1 | <0.001 | ||
| Pointing number, quadratic trend * Lens type | 623.07 | 1 | <0.001 | ||
| (b) | Estimate | SE | df | t | Pr (>|t|) |
| (Intercept) | −0.73 | 0.1 | 46 | −7.01 | <0.001 |
| Pointing number, linear trend | 44.88 | 1.63 | 4364 | 27.45 | <0.001 |
| Pointing number, quadratic trend | −31.80 | 1.63 | 4364 | −19.45 | <0.001 |
| Lens type Prismatic vs. Sham | 0.8 | 0.15 | 46 | 5.456 | <0.001 |
| Pointing number, linear trend * Prismatic vs. Sham | −45.49 | 2.31 | 4364 | −19.672 | <0.001 |
| Pointing number, quadratic trend: Prismatic vs. Sham | −35.53 | 2.31 | 4364 | 15.365 | <0.001 |
| (a) | χ2 | df | Pr (>χ2) | ||
|---|---|---|---|---|---|
| Pointing number, quadratic trend | 10.46 | 2 | 0.005 | ||
| Lens type | 16.72 | 1 | <0.001 | ||
| Pointing number, quadratic trend * Lens type | 14.3 | 2 | <0.001 | ||
| (b) | Estimate | SE | df | t | Pr (>|t|) |
| (Intercept) | 2.34 | 0.34 | 46 | 6.81 | <0.001 |
| Pointing number, linear trend | 25.58 | 6.15 | 4364 | 4.16 | <0.001 |
| Pointing number, quadratic trend | 10.67 | 6.149 | 4364 | 1.736 | 0.08 |
| Lens type Prismatic vs. Sham | −1.98 | 0.49 | 46 | −4.086 | <0.001 |
| Pointing number, linear trend * Prismatic vs. Sham | −32.74 | 8.7 | 4364 | −3.76 | <0.001 |
| Pointing number, quadratic trend: Prismatic vs. Sham | −0.34 | 8.7 | 4364 | −0.039 | 0.9 |
| (a) | χ2 | df | Pr (>χ2) | ||
|---|---|---|---|---|---|
| Group | 4.3082 | 1 | <0.03 | ||
| Pointing number, quadratic trend | 286.4993 | 2 | <0.001 | ||
| Pointing number, quadratic trend * Group | 12.7584 | 2 | <0.002 | ||
| (b) | Estimate | SE | df | t | Pr (>|t|) |
| (Intercept) | 1.21 | 1.59 | 23 | 0.76 | 0.45 |
| Pointing number, linear trend | 23.22 | 14.1 | 571 | 1.65 | 0.1 |
| Pointing number, quadratic trend | −72.97 | 14.1 | 571 | −5.17 | <0.001 |
| TMA vs. healthy participants | −3.36 | 1.62 | 23 | −2.08 | 0.04 |
| Pointing number, linear trend * TMA vs. healthy participants | 17.21 | 14.4 | 571 | 1.2 | 0.23 |
| Pointing number, quadratic trend * TMA vs. healthy participants | 48.45 | 14.4 | 571 | 3.37 | <0.001 |
| (a) | χ2 | df | Pr (>χ2) | ||
|---|---|---|---|---|---|
| Group | 19.36 | 1 | <0.001 | ||
| Pointing number, quadratic trend | 647.31 | 2 | <0.001 | ||
| Pointing number, quadratic trend * Group | 17.71 | 2 | <0.002 | ||
| (b) | Estimate | SE | df | t. | Pr (>|t|) |
| (Intercept) | −0.73 | 0.14 | 23 | −5.17 | <0.001 |
| Pointing number, linear trend | 32.39 | 1.55 | 2271 | 20.84 | <0.001 |
| Pointing number, quadratic trend | −22.95 | 1.55 | 2271 | −14.77 | <0.001 |
| TMA vs. healthy participants | 3.1 | 0.7 | 23 | 4.4 | <0.001 |
| Pointing number, linear trend * TMA vs. healthy participants | 14.94 | 7.77 | 2271 | 1.92 | 0.05 |
| Pointing number, quadratic trend * TMA vs. healthy participants | 29.08 | 7.77 | 2271 | 3.74 | <0.001 |
| (a) | χ2 | df | Pr (>χ2) | ||
|---|---|---|---|---|---|
| Group | 9.37 | 2 | 0.009 | ||
| Pointing number, quadratic trend | 18.93 | 1 | <0.001 | ||
| Pointing number, quadratic trend * Group | 13.01 | 2 | 0.001 | ||
| (b) | Estimate | SE | df | t. | Pr (>|t|) |
| (Intercept) | 0.07 | 0.04 | 23 | 1.73 | 0 |
| Pointing number, linear trend | −0.43 | 0.85 | 2271 | −0.514 | 0.61 |
| Pointing number, quadratic trend | 2.68 | 0.85 | 2271 | 3.147 | 0.002 |
| TMA vs. healthy participants | 0.92 | 0.21 | 23 | 4.35 | <0.001 |
| Pointing number, linear trend * TMA vs. healthy participants | −13.21 | 4.27 | 2271 | −3.094 | 0.002 |
| Pointing number, quadratic trend * TMA vs. healthy participants | −7.92 | 4.27 | 2271 | −1.85 | 0.06 |
| (a) | χ2 | df | Pr (>χ2) | ||
|---|---|---|---|---|---|
| Group | 5.99 | 1 | <0.001 | ||
| Pointing number, quadratic trend | 16.16 | 2 | <0.001 | ||
| Pointing number, quadratic trend * Group | 6.42 | 2 | 0.04 | ||
| (b) | Estimate | SE | df | t. | Pr (>|t|) |
| (Intercept) | 2.34 | 0.46 | 23 | 5.1 | <0.001 |
| Pointing number, linear trend | 18.46 | 5.61 | 2271 | 3.29 | 0.001 |
| Pointing number, quadratic trend | 7.7 | 5.61 | 2271 | 1.37 | 0.16 |
| TMA vs. control participants | 5.61 | 2.29 | 23 | 2.44 | 0.02 |
| Pointing number, linear trend * TMA vs. control participants | 65.65 | 28.05 | 2271 | 2.34 | 0.01 |
| Pointing number, quadratic trend * TMA vs. control participants | −27.32 | 28.05 | 2271 | −0.97 | 0.33 |
| (a) | χ2 | df | Pr (>χ2) | ||
|---|---|---|---|---|---|
| Group | 10.7 | 2 | 0.004 | ||
| Pointing number, quadratic trend | 5.1 | 1 | 0.02 | ||
| Pointing number, quadratic trend * Group | 7.87 | 2 | 0.01 | ||
| (b) | Estimate | SE | df | t. | Pr (>|t|) |
| (Intercept) | 0.35 | 0.16 | 23 | 2.22 | 0.03 |
| Pointing number, linear trend | −5.16 | 2.74 | 2271 | −1.88 | 0.05 |
| Pointing number, quadratic trend | 7.46 | 2.74 | 2271 | 2.719 | 0.007 |
| TMA vs. control participants | 1.81 | 0.8 | 23 | 2.64 | 0.008 |
| Pointing number, linear trend * TMA vs. control participants | 36.27 | 13.71 | 2271 | 2.64 | 0.008 |
| Pointing number, quadratic trend * TMA vs. control participants | 12.83 | 13.71 | 2271 | 0.93 | 0.34 |
References
- Frassinetti, F.; Bolognini, N.; Ladavas, E. Enhancement of visual perception by crossmodal visuo-auditory interaction. Exp. Brain Res. 2002, 147, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, N.; Convento, S.; Fusaro, M.; Vallar, G. The sound-induced phosphene illusion. Exp. Brain Res. 2013, 231, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Hairston, W.D.; Laurienti, P.J.; Mishra, G.; Burdette, J.H.; Wallace, M.T. Multisensory enhancement of localization under conditions of induced myopia. Exp. Brain Res. 2003, 152, 404–408. [Google Scholar] [CrossRef]
- Leo, F.; Bolognini, N.; Passamonti, C.; Stein, B.E.; Làdavas, E. Cross-modal localization in hemianopia: New insights on multisensory integration. Brain 2008, 131. [Google Scholar] [CrossRef][Green Version]
- Colonius, H.; Arndt, P. A two-stage model for visual-auditory interaction in saccadic latencies. Percept. Psychophys. 2001, 63, 126–147. [Google Scholar] [CrossRef]
- Hughes, H.C.; Nelson, M.D.; Aronchick, D.M. Spatial characteristics of visual-auditory summation in human saccades. Vis. Res. 1998, 38, 3955–3963. [Google Scholar] [CrossRef]
- Harrington, L.K.; Peck, C.K. Spatial disparity affects visual-auditory interactions in human sensorimotor processing. Exp. Brain Res. 1998, 122, 247–252. [Google Scholar] [CrossRef]
- Corneil, B.D.; Van Wanrooij, M.; Munoz, D.P.; van Opstal, A.J. Auditory-Visual Interactions Subserving Goal-Directed Saccades in a Complex Scene. J. Neurophysiol. 2002, 88, 438–454. [Google Scholar] [CrossRef]
- Vallar, G.; Ronchi, R. Unilateral Spatial Neglect. In Encyclopedia of Behavioral Neuroscience, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2–3, pp. 605–618. [Google Scholar]
- Vallar, G.; Calzolari, E. Unilateral spatial neglect after posterior parietal damage. Handb. Clin. Neurol. 2018, 151, 287–312. [Google Scholar] [CrossRef]
- Halligan, P.W.; Fink, G.R.; Marshall, J.C.; Vallar, G. Spatial cognition: Evidence from visual neglect. Trends Cogn. Sci. 2003, 7, 125–133. [Google Scholar] [CrossRef]
- Zeki, S.; Leff, A. The striate cortex and hemianopia. Handb. Clin. Neurol. 2021, 178, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Van Vleet, T.M.; Robertson, L.C. Cross-modal Interactions in Time and Space: Auditory Influence on Visual Attention in Hemispatial Neglect. J. Cogn. Neurosci. 2006, 18, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, N.; Rasi, F.; Coccia, M.; Làdavas, E. Visual search improvement in hemianopic patients after audio-visual stimulation. Brain 2005, 128, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, F.; Pavani, F.; Làdavas, E. Acoustical Vision of Neglected Stimuli: Interaction among Spatially Converging Audiovisual Inputs in Neglect Patients. J. Cogn. Neurosci. 2002, 14, 62–69. [Google Scholar] [CrossRef]
- Frassinetti, F.; Bolognini, N.; Bottari, D.; Bonora, A.; Làdavas, E. Audiovisual Integration in Patients with Visual Deficit. J. Cogn. Neurosci. 2005, 17, 1442–1452. [Google Scholar] [CrossRef]
- Calzolari, E.; Albini, F.; Bolognini, N.; Vallar, G. Multisensory and Modality-Specific Influences on Adaptation to Optical Prisms. Front. Hum. Neurosci. 2017, 11, 568. [Google Scholar] [CrossRef]
- Zigiotto, L.; Damora, A.; Albini, F.; Casati, C.; Scrocco, G.; Mancuso, M.; Tesio, L.; Vallar, G.; Bolognini, N. Multisensory stimulation for the rehabilitation of unilateral spatial neglect. Neuropsychol. Rehabil. 2020, 31, 1410–1443. [Google Scholar] [CrossRef]
- Redding, G.M.; Rossetti, Y.; Wallace, B. Applications of prism adaptation: A tutorial in theory and method. Neurosci. Biobehav. Rev. 2005, 29, 431–444. [Google Scholar] [CrossRef]
- Frassinetti, F.; Angeli, V.; Meneghello, F.; Avanzi, S.; Ladavas, E. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain 2002, 125, 608–623. [Google Scholar] [CrossRef]
- Rossetti, Y.; Rode, G.; Pisella, L.; Farne’, A.; Li, L.; Boisson, D.; Perenin, M.-T. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 1998, 395, 166–169. [Google Scholar] [CrossRef]
- Fortis, P.; Maravita, A.; Gallucci, M.; Ronchi, R.; Grassi, E.; Senna, I.; Olgiati, E.; Perucca, L.; Banco, E.; Posteraro, L.; et al. Rehabilitating patients with left spatial neglect by prism exposure during a visuomotor activity. Neuropsychology 2010, 24, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Fortis, P.; Ronchi, R.; Velardo, V.; Calzolari, E.; Banco, E.; Algeri, L.; Spada, M.S.; Vallar, G. A home-based prism adaptation training for neglect patients. Cortex 2018, 122, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Redding, G.M.; Wallace, B. Prism adaptation and unilateral neglect: Review and analysis. Neuropsychologia 2006, 44, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Redding, G.M.; Wallace, B. Strategie Calibration and Spatial Alignment: A Model From Prism Adaptation. J. Mot. Behav. 2002, 34, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Redding, G.M.; Wallace, B. Generalization of prism adaptation. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 1006–1022. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.I. Fundamental Components of Attention. Annu. Rev. Neurosci. 2007, 30, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Bachus, L.; Knoth, E.; O’Neill, W.E.; Paige, G.D. Eye position and cross-sensory learning both contribute to prism adaptation of auditory space. Prog. Brain Res. 2008, 171, 265–270. [Google Scholar] [CrossRef]
- Michel, C.; Bonnet, C.; Podor, B.; Bard, P.; Poulin-Charronnat, B. Wearing prisms to hear differently: After-effects of prism adaptation on auditory perception. Cortex 2019, 115, 123–132. [Google Scholar] [CrossRef]
- A Bornschlegl, M.; Fahle, M.; Redding, G.M. The Role of Movement Synchronization with an Auditory Signal in Producing Prism Adaptation. Perception 2012, 41, 950–962. [Google Scholar] [CrossRef]
- Wallace, M.T.; Woynaroski, T.G.; Stevenson, R.A. Multisensory Integration as a Window into Orderly and Disrupted Cognition and Communication. Annu. Rev. Psychol. 2020, 71, 193–219. [Google Scholar] [CrossRef]
- Luauté, J.; Schwartz, S.; Rossetti, Y.; Spiridon, M.; Rode, G.; Boisson, D.; Vuilleumier, P. Dynamic Changes in Brain Activity during Prism Adaptation. J. Neurosci. 2009, 29, 169–178. [Google Scholar] [CrossRef]
- Pisella, L.; Rode, G.; Farne’, A.; Tilikete, C.; Rossetti, Y. Prism adaptation in the rehabilitation of patients with visuo-spatial cognitive disorders. Curr. Opin. Neurol. 2006, 19, 534–542. [Google Scholar] [CrossRef]
- Milner, D.; Goodale, M. The Visual Brain in Action, 2nd ed.; Oxford University Press: Oxford, UK, 2006; ISBN 9780198524724. [Google Scholar]
- Milner, A.; Goodale, M. Two visual systems re-viewed. Neuropsychologia 2008, 46, 774–785. [Google Scholar] [CrossRef]
- Milner, A.D. How do the two visual streams interact with each other? Exp. Brain Res. 2017, 235, 1297–1308. [Google Scholar] [CrossRef]
- Milner, A.D.; Cavina-Pratesi, C. Perceptual deficits of object identification: Apperceptive agnosia. Handb. Clin. Neurol. 2018, 151, 269–286. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Siddhartha, G.; Vijay, P. R-CHOP versus R-CVP in the treatment of follicular lymphoma: A meta-analysis and critical appraisal of current literature. J. Hematol. Oncol. 2009, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Felli, V.; Di Sibio, A.; Anselmi, M.; Gennarelli, A.; Sucapane, P.; Splendiani, A.; Catalucci, A.; Marini, C.; Gallucci, M. Progressive Multifocal Leukoencephalopathy following Treatment with Rituximab in an HIV-Negative Patient with Non-Hodgkin Lymphoma. Neuroradiol. J. 2014, 27, 657–664. [Google Scholar] [CrossRef]
- Bisiach, E.; Vallar, G.; Perani, D.; Papagno, C.; Berti, A. Unawareness of disease following lesions of the right hemisphere: Anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia 1986, 24, 471–482. [Google Scholar] [CrossRef]
- Pieri, V.; Sanvito, F.; Riva, M.; Petrini, A.; Rancoita, P.M.V.; Cirillo, S.; Iadanza, A.; Bello, L.; Castellano, A.; Falini, A. Along-tract statistics of neurite orientation dispersion and density imaging diffusion metrics to enhance MR tractography quantitative analysis in healthy controls and in patients with brain tumors. Hum. Brain Mapp. 2020, 42, 1268–1286. [Google Scholar] [CrossRef]
- Warrington, E.K.; James, M. Visual Object and Space Perception Battery (VOSP); Pearson Clinical Assessment UK: London, England, 1991. [Google Scholar]
- Benton, A.; Hannay, H.J.; Varney, N.R. Visual perception of line direction in patients with unilateral brain disease. Neurology 1975, 25, 907. [Google Scholar] [CrossRef] [PubMed]
- Riddoch, J.M.; Humphreys, G.W. BORB: Birmingham Object Recognition Battery; Lawrence Erbaum Associates: Mahwah, NJ, USA, 1993. [Google Scholar]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M.; Vallar, G.; Cappa, S.F. Tre Test Clinici Di Ricerca e Produzione Lessicale. Taratura Su Soggetti Normali. Archivio di Psicologia, Neurologia e Psichiatria 1986, 47, 477–506. [Google Scholar]
- Capasso, R.; Miceli, G. Esame Neuropsicologico per l’afasia (E.N.P.A.); Springer: Milano, Italy, 2001. [Google Scholar]
- De Renzi, E.; Faglioni, P. Normative Data and Screening Power of a Shortened Version of the Token Test. Cortex 1978, 14, 41–49. [Google Scholar] [CrossRef]
- Kaplan, E.; Goodglass, H. Weintraub Sandra The Boston Naming Test, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G.; Nocentini, U. Batteria per La Valutazione Del Deterioramento Mentale (Parte II): Standardizzazione e Affidabilità Diagnostica Nell’identificazione Di Pazienti Affetti Da Sindrome Demenziale. Archivio di Psicologia, Neurologia e Psichiatria 1995, 56, 471–488. [Google Scholar]
- Orsini, A.; Grossi, D.; Capitani, E.; Laiacona, M.; Papagno, C.; Vallar, G. Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Neurol. Sci. 1987, 8, 537–548. [Google Scholar] [CrossRef]
- Capitani, E.; Grossi, D.; Lucca, U.; Orsini, A.; Spinnler, H. Spatial and color cues in a route-learning task. Acta Neurol. 1980, 2. [Google Scholar]
- Albert, M.L. A simple test of visual neglect. Neurology 1973, 23, 658. [Google Scholar] [CrossRef]
- Diller, L.; Weinberg, J. Hemi-Inattention in Rehabilitation: The Evolution of a Rational Remediation Program. Adv. Neurol. 1977, 18, 63–82. [Google Scholar]
- Vallar, G.; Rusconi, M.L.; Fontana, S.; Musicco, M. Tre Test Di Esplorazione Visuo-Spaziale: Taratura Su 212 Soggetti Normali. Arch. Psicol. Neurol. Psichiatr. 1994, 55, 827–841. [Google Scholar]
- Servos, P.; Goodale, M.; Humphrey, G. The drawing of objects by a visual form agnosic: Contribution of surface properties and memorial representations. Neuropsychologia 1993, 31, 251–259. [Google Scholar] [CrossRef]
- Baayen, H.; Davidson, D.; Bates, D. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using Eigen and S4; (Version 1.1-7), [Computer Software]; The R Foundation: Vienna, Austria, 2014. [Google Scholar]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel Hierarchical Models; Cambridge University Press: New York, NY, USA, 2007; Volume 1. [Google Scholar]
- Huber, S.; Klein, E.; Moeller, K.; Willmes, K. Comparing a single case to a control group – Applying linear mixed effects models to repeated measures data. Cortex 2015, 71, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Estimating regression models with unknown break-points. Stat. Med. 2003, 22, 3055–3071. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Segmented: An R Package to Fit Regression Models with Broken-Line Relationships. R news 2008, 8, 20–25. [Google Scholar]
- Terruzzi, S.; Crivelli, D.; Pisoni, A.; Mattavelli, G.; Lauro, L.J.R.; Bolognini, N.; Vallar, G. The role of the right posterior parietal cortex in prism adaptation and its aftereffects. Neuropsychologia 2020, 150, 107672. [Google Scholar] [CrossRef]
- Terruzzi, S.; Crivelli, D.; Campana, E.; Pisoni, A.; Lauro, L.J.R.; Bolognini, N.; Vallar, G. Exploring the time-course and the reference frames of adaptation to optical prisms and its aftereffects. Cortex 2021, 141, 16–35. [Google Scholar] [CrossRef]
- Rossetti, Y.; Pisella, L. Optic ataxia: Beyond the dorsal stream cliché. Handb. Clin. Neurol. 2018, 151, 225–247. [Google Scholar] [CrossRef]
- Perenin, M.-T.; Vighetto, A. Optic Ataxia: A Specific Disruption in Visuomotor Mechanisms. Brain 1988, 111, 643–674. [Google Scholar] [CrossRef]
- Rossetti, Y.; Pisella, L.; McIntosh, R.D. Definition: Optic ataxia. Cortex 2019, 121, 481. [Google Scholar] [CrossRef]
- Khan, A.Z.; Pisella, L.; Delporte, L.; Rode, G.; Rossetti, Y. Testing for optic ataxia in a blind field. Front. Hum. Neurosci. 2013, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.J.S.; E Black, S. Line bisection in hemianopia. J. Neurol. Neurosurg. Psychiatry 1998, 64, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.J.; Behrmann, M.; Black, S. Ocular search during line bisection. The effects of hemi-neglect and hemianopia. Brain 1998, 121, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Quentin, R.; Awosika, O.; Cohen, L.G. Plasticity and recovery of function. Handb. Clin. Neurol. 2019, 163, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.E.; Stanford, T.R. Multisensory integration: Current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 2008, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Laurienti, P.J.; Kraft, R.A.; Maldjian, J.A.; Burdette, J.H.; Wallace, M.T. Semantic congruence is a critical factor in multisensory behavioral performance. Exp. Brain Res. 2004, 158, 405–414. [Google Scholar] [CrossRef]
- Striemer, C.L.; Russell, K.; Nath, P. Prism adaptation magnitude has differential influences on perceptual versus manual responses. Exp. Brain Res. 2016, 234, 2761–2772. [Google Scholar] [CrossRef]
- Wilf, M.; Cheraka, M.C.; Jeanneret, M.; Ott, R.; Perrin, H.; Crottaz-Herbette, S.; Serino, A. Combined virtual reality and haptic robotics induce space and movement invariant sensorimotor adaptation. Neuropsychologia 2020, 150, 107692. [Google Scholar] [CrossRef]
- Michel, C.; Pisella, L.; Prablanc, C.; Rode, G.; Rossetti, Y. Enhancing Visuomotor Adaptation by Reducing Error Signals: Single-step (Aware) versus Multiple-step (Unaware) Exposure to Wedge Prisms. J. Cogn. Neurosci. 2007, 19, 341–350. [Google Scholar] [CrossRef]
- Panico, F.; Rossetti, Y.; Trojano, L. On the mechanisms underlying Prism Adaptation: A review of neuro-imaging and neuro-stimulation studies. Cortex 2020, 123, 57–71. [Google Scholar] [CrossRef]
- Saj, A.; Cojan, Y.; Vocat, R.; Luauté, J.; Vuilleumier, P. Prism adaptation enhances activity of intact fronto-parietal areas in both hemispheres in neglect patients. Cortex 2013, 49, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Boukrina, O.; Chen, P. Neural Mechanisms of Prism Adaptation in Healthy Adults and Individuals with Spatial Neglect after Unilateral Stroke: A Review of fMRI Studies. Brain Sci. 2021, 11, 1468. [Google Scholar] [CrossRef] [PubMed]
- Schintu, S.; Freedberg, M.; Gotts, S.J.; Cunningham, C.A.; Alam, Z.M.; Shomstein, S.; Wassermann, E.M. Prism Adaptation Modulates Connectivity of the Intraparietal Sulcus with Multiple Brain Networks. Cereb. Cortex 2020, 30, 4747–4758. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, K.; Mizuno, K.; Nishida, D.; Tahara, M.; Yamada, E.; Shindo, S.; Kasuga, S.; Liu, M. Prism adaptation changes resting-state functional connectivity in the dorsal stream of visual attention networks in healthy adults: A fMRI study. Cortex 2019, 119, 594–605. [Google Scholar] [CrossRef]
- Wilf, M.; Serino, A.; Clarke, S.; Crottaz-Herbette, S. Prism adaptation enhances decoupling between the default mode network and the attentional networks. NeuroImage 2019, 200, 210–220. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Buccione, I.; Fadda, L.; Graceffa, A.; Mauri, M.; Lorusso, S.; Bevilacqua, G.; Caltagirone, C. Normative Data of Two Memory Tasks: Short-Story Recall and Rey’s Figure. Nuova Riv. Neurol. 2002, 12, 1–13. [Google Scholar]
- Spinnler, H.R.; Tognoni, G. Standardizzazione e Taratura Italiana Di Test Neuropsicologici. Ital. J. Neurol. Sci. 1987, 8, S1–S120. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albini, F.; Pisoni, A.; Salvatore, A.; Calzolari, E.; Casati, C.; Marzoli, S.B.; Falini, A.; Crespi, S.A.; Godi, C.; Castellano, A.; et al. Aftereffects to Prism Exposure without Adaptation: A Single Case Study. Brain Sci. 2022, 12, 480. https://doi.org/10.3390/brainsci12040480
Albini F, Pisoni A, Salvatore A, Calzolari E, Casati C, Marzoli SB, Falini A, Crespi SA, Godi C, Castellano A, et al. Aftereffects to Prism Exposure without Adaptation: A Single Case Study. Brain Sciences. 2022; 12(4):480. https://doi.org/10.3390/brainsci12040480
Chicago/Turabian StyleAlbini, Federica, Alberto Pisoni, Anna Salvatore, Elena Calzolari, Carlotta Casati, Stefania Bianchi Marzoli, Andrea Falini, Sofia Allegra Crespi, Claudia Godi, Antonella Castellano, and et al. 2022. "Aftereffects to Prism Exposure without Adaptation: A Single Case Study" Brain Sciences 12, no. 4: 480. https://doi.org/10.3390/brainsci12040480
APA StyleAlbini, F., Pisoni, A., Salvatore, A., Calzolari, E., Casati, C., Marzoli, S. B., Falini, A., Crespi, S. A., Godi, C., Castellano, A., Bolognini, N., & Vallar, G. (2022). Aftereffects to Prism Exposure without Adaptation: A Single Case Study. Brain Sciences, 12(4), 480. https://doi.org/10.3390/brainsci12040480







