Abstract
Although pontine infarction is the most common subtype of posterior circulation stroke, there has been little research focusing on recurrent pontine infarction. Our study aimed to investigate the factors associated with site and mechanism of recurrent pontine infarction. Patients with acute isolated pontine infarction were enrolled and followed up for one year. Lesion topography was determined by diffusion-weighted imaging. Mechanisms were determined based on lesion topography and other vascular, cardiologic and laboratory results. A total of 562 patients with pontine infarction were included, with 67 patients experiencing recurrence during the follow-up period. Forty-one recurrences occurred at the same site as index pontine infarction (41/67, 61.2%). Results indicated that the mechanism of index pontine infarction was significantly associated with the recurrent sites (p = 0.041, OR 2.938, 95% CI 1.044–8.268), and also with the mechanisms of recurrence (p = 0.004, OR 6.056, 95% CI 1.774–20.679). Branch atheromatous disease-induced index pontine infarction was likely to recur at the same site and with the same mechanism. Moreover, if recurrence occurred at the same site, the mechanism was probably the same as that of the index stroke (p = 0.000). Our study may help physicians treat patients with pontine infarction by predicting the site and mechanism of recurrence.
1. Introduction
The first and key step of preventing recurrent stroke is to understand its knowledge, especially where and why it would recur [1]. Previous studies have shown that intracranial large-artery disease would cause recurrence at the same site or adjacent to the index stroke [2], which made it possible to predict recurrence. However, the site and mechanism of recurrence caused by branch atheromatous disease (BAD) and small vessel disease (SVD) remain unclear.
Pontine infarction is the most common stroke subtype in the posterior circulation [3]. Referring to stroke etiology, BAD was the most frequent mechanism in pontine infarction (39%–44%), followed by SVD (34%) [4,5,6,7]. Large artery disease (LAD) and other mechanisms, such as potential cardiac sources of embolism (PCSE), were less common [4,5,6,7]. Pontine infarction etiology has been proven to be correlated with lesion topography [8,9]. BAD was particularly associated with large ventral infarctions reaching the pontine surface [7]. SVD was usually defined as small (<15 mm) lesions sparing the surface [3]. The prognosis of pontine infarction varies among different mechanisms. Short-term (30 days to 3 months) and long-term (9 months to 5 years) outcomes were relatively benign in patients with SVD but worse in patients with BAD [4,6,10,11,12].
Patients with BAD or SVD might have a high risk of recurrence, and some previous studies suggested a specific stroke pathophysiology in these patient subgroups [13]. In our previous publication, we conducted a multicenter study that recruited the largest cohort of patients with pontine infarction [5]. In the present study, we analyzed one-year follow-up data to elucidate the topographic locations and mechanisms of recurrent pontine infarction. To our knowledge, this study is the first to investigate the factors associated with site and mechanism of recurrent pontine stroke.
2. Materials and Methods
2.1. Study Design
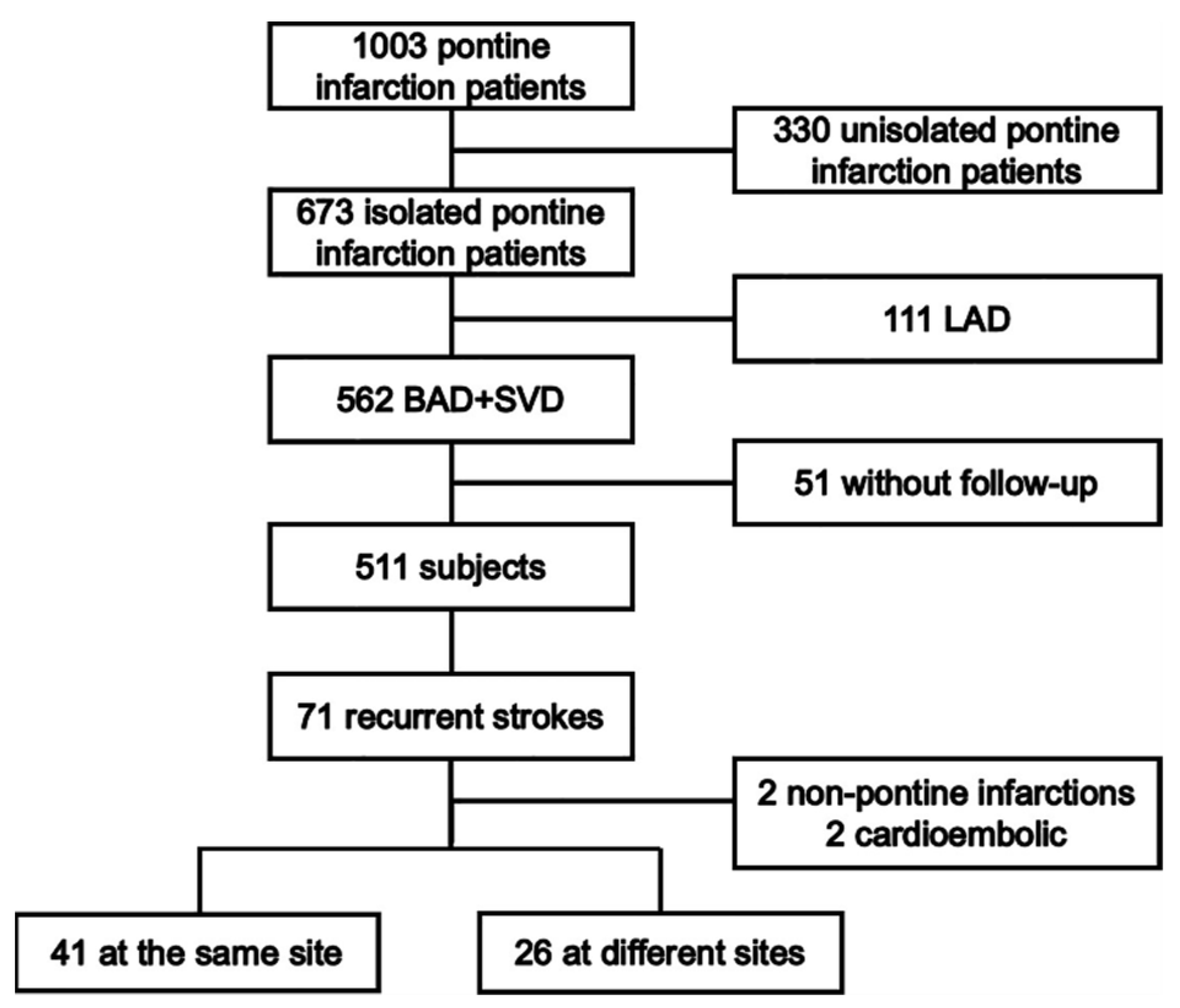
A schematic diagram of the study design is shown in Figure 1. This was a multicenter hospital-based follow-up study that enrolled patients with index pontine infarction admitted from 1 May 2003 to 31 October 2017 (The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou Panyu District Hexian Memorial Hospital, Shanghai General Hospital, Wuxi People’s Hospital, the First Affiliated Hospital of Suzhou University, and the First Affiliated Hospital of Sun Yat-sen University). The research protocol was reviewed and approved by the ethics committees of each institution. Written consent was obtained from patients or their authorized relatives before enrollment. Details were previously reported [5].
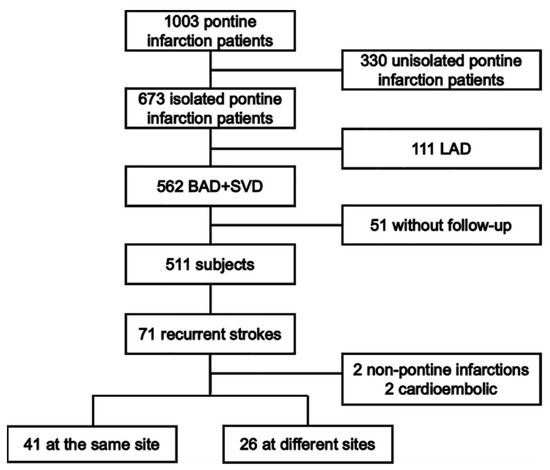
Figure 1.
The schematic diagram of the study design.
2.2. Study Population
Patients that met the following criteria were included: (1) over 18 years old; (2) with symptoms of stroke; (3) admitted within 7 days after onset and diagnosed with acute pontine infarction by diffusion weighted imaging (DWI); (4) with intracranial and extracranial cerebral arteries visualized by ultrasound, magnetic resonance angiography (MRA), computed tomography angiography (CTA) or digital subtraction angiography (DSA); and (5) index stroke mechanism was classified as BAD or SVD. Patients were excluded if they met any of the following criteria: (1) missing clinical or imaging information, or images could not be identified; (2) tumor, parasites, or hemangioma in the pons; (3) pontine hemorrhage or trauma; (4) a previous history of atrial fibrillation or newly diagnosed atrial fibrillation; (5) two or more mechanisms; (6) nonatherosclerotic stroke; or (7) index or recurrent stroke involving the regions outside of the pons. The patients received standard therapy including thrombolytic therapy, embolectomy or other medications at the acute stage of index stroke, and then they were given standard secondary prevention including stroke risk control, antiplatelet drugs (aspirin and clopidogrel for 3 weeks following by aspirin or clopidogrel) and standard dose of statin. All patients were followed up for 12 months (at 1, 3, 6, and 12 months after discharge) by telephone or in the outpatient department. If any suspected new neurological symptoms emerged, the patients were evaluated by one senior neurologist. If diagnosed as recurrent stroke by the senior neurologist, MRI scanning was performed to confirm the sites of new infarction at 72 h—7 d after onset.
2.3. Demographic Characteristics
On admission, demographic information (including age and gender), risk factors for stroke (including current smoking or drinking, hypertension, hyperlipidemia, diabetes, and previous stroke or transient ischemic attack (TIA)), and current medications on admission were acquired. Current drinking was defined as heavy intake (≥14 drinks/week in women or 21 drinks/week in men) or episodic heavy intake (≥5 drinks/episode at least once per month) [14]. Blood glucose, blood cholesterol, and cardiac examinations (electrocardiogram and echocardiogram) were also performed.
2.4. The Site and Mechanisms of Pontine Infarction
All patients underwent MRI using either 1.5 T or 3.0 T MRI unit. Standard axial and sagittal MRI sequences, including T1-weighted imaging, T2-weighted imaging, DWI, fluid-attenuated inversion recovery sequence, apparent diffusion coefficient maps, and time-of-flight MRA covering the circle of Willis were applied. Lesion topography was determined based on DWI with reference to other sequences. Sagittal sections were used to confirm whether there was associated infarcts in the medulla oblongata, mesencephalon and thalamus or sporadic infarct in distant areas. There were usually three axial images from rostral to caudal pons. All sections were interpreted by two independent imaging physicians who were blinded to the study design.
Based on their clinical symptoms, infarction site and size on DWI and the results of vascular, cardiologic and laboratory results, the mechanisms of index or recurrent pontine infarction could be divided into 5 categories: (1) LAD was presumed in patients with a stenosis of more than 50% of the lumen diameter in the large artery (vertebral or basilar artery) corresponding to the infarct territory, (2) BAD was presumed in patients with an infarct reaching or approaching the ventral pontine surface in the absence of LAD corresponding to the infarct and potential sources of cardio-embolism, (3) SVD was considered if the patients had a small (less than 15 mm in diameter) deep (sparing the surface of the pons) infarct lesion, in the absence of other etiologies, (4) PCSE mainly included nonvalvular atrial fibrillation, mitral stenosis, a prosthetic valve, myocardial infarction within 6 weeks, intracardiac clot, ventricular aneurysm and bacterial endocarditis, and (5) other undetermined etiologies [6].
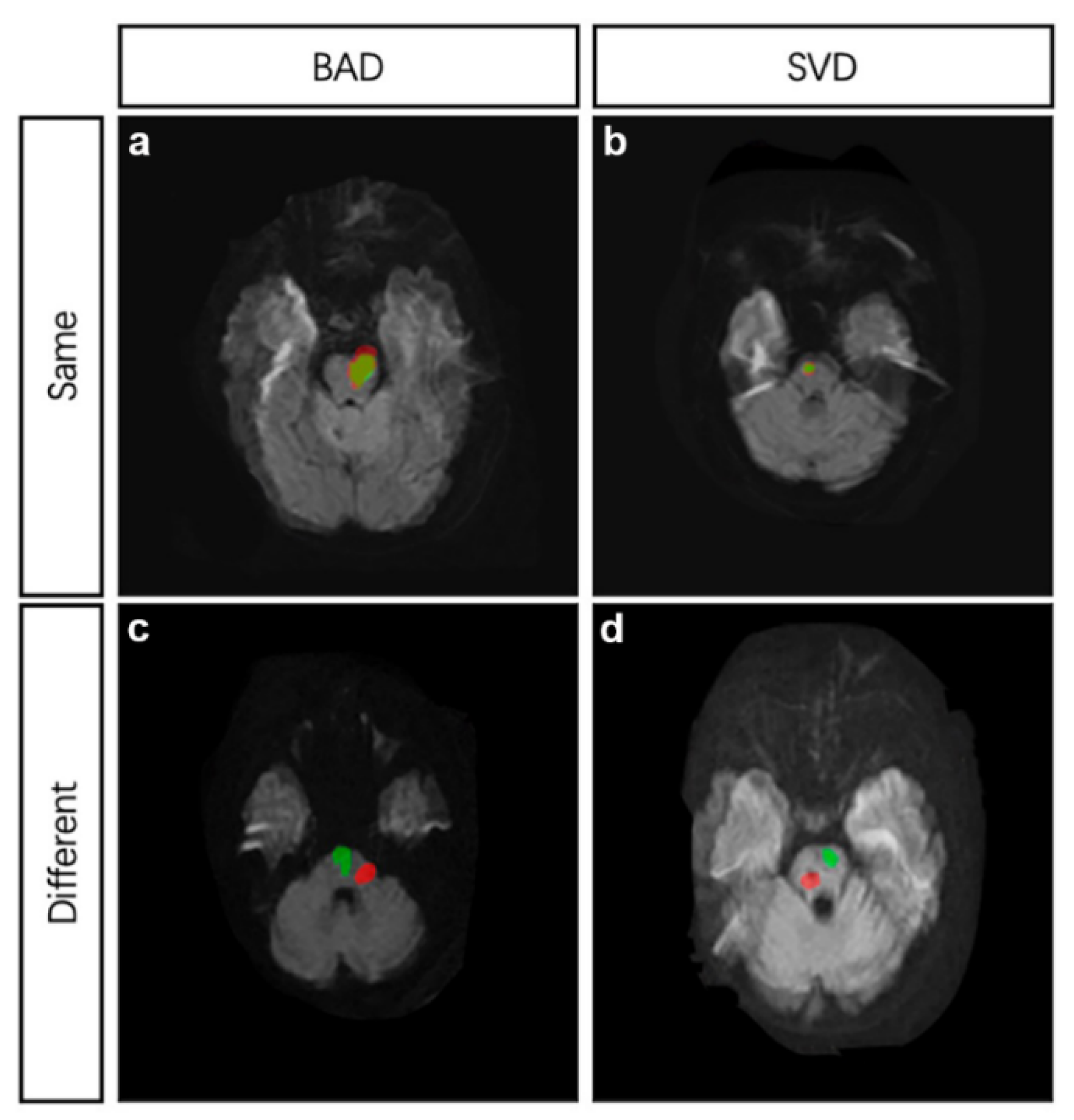
To compare the site of recurrence, two DWI images in TIFF from index and recurrent stroke at the same cross section were dealt with in Photoshop 7.0. Briefly, open the first DWI image; add the secondary DWI image to another layer in the same project; adjust the transparency of the second image; and resize the second image with reference to the basilar artery and the fourth ventricle. The same site of two strokes was defined as follows (Figure 2): (1) if the index and recurrent infarctions were both BAD, and the recurrence was confirmed to be caused by the same branch artery; (2) if both the index and recurrent infarctions were SVD, and the recurrent infarction mostly overlapped with the index infarction; (3) if index infarction was SVD and recurrent infarction was BAD, and the recurrent infarction area covered the index infarction; and (4) if index infarction was BAD and recurrent infarction was SVD, only if the recurrent infarction located within the range of index infarction area would the infarct site be considered the same as index stroke. Cohen’s kappa was used to determine the intra-observer agreement. The kappa value was 0.91 (p < 0.05).
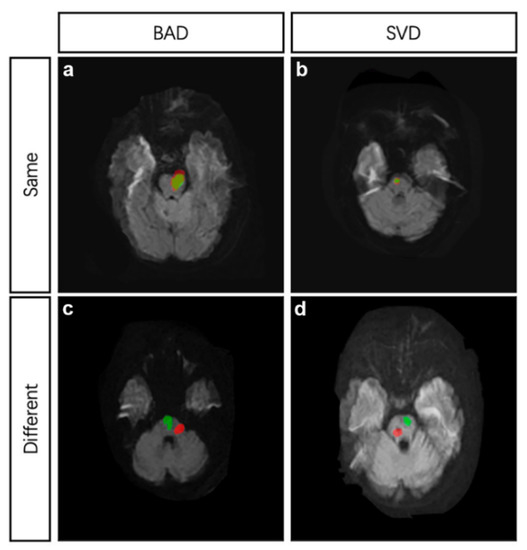
Figure 2.
MRI demonstrations of four different conditions. (a), the index stroke and recurrent stroke were both attributed to BAD and located at the same site. (b), the index stroke and recurrent stroke were both attributed to SVD and located at the same site. (c), the index stroke and recurrent stroke were both attributed to BAD but located at different sites. (d), the index stroke and recurrent stroke were both attributed to SVD but located at different sites. Red area indicates the index infarction while green area indicates the recurrent infarction. BAD, branch atheromatous disease, SVD, small vessel disease.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS (version 20.0). Continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed continuous variables are expressed as the mean ± standard deviation, while categorical variables are expressed as percentages. Differences in continuous variables were compared by Student’s t test (normally distributed) or the equivalent nonparametric test (nonnormally distributed). Univariate and multivariate logistic regression were performed to confirm the factors independently associated with the site and mechanism of recurrence. Variables with a potential association from univariate analysis (p < 0.2) were used for multivariate analysis. Pearson’s chi-square test was used to assess differences in the mechanisms of index and recurrent pontine infarctions. p < 0.05 was considered statistically significant.
3. Result
3.1. Participants
A total of 1003 patients with pontine infarction were screened, as shown in Figure 1. Of these, 330 patients with confirmed cases of unisolated pontine infarction were excluded. For the remaining 673 patients with isolated pontine infarction, 111 patients with index pontine infarction attributed to LAD were excluded. During the 12-month follow-up, 51 patients were lost. A total of 71 recurrent strokes occurred, with two located in the regions outside of the pons and two derived from cardio-embolism. The rate of recurrent pontine infarction was 13.1% (67/511). There were 21 (4.1%), 32 (6.3%), 40 (7.8%) and 67 (13.1%) stroke recurrence on 1, 3, 6, and 12 months after the index pontine infarction, respectively. There were more recurrences at the same site than at different sites (41 vs. 26). The demographic and clinical characteristics are presented in Table 1.

Table 1.
Site of recurrence.
3.2. Mechanism of Index Pontine Infarction Associated with Site of Recurrence
In the univariate logistic regression analysis, antihypertensives usage and mechanisms of index pontine infarction were potentially associated with the same site of recurrence (p < 0.2). In the multivariate logistic regression analysis, BAD as the mechanism of index pontine infarction was significantly associated with the same site of recurrence (p = 0.041, OR 2.938, 95% CI 1.044–8.268, Table 1).
3.3. Mechanism of Index Pontine Infarction Associated with Mechanism of Recurrence
In the univariate logistic regression analysis, hypertension, diabetes, index stroke mechanism and site of recurrence were potentially associated with the mechanism of recurrence (p < 0.2). In the multivariate logistic regression analysis, hypertension (p = 0.038, OR 6.010, 95% CI 1.100–32.850, Table 2), BAD of index stroke (p = 0.004, OR 6.056, 95% CI 1.774–20.679, Table 2) and the same site of recurrence (p = 0.045, OR 3.710, 95% CI 1.030–13.364, Table 2) were significantly associated with BAD of recurrent pontine infarction. For the subgroup analysis, if recurrence of BAD-induced index stroke occurred at the same site, the mechanism of recurrence was probably BAD (p = 0.000, Table 3), and if recurrence of SVD occurred at the same site, it was likely to be another SVD (p = 0.000, Table 3). However, if recurrence occurred at different sites, the mechanism was not significantly related to the mechanism of index pontine infarction (p > 0.05, Table 3).

Table 2.
Mechanism of recurrence.

Table 3.
Relationship of the mechanism of index pontine infarction with the site and mechanism of recurrent pontine infarction.
4. Discussion
The present study investigated the factors associated with the site and mechanism of recurrent pontine stroke. Our data showed that 13.1% of patients with pontine infarction had recurrence in the first year. The majority of recurrences occurred at the same site of the index pontine infarction. Recurrence in patients with BAD was likely to occur at the same site, while there was no such feature in patients with SVD. The mechanism of recurrence was significantly related to that of index pontine infarction. The main strength of our study was to demonstrate the relationship of the site and mechanism of index pontine infarction with the site and mechanism of recurrent pontine infarction, which would help physicians predict where and why recurrent pontine infarction is going to occur.
Pontine infarction was found to tend to recur, while few researches regarding the recurrence rate of pontine stroke has been conducted. Based on our data, the one-month, three-month, six-month and one-year recurrence rate of pontine infarction caused by BAD and SVD were 4.1%, 6.3%, 7.8% and 13.1%, respectively, which were similar to the recurrence rates of posterior circulation stroke [4,10] and higher than that of anterior circulation stroke [15,16,17]. The high rate of recurrence warrants additional research. Some factors such as concomitant presence of coronary heart disease and higher number of risk factors for atherosclerosis were found to be related to the recurrence of pontine stroke [18]. To explore the characteristics of recurrent pontine infarction, the first question was to determine where recurrent pontine infarction would happen.
In the present study, we found that 61.2% of recurrences occurred at the same site as the index stroke. Previous studies have demonstrated that large intracranial artery disease including basilar artery disease always caused recurrent stroke in the same area [1,19,20]. The recurrence pattern of large intracranial artery disease could be explained by artery-to-artery embolism [21]. For branch artery and small artery diseases, no such evidence has been reported. In this study, demographic information (including age and gender), risk factors for stroke (including current smoker or drinker, the presence of hypertension, hyperlipidemia, diabetes, and stroke or TIA history), current medications (including antihypertensives, hypoglycemics, statins, antiplatelets), and the mechanisms of index pontine infarction (including BAD and SVD) were included in univariate logistic regression analysis, then multivariate logistic regression analysis, to investigate the potential factors associated with the site and mechanism of recurrent pontine stroke. The results showed that the mechanism of index stroke was associated with the site of recurrence. Patients with BAD in the first stroke were likely to be attacked at the same site during recurrence, while there was no such feature in patients with SVD. The underlying mechanism remained undetermined. The possible reason might be that BAD was an occlusion or stenosis at the origin of one penetrating artery without affecting other penetrating arteries [3]. Thus, recurrence would be caused by thrombosis formed at the origin of the same penetrating artery. In SVD referring to random terminal artery occlusion due to lipohyalinosis or other pathogenies, there would be equal opportunity to cause recurrence at any site [22]. Thus, it was assumed that the mechanism of recurrence would be related to that of index strokes.
In the next step of the analysis, hypertension, mechanism of index stroke and recurrence at the same site were found to be significantly associated with the mechanism of recurrent pontine infarction. It is beyond our expectation that hypertension was related to the mechanism of recurrent stroke, since it is the risk factor for both branch atheromatous disease and small vessel diseases [6,7]. As expected, BAD in the first stroke was likely to cause another BAD during recurrence. In the subgroup analysis, if the recurrence occurred at the same site as the index stroke, the mechanism was probably the same as the mechanism of the index stroke. However, if recurrence occurred at different sites, the mechanism of recurrence was unpredictable.
There were some limitations to this study. First, even with the largest sampling number involving recurrence of pontine stroke, this study included a relatively small number of patients with recurrence, especially in the subgroup analysis. Then, index and recurrent pontine infarction were strictly defined according to the MRI findings. This would cause potential bias for some patients unable to receive MRI due to restrictions, such as having a pacemaker.
In conclusion, our data indicated that 13.1% of patients with pontine infarction experienced recurrence during the first year, the site and mechanism of which was associated with the index pontine infarction mechanism. Patients with BAD-induced index pontine infarction were likely to be affected at the same site during recurrence, and patients with recurrence at the same site were more likely to have the same mechanism as index pontine infarction. These phenomena might give neurologists a suggestion in predicting the site and mechanisms of recurrent pontine infarction and help them adapt appropriate prevention measures to these patients according to the infarct site and mechanism.
Author Contributions
Conceptualization, Y.J. and L.W.; methodology, Y.J. and Z.Y.; software, Y.L., C.L. (Chenghao Li) and C.L. (Chaowei Lie); validation, Y.J. and L.W.; formal analysis, Y.L.; investigation, Y.L., Z.Y., D.L., Z.D., J.Z. and H.C.; resources, Y.J., L.W. and Z.Y.; data curation, Y.L.; writing—original draft preparation, L.W.; writing—review and editing, Y.J.; visualization, Y.L.; supervision, Y.J.; project administration, Y.J.; funding acquisition, Y.J., L.W. and Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation of China (grant number 81870933), GuangDong Basic and Applied Basic Research Foundation (grant number 2021A1515011351), Guangzhou Science and Technology Project (grant number 202102010127), and the Opening Lab Program of Guangzhou Medical University (grant number 0506308) to Y Jiang, and by the National Science Foundation of China (grant number 81501009), the Scientific Program of Guangzhou Municipal Health Commission (grant number 20211A011080) and the Guangdong Provincial Medical Research Fund (grant number A2021406) to L Wu, and by the Science and Technology Planning Project of Panyu District (grant number 2019-Z04-11) to Z Ye.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of each institute (the Second Affiliated Hospital of Guangzhou Medical University, protocol code: 2002-wz-12, date of approval: 9 September 2002; Guangzhou Panyu District Hexian Memorial Hospital, protocol code: 2002100018, date of approval: 11 October 2002; the First Affiliated Hospital, Sun Yat-Sen University, protocol code: ct-2002-12-11, date of approval: 30 December 2002; the First Affiliated Hospital of Soochow University, protocol code: 200200112, date of approval: 12 December 2002; Wuxi People’s Hospital, protocol code: lcsy-2002-11-021, date of approval: 10 November 2002; and Shanghai General Hospital, protocol code: 200211101312, date of approval: 13 October 2002).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Shin, D.H.; Lee, P.H.; Bang, O.Y. Mechanisms of recurrence in subtypes of ischemic stroke: A hospital-based follow-up study. Arch. Neurol. 2005, 62, 1232–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, T.W.; Wang, L.; Soo, Y.O.; Ip, V.H.; Chan, A.Y.; Au, L.W.; Fan, F.S.; Lau, A.Y.; Leung, H.; Abrigo, J.; et al. Evolution of intracranial atherosclerotic disease under modern medical therapy. Ann. Neurol. 2015, 77, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, X.; Chen, W.; Lin, J.; Chai, Z.; Yi, X. Stroke Subtypes and Topographic Locations Associated with Neurological Deterioration in Acute Isolated Pontine Infarction. J. Stroke Cerebrovasc. Dis. 2016, 25, 206–213. [Google Scholar] [CrossRef]
- Vemmos, K.N.; Spengos, K.; Tsivgoulis, G.; Manios, E.; Zis, V.; Vassilopoulos, D. Aetiopathogenesis and long-term outcome of isolated pontine infarcts. J. Neurol. 2005, 252, 212–217. [Google Scholar] [CrossRef]
- Huang, J.; Qiu, Z.; Zhou, P.; Li, J.; Chen, Y.; Huang, R.; Li, C.; Ouyang, X.; Feng, H.; Xu, H.; et al. Topographic location of unisolated pontine infarction. BMC Neurol. 2019, 19, 186. [Google Scholar] [CrossRef] [Green Version]
- Kumral, E.; Bayulkem, G.; Evyapan, D. Clinical spectrum of pontine infarction. Clinical-MRI correlations. J. Neurol. 2002, 249, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Gökçal, E.; Niftaliyev, E.; Baran, G.; Deniz, Ç.; Asil, T. Progressive deficit in isolated pontine infarction: The association with etiological subtype, lesion topography and outcome. Acta Neurol. Belg. 2017, 117, 649–654. [Google Scholar] [CrossRef]
- Klein, I.F.; Lavallée, P.C.; Mazighi, M.; Schouman-Claeys, E.; Labreuche, J.; Amarenco, P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: A high-resolution MRI study. Stroke 2010, 41, 1405–1409. [Google Scholar] [CrossRef] [Green Version]
- Frid, P.; Drake, M.; Giese, A.K.; Wasselius, J.; Schirmer, M.D.; Donahue, K.L.; Cloonan, L.; Irie, R.; Bouts, M.J.; McIntosh, E.C.; et al. Detailed phenotyping of posterior vs. anterior circulation ischemic stroke: A multi-center MRI study. J. Neurol. 2020, 267, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, S.; Jordan, J.; Mishra, S. Clinical presentation as a guide to early prognosis in vertebrobasilar stroke. Stroke 1992, 23, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Lin, Q.; Lu, J.; Rong, D.; Zhao, Z.; Ma, Q.; Liu, H.; Shu, N.; He, Y.; Li, K. Pontine infarction: Diffusion-tensor imaging of motor pathways—A longitudinal study. Radiology 2015, 274, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Hussain, M.; Asmaro, K.; Zhao, X.; Liu, L.; Li, J.; Wang, Y. Clinical and imaging characteristics of isolated pontine infarcts: A one-year follow-up study. Neurol. Res. 2013, 35, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.M. The ‘herald hemiparesis’ of basilar artery occlusion. Arch. Neurol. 1988, 45, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhu, M.; Shi, W.; Li, M.; Chen, W.; Dai, Q.; Jiang, Y.; Liu, X. The Incidence and Risk Factors of In-Stent Restenosis for Vertebrobasilar Artery Stenting. World Neurosurg. 2018, 110, e937–e941. [Google Scholar] [CrossRef]
- Boulanger, M.; Béjot, Y.; Rothwell, P.M.; Touzé, E. Long-Term Risk of Myocardial Infarction Compared to Recurrent Stroke After Transient Ischemic Attack and Ischemic Stroke: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e007267. [Google Scholar] [CrossRef] [Green Version]
- Amarenco, P.; Lavallée, P.C.; Labreuche, J.; Albers, G.W.; Bornstein, N.M.; Canhão, P.; Caplan, L.R.; Donnan, G.A.; Ferro, J.M.; Hennerici, M.G.; et al. One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N. Engl. J. Med. 2016, 374, 1533–1542. [Google Scholar] [CrossRef]
- Khanevski, A.N.; Bjerkreim, A.T.; Novotny, V.; Næss, H.; Thomassen, L.; Logallo, N.; Kvistad, C.E.; NOR-STROKE Study Group. Recurrent ischemic stroke: Incidence, predictors, and impact on mortality. Acta Neurol. Scand. 2019, 140, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S. Recurrent pontine base infarction: A controlled study. Cerebrovasc. Dis. 2002, 13, 257–261. [Google Scholar] [CrossRef]
- Sangha, R.S.; Prabhakaran, S.; Feldmann, E.; Honda, T.; Nizam, A.; Cotsonis, G.A.; Campo-Bustillo, I.; Romano, J.G.; Liebeskind, D.S. Imaging Patterns of Recurrent Infarction in the Mechanisms of Early Recurrence in Intracranial Atherosclerotic Disease (MyRIAD) Study. Front. Neurol. 2020, 11, 615094. [Google Scholar] [CrossRef]
- Raghuram, K.; Durgam, A.; Kohlnhofer, J.; Singh, A. Relationship between stroke recurrence, infarct pattern, and vascular distribution in patients with symptomatic intracranial stenosis. J. Neurointerv. Surg. 2018, 10, 1161–1163. [Google Scholar] [CrossRef]
- López-Cancio, E.; Matheus, M.G.; Romano, J.G.; Liebeskind, D.S.; Prabhakaran, S.; Turan, T.N.; Cotsonis, G.A.; Lynn, M.J.; Rumboldt, Z.; et al.; Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial investigators Infarct patterns, collaterals and likely causative mechanisms of stroke in symptomatic intracranial atherosclerosis. Cerebrovasc. Dis. 2014, 37, 417–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regenhardt, R.W.; Das, A.S.; Ohtomo, R.; Lo, E.H.; Ayata, C.; Gurol, M.E. Pathophysiology of Lacunar Stroke: History’s Mysteries and Modern Interpretations. J. Stroke Cerebrovasc. Dis. 2019, 28, 2079–2097. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).