Current Clinical Trials in Traumatic Brain Injury
Abstract
:1. Introduction
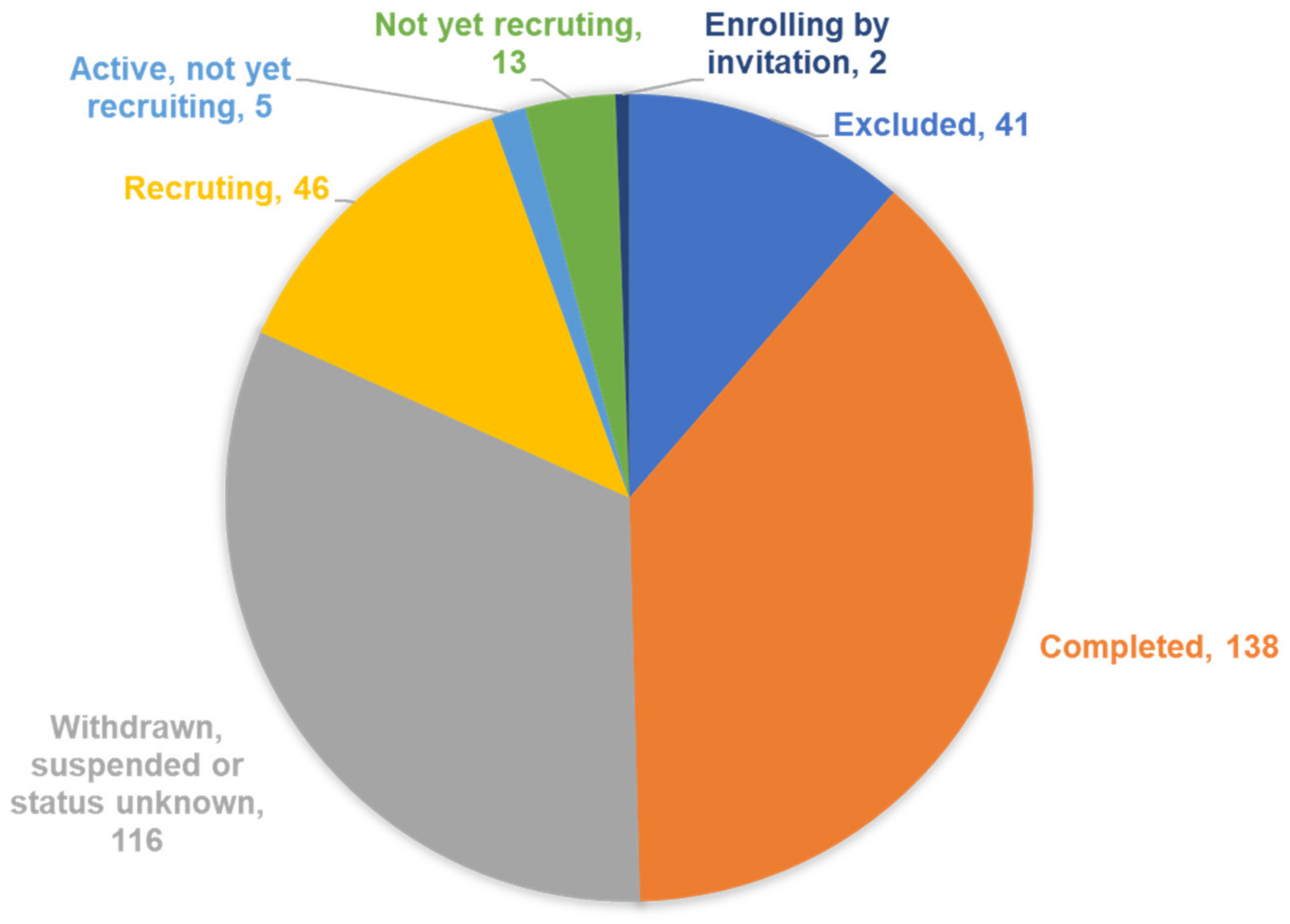
2. Trials Registered in ClinicalTrials
2.1. Completed Studies
2.2. Phase IV Studies
3. Characteristics of Completed Studies
3.1. Completed Studies with Results
3.2. Summary of Evidence for Drugs Appearing in Three or More TBI Studies
3.2.1. Amantadine
3.2.2. Botulinum Toxin Type A
3.2.3. Hyperbaric Oxygen
3.2.4. Methylphenidate
3.2.5. NNZ-2566
3.2.6. Rivastigmine
3.2.7. Tranexamic Acid (TXA)
3.3. Ongoing Studies
3.4. Summary of Drugs to Be Used in Large TBI Studies That Are Currently Active
3.4.1. Erythropoietin
3.4.2. Phenytoin Sodium
3.4.3. Dalteparin
3.4.4. Propanolol
3.4.5. NT201
3.4.6. Dexamethasone
4. Discussion
Future Clinical Trial Designs
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruns, J., Jr.; Hauser, W.A. The epidemiology of traumatic brain injury: A review. Epilepsia 2003, 44, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Buki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Zaloshnja, E.; Miller, T.; Langlois, J.A.; Selassie, A.W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008, 23, 394–400. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Cernak, I.; Noble-Haeusslein, L.; Potolicchio, S.; Temkin, N. Long-term neurologic outcomes after traumatic brain injury. J. Head Trauma Rehabil. 2009, 24, 439–451. [Google Scholar] [CrossRef]
- Andriessen, T.M.; Jacobs, B.; Vos, P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010, 14, 2381–2392. [Google Scholar] [CrossRef] [Green Version]
- Skandsen, T.; Finnanger, T.G.; Andersson, S.; Lydersen, S.; Brunner, J.F.; Vik, A. Cognitive impairment 3 months after moderate and severe traumatic brain injury: A prospective follow-up study. Arch. Phys. Med. Rehabil. 2010, 91, 1904–1913. [Google Scholar] [CrossRef]
- Glaesser, J.; Neuner, F.; Lutgehetmann, R.; Schmidt, R.; Elbert, T. Posttraumatic Stress Disorder in patients with traumatic brain injury. BMC Psychiatry 2004, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.H.; Meaney, D.F.; Shull, W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003, 18, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Saatman, K.E.; Duhaime, A.C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T.; Workshop Scientific, T.; Advisory Panel, M. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.K.; Dixon, C.E.; Banik, N.L. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol. Histopathol. 2002, 17, 1137–1152. [Google Scholar] [CrossRef]
- Rosenfeld, J.V.; Maas, A.I.; Bragge, P.; Morganti-Kossmann, M.C.; Manley, G.T.; Gruen, R.L. Early management of severe traumatic brain injury. Lancet 2012, 380, 1088–1098. [Google Scholar] [CrossRef]
- Thomsen, I.V. Late outcome of very severe blunt head trauma: A 10–15 year second follow-up. J. Neurol. Neurosurg. Psychiatry 1984, 47, 260–268. [Google Scholar] [CrossRef] [Green Version]
- DeGrauw, X.; Thurman, D.; Xu, L.; Kancherla, V.; DeGrauw, T. Epidemiology of traumatic brain injury-associated epilepsy and early use of anti-epilepsy drugs: An analysis of insurance claims data, 2004–2014. Epilepsy Res. 2018, 146, 41–49. [Google Scholar] [CrossRef]
- Ferguson, P.L.; Pickelsimer, E.E.; Corrigan, J.D.; Bogner, J.A.; Wald, M. Prevalence of traumatic brain injury among prisoners in South Carolina. J. Head Trauma Rehabil. 2012, 27, E11–E20. [Google Scholar] [CrossRef]
- Draper, K.; Ponsford, J.; Schönberger, M. Psychosocial and emotional outcomes 10 years following traumatic brain injury. J. Head Trauma Rehabil. 2007, 22, 278–287. [Google Scholar] [CrossRef]
- Corrigan, J.D.; Whiteneck, G.; Mellick, D. Perceived needs following traumatic brain injury. J. Head Trauma Rehabil. 2004, 19, 205–216. [Google Scholar] [CrossRef]
- Andelic, N.; Soberg, H.L.; Berntsen, S.; Sigurdardottir, S.; Roe, C. Self-perceived health care needs and delivery of health care services 5 years after moderate-to-severe traumatic brain injury. PMR 2014, 6, 1013–1021. [Google Scholar] [CrossRef]
- Andelic, N.; Hammergren, N.; Bautz-Holter, E.; Sveen, U.; Brunborg, C.; Røe, C. Functional outcome and health-related quality of life 10 years after moderate-to-severe traumatic brain injury. Acta Neurol. Scand. 2009, 120, 16–23. [Google Scholar] [CrossRef]
- Andelic, N.; Howe, E.I.; Hellstrøm, T.; Sanchez, M.F.; Lu, J.; Løvstad, M.; Røe, C. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018, 8, e01018. [Google Scholar] [CrossRef]
- Warden, D.L.; Gordon, B.; McAllister, T.W.; Silver, J.M.; Barth, J.T.; Bruns, J.; Drake, A.; Gentry, T.; Jagoda, A.; Katz, D.I.; et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma 2006, 23, 1468–1501. [Google Scholar] [CrossRef] [Green Version]
- Meythaler, J.M.; Brunner, R.C.; Johnson, A.; Novack, T.A. Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: A pilot double-blind randomized trial. J. Head Trauma Rehabil. 2002, 17, 300–313. [Google Scholar] [CrossRef]
- Schneider, W.N.; Drew-Cates, J.; Wong, T.M.; Dombovy, M.L. Cognitive and behavioural efficacy of amantadine in acute traumatic brain injury: An initial double-blind placebo-controlled study. Brain Inj. 1999, 13, 863–872. [Google Scholar] [CrossRef]
- Giacino, J.T.; Whyte, J.; Bagiella, E.; Kalmar, K.; Childs, N.; Khademi, A.; Eifert, B.; Long, D.; Katz, D.I.; Cho, S.; et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 2012, 366, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Kraus, M.F.; Smith, G.S.; Butters, M.; Donnell, A.J.; Dixon, E.; Yilong, C.; Marion, D. Effects of the dopaminergic agent and NMDA receptor antagonist amantadine on cognitive function, cerebral glucose metabolism and D2 receptor availability in chronic traumatic brain injury: A study using positron emission tomography (PET). Brain Inj. 2005, 19, 471–479. [Google Scholar] [CrossRef]
- Reddy, C.C.; Collins, M.; Lovell, M.; Kontos, A.P. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J. Head Trauma Rehabil. 2013, 28, 260–265. [Google Scholar] [CrossRef]
- Hammond, F.M.; Sherer, M.; Malec, J.F.; Zafonte, R.D.; Dikmen, S.; Bogner, J.; Bell, K.R.; Barber, J.; Temkin, N. Amantadine Did Not Positively Impact Cognition in Chronic Traumatic Brain Injury: A Multi-Site, Randomized, Controlled Trial. J. Neurotrauma 2018, 35, 2298–2305. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Truong, D.D.; Jost, W.H. Botulinum toxin: Clinical use. Park. Relat. Disord. 2006, 12, 331–355. [Google Scholar] [CrossRef]
- Pavesi, G.; Brianti, R.; Medici, D.; Mammi, P.; Mazzucchi, A.; Mancia, D. Botulinum toxin type A in the treatment of upper limb spasticity among patients with traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 1998, 64, 419–420. [Google Scholar] [CrossRef] [Green Version]
- Francis, H.P.; Wade, D.T.; Turner-Stokes, L.; Kingswell, R.S.; Dott, C.S.; Coxon, E.A. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum neurotoxins for post-stroke spasticity in adults: A systematic review. Mov. Disord. 2009, 24, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Foley, N.; Pereira, S.; Salter, K.; Fernandez, M.M.; Speechley, M.; Sequeira, K.; Miller, T.; Teasell, R. Treatment with botulinum toxin improves upper-extremity function post stroke: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, T.; Hu, X.; Wang, T. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 256–267. [Google Scholar] [CrossRef]
- Gracies, J.M.; Brashear, A.; Jech, R.; McAllister, P.; Banach, M.; Valkovic, P.; Walker, H.; Marciniak, C.; Deltombe, T.; Skoromets, A.; et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: A double-blind randomised controlled trial. Lancet Neurol. 2015, 14, 992–1001. [Google Scholar] [CrossRef]
- Dashtipour, K.; Chen, J.J.; Walker, H.W.; Lee, M.Y. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am. J. Phys. Med. Rehabil. 2015, 94, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Jacinto, J.; Varriale, P.; Pain, E.; Lysandropoulos, A.; Esquenazi, A. Patient Perspectives on the Therapeutic Profile of Botulinum Neurotoxin Type A in Spasticity. Front. Neurol. 2020, 11, 388. [Google Scholar] [CrossRef]
- Hu, Q.; Manaenko, A.; Xu, T.; Guo, Z.; Tang, J.; Zhang, J.H. Hyperbaric oxygen therapy for traumatic brain injury: Bench-to-bedside. Med. Gas. Res. 2016, 6, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Shandley, S.; Wolf, E.G.; Schubert-Kappan, C.M.; Baugh, L.M.; Richards, M.F.; Prye, J.; Arizpe, H.M.; Kalns, J. Increased circulating stem cells and better cognitive performance in traumatic brain injury subjects following hyperbaric oxygen therapy. Undersea Hyperb. Med. 2017, 44, 257–269. [Google Scholar] [CrossRef]
- Geng, F.; Ma, Y.; Xing, T.; Zhuang, X.; Zhu, J.; Yao, L. Effects of Hyperbaric Oxygen Therapy on Inflammasome Signaling after Traumatic Brain Injury. Neuroimmunomodulation 2016, 23, 122–129. [Google Scholar] [CrossRef]
- Zhong, X.; Shan, A.; Xu, J.; Liang, J.; Long, Y.; Du, B. Hyperbaric oxygen for severe traumatic brain injury: A randomized trial. J. Int. Med. Res. 2020, 48, 300060520939824. [Google Scholar] [CrossRef]
- Hadanny, A.; Abbott, S.; Suzin, G.; Bechor, Y.; Efrati, S. Effect of hyperbaric oxygen therapy on chronic neurocognitive deficits of post-traumatic brain injury patients: Retrospective analysis. BMJ Open 2018, 8, e023387. [Google Scholar] [CrossRef] [Green Version]
- Leonard, B.E.; McCartan, D.; White, J.; King, D.J. Methylphenidate: A review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum. Psychopharmacol. 2004, 19, 151–180. [Google Scholar] [CrossRef]
- Weber, P.; Lutschg, J. Methylphenidate treatment. Pediatr. Neurol. 2002, 26, 261–266. [Google Scholar] [CrossRef]
- Sivan, M.; Neumann, V.; Kent, R.; Stroud, A.; Bhakta, B.B. Pharmacotherapy for treatment of attention deficits after non-progressive acquired brain injury. A systematic review. Clin. Rehabil. 2010, 24, 110–121. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, C.C.; Sun, C.K.; Lin, G.H.; Hou, W.H. Methylphenidate on Cognitive Improvement in Patients with Traumatic Brain Injury: A Meta-Analysis. Curr. Neuropharmacol. 2016, 14, 272–281. [Google Scholar] [CrossRef]
- Frenette, A.J.; Kanji, S.; Rees, L.; Williamson, D.R.; Perreault, M.M.; Turgeon, A.F.; Bernard, F.; Fergusson, D.A. Efficacy and safety of dopamine agonists in traumatic brain injury: A systematic review of randomized controlled trials. J. Neurotrauma 2012, 29, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Iaccarino, M.A.; Philpotts, L.L.; Zafonte, R.; Biederman, J. Stimulant Use in the Management of Mild Traumatic Brain Injury: A Qualitative Literature Review. J. Atten. Disord. 2020, 24, 309–317. [Google Scholar] [CrossRef]
- Johansson, B.; Wentzel, A.P.; Andrell, P.; Odenstedt, J.; Mannheimer, C.; Ronnback, L. Evaluation of dosage, safety and effects of methylphenidate on post-traumatic brain injury symptoms with a focus on mental fatigue and pain. Brain Inj. 2014, 28, 304–310. [Google Scholar] [CrossRef]
- Johansson, B.; Wentzel, A.P.; Andrell, P.; Mannheimer, C.; Ronnback, L. Methylphenidate reduces mental fatigue and improves processing speed in persons suffered a traumatic brain injury. Brain Inj. 2015, 29, 758–765. [Google Scholar] [CrossRef]
- McAllister, T.W.; Zafonte, R.; Jain, S.; Flashman, L.A.; George, M.S.; Grant, G.A.; He, F.; Lohr, J.B.; Andaluz, N.; Summerall, L.; et al. Randomized Placebo-Controlled Trial of Methylphenidate or Galantamine for Persistent Emotional and Cognitive Symptoms Associated with PTSD and/or Traumatic Brain Injury. Neuropsychopharmacology 2016, 41, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Johansson, B.; Wentzel, A.P.; Andrell, P.; Ronnback, L.; Mannheimer, C. Long-term treatment with methylphenidate for fatigue after traumatic brain injury. Acta Neurol. Scand. 2017, 135, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Bickerdike, M.J.; Thomas, G.B.; Batchelor, D.C.; Sirimanne, E.S.; Leong, W.; Lin, H.; Sieg, F.; Wen, J.; Brimble, M.A.; Harris, P.W.; et al. NNZ-2566: A Gly-Pro-Glu analogue with neuroprotective efficacy in a rat model of acute focal stroke. J. Neurol. Sci. 2009, 278, 85–90. [Google Scholar] [CrossRef]
- Lu, X.C.; Chen, R.W.; Yao, C.; Wei, H.; Yang, X.; Liao, Z.; Dave, J.R.; Tortella, F.C. NNZ-2566, a glypromate analog, improves functional recovery and attenuates apoptosis and inflammation in a rat model of penetrating ballistic-type brain injury. J. Neurotrauma 2009, 26, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.C.; Si, Y.; Williams, A.J.; Hartings, J.A.; Gryder, D.; Tortella, F.C. NNZ-2566, a glypromate analog, attenuates brain ischemia-induced non-convulsive seizures in rats. J. Cereb. Blood Flow Metab. 2009, 29, 1924–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.H.; Lu, X.C.; Shear, D.A.; Waghray, A.; Yao, C.; Tortella, F.C.; Dave, J.R. NNZ-2566 treatment inhibits neuroinflammation and pro-inflammatory cytokine expression induced by experimental penetrating ballistic-like brain injury in rats. J. NeuroInflamm. 2009, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartagena, C.M.; Phillips, K.L.; Williams, G.L.; Konopko, M.; Tortella, F.C.; Dave, J.R.; Schmid, K.E. Mechanism of action for NNZ-2566 anti-inflammatory effects following PBBI involves upregulation of immunomodulator ATF3. Neuromol. Med. 2013, 15, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, J.M.; Koumaras, B.; Chen, M.; Mirski, D.; Potkin, S.G.; Reyes, P.; Warden, D.; Harvey, P.D.; Arciniegas, D.; Katz, D.I.; et al. Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology 2006, 67, 748–755. [Google Scholar] [CrossRef]
- Silver, J.M.; Koumaras, B.; Meng, X.; Potkin, S.G.; Reyes, P.F.; Harvey, P.D.; Katz, D.I.; Gunay, I.; Arciniegas, D.B. Long-term effects of rivastigmine capsules in patients with traumatic brain injury. Brain Inj. 2009, 23, 123–132. [Google Scholar] [CrossRef]
- Tenovuo, O.; Alin, J.; Helenius, H. A randomized controlled trial of rivastigmine for chronic sequels of traumatic brain injury-what it showed and taught? Brain Inj. 2009, 23, 548–558. [Google Scholar] [CrossRef]
- Brawman-Mintzer, O.; Tang, X.C.; Bizien, M.; Harvey, P.D.; Horner, M.D.; Arciniegas, D.B.; Raskind, M.; Johnson-Greene, L.; Martineau, R.J.; Hamner, M.; et al. Rivastigmine Transdermal Patch Treatment for Moderate to Severe Cognitive Impairment in Veterans with Traumatic Brain Injury (RiVET Study): A Randomized Clinical Trial. J. Neurotrauma 2021, 38, 1943–1952. [Google Scholar] [CrossRef]
- Perel, P.; Al-Shahi Salman, R.; Kawahara, T.; Morris, Z.; Prieto-Merino, D.; Roberts, I.; Sandercock, P.; Shakur, H.; Wardlaw, J. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: The effect of tranexamic acid in traumatic brain injury—A nested randomised, placebo-controlled trial. Health Technol. Assess. 2012, 16, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Harhangi, B.S.; Kompanje, E.J.; Leebeek, F.W.; Maas, A.I. Coagulation disorders after traumatic brain injury. Acta Neurochir. 2008, 150, 165–175, discussion 175. [Google Scholar] [CrossRef]
- Bayir, A.; Kalkan, E.; Kocak, S.; Ak, A.; Cander, B.; Bodur, S. Fibrinolytic markers and neurologic outcome in traumatic brain injury. Neurol. India 2006, 54, 363–365. [Google Scholar] [CrossRef]
- Collaborators, C.-T.; Shakur, H.; Roberts, I.; Bautista, R.; Caballero, J.; Coats, T.; Dewan, Y.; El-Sayed, H.; Gogichaishvili, T.; Gupta, S.; et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar] [CrossRef]
- Henry, D.A.; Carless, P.A.; Moxey, A.J.; O’Connell, D.; Stokes, B.J.; McClelland, B.; Laupacis, A.; Fergusson, D. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst. Rev. 2007, 4, CD001886. [Google Scholar] [CrossRef]
- Lawati, K.A.; Sharif, S.; Maqbali, S.A.; Rimawi, H.A.; Petrosoniak, A.; Belley-Cote, E.P.; Sharma, S.V.; Morgenstern, J.; Fernando, S.M.; Owen, J.J.; et al. Efficacy and safety of tranexamic acid in acute traumatic brain injury: A systematic review and meta-analysis of randomized-controlled trials. Intensive Care Med. 2021, 47, 14–27. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Steyerberg, E.W.; Citerio, G. Tranexamic acid in traumatic brain injury: Systematic review and meta-analysis trumps a large clinical trial? Intensive Care Med. 2021, 47, 74–76. [Google Scholar] [CrossRef]
- Bossers, S.M.; Loer, S.A.; Bloemers, F.W.; Den Hartog, D.; Van Lieshout, E.M.M.; Hoogerwerf, N.; van der Naalt, J.; Absalom, A.R.; Peerdeman, S.M.; Schwarte, L.A.; et al. Association Between Prehospital Tranexamic Acid Administration and Outcomes of Severe Traumatic Brain Injury. JAMA Neurol. 2021, 78, 338–345. [Google Scholar] [CrossRef]
- Jelkmann, W. Physiology and pharmacology of erythropoietin. Transfus. Med. Hemother. 2013, 40, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Bramlet, H.M.; Dietrich, W.D.; Dixosn, C.E.; Shear, D.A.; Schmid, K.E.; Modello, S.; Wang, K.K.; Hayes, R.L.; Povlishock, J.T.; Tortella, F.C.; et al. Erythropoietin treatment in trauamtic brain injury: Operation brain trauma therapy. J. Neurotrauma 2016, 33, 538–552. [Google Scholar] [CrossRef]
- Cherian, L.; Goodman, J.C.; Robertson, C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J. Pharm. Exp. 2007, 322, 789–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Xing, Z.; Yang, J.; Wang, Y.; Wang, W.; Huang, W. The efficacy of erythropoietin in treating experimental traumatic brain injury: A systematic review of controlled trials in animal models. J. Neurosurg. 2014, 121, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Kabadi, S.V.; Faden, A.I. Neuroprotective strategies for traumatic brain injury: Improving clinical translation. Int. J. Mol. Sci. 2014, 15, 1216–1236. [Google Scholar] [CrossRef] [Green Version]
- Grasso, G.; Alafaci, C.; Buemi, M. Erythropoietin in traumatic brain injury: An answer will come soon. World Neurosurg. 2015, 84, 1491–1492. [Google Scholar] [CrossRef]
- Liu, W.C.; Wen, L.; Xie, T.; Wang, H.; Gong, J.B.; Yang, X.F. Therapeutic effect of erythropoietin in patients with traumatic brain injury: A meta-analysis of randomized controlled trials. J. Neurosurg. 2016, 127, 8–15. [Google Scholar] [CrossRef]
- Liu, M.; Wang, A.J.; Chen, C.; Zhao, G.; Jiang, Z.; Wang, X.; Shi, D.; Zhang, T.; Sun, B.; He, S.; et al. Efficacy and safety of erythropoietin for traumatic brain injury. BMC Neurol. 2020, 20, 399. [Google Scholar] [CrossRef]
- Temkin, N.R.; Dikmen, S.S.; Wilensky, A.J.; Keihm, J.; Chabal, S.; Winn, R. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N. Engl. J. Med. 1990, 323, 497–502. [Google Scholar] [CrossRef]
- Khan, S.A.; Bhatti, S.N.; Khan, A.A.; Afridi, E.A.K.; Muhammad, G.; Gul, N.; Zadran, K.K.; Alam, S.; Aurangzeb, A. Comparison of efficacy of Phenytoin and Levetiracetam for prevention of early post traumatic seizures. J. Ayub. Med. Coll. Abbottabad 2016, 28, 455–460. [Google Scholar] [PubMed]
- McGinn, R.J.; Aljoghaiman, M.S.; Sharma, S.V. Levetiracetam vs phenytoin prophylaxis in severe traumatic brain injury: Systematic review and meta-analysis. Interdiscip. Neurosurg. 2022, 27, 101394. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Hussein, M.; Lin, J.; Battleman, D. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb. Haemost. 2009, 102, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaible, E.-V.; Thal, S.C. Anticoagulation in patients with traumatic brain injury. Curr. Opin. Anaesthesiol. 2013, 26, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.R.; Azi, I.; Bonnici, A.; Saluja, R.S.; Lamoureux, J.; Kalmovitch, B.; Gursahaney, A.; Razek, T. Early Venous Thromboembolic Event Prophylaxis in Traumatic Brain Injury with Low-Molecular-Weight Heparin: Risks and Benefits. J. Neurotrauma 2010, 27, 12. [Google Scholar] [CrossRef]
- 8Margilick, J.; Dandurand, C.; Duncan, K.; Chen, W.; Evans, D.C.; Sekhon, M.S.; Garraway, N.; Griesdale, D.E.G.; Gooderham, P.; Hameed, S.M. A Systematic Review of the Risks and Benefits of Venous Thromboembolism Prophylaxis in Traumatic Brain Injury. Can. J. Neurol. Sci. 2018, 45, 432–444. [Google Scholar] [CrossRef]
- Gunning, A.C.; Maier, R.V.; de Rooij, D.; de Leenen, D.; Leenen, L.P.H.; Hietbrink, F. Venous thromboembolism (VTE) prophylaxis in severely injured patients: An international comparative assessment. Eur. J. Trauma Emerg. Surg. 2021, 47, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Woolf, P.D.; Hamill, R.W.; Lee, L.A.; Cox, C.; McDonald, J.V. The predictive value of catecholamines in assessing outcome in traumatic brain injury. J. Neurosurg. 1987, 66, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Woolf, P.D.; McDonald, J.V.; Feliciano, D.V.; Kelly, M.M.; Nichols, D.; Cox, C. The catecholamine response to multisystem trauma. Arch. Surg. 1992, 127, 899–903. [Google Scholar] [CrossRef]
- Perkes, I.; Baguley, I.J.; Nott, M.T.; Menon, D.K. A review of paroxysmal sympathetic hyperactivity after acquired brain injury. Ann. Neurol. 2010, 68, 126–135. [Google Scholar] [CrossRef]
- Riordan, W.P.; Cotton, B.A.; Norris, P.R.; Waitman, L.R.; Jenkins, J.M.; Morris, J.A. Beta-blocker exposure in patients with severe traumatic brain injury (TBI) and cardiac uncoupling. J. Trauma 2007, 63, 503–510. [Google Scholar] [CrossRef]
- Nott, M.T.; Chapparo, C.; Baguley, I.J. Agitation following traumatic brain injury: An Australian sample. Brain Inj. 2006, 20, 1175–1182. [Google Scholar] [CrossRef]
- Baguley, I.J.; Slewa-Younan, S.; Heriseanu, R.E.; Nott, M.T.; Mudaliar, Y.; Nayyar, V. The incidence of dysautonomia and its relationship with autonomic arousal following traumatic brain injury. Brain Inj. 2007, 21, 1175–1181. [Google Scholar] [CrossRef]
- Liu, M.Y. Protective effects of propranolol on experimentally head-injured mouse brains. J. Med. Assoc. 1995, 94, 386–390. [Google Scholar]
- Ley, E.J.; Park, R.; Dagliyan, G.; Palestrant, D.; Miller, C.M.; Conti, P.S.; Margulies, D.R.; Salim, A. In vivo effect of propranolol dose and timing on cerebral perfusion after traumatic brain injury. J. Trauma 2010, 68, 353–356. [Google Scholar] [CrossRef]
- Ley, E.J.; Scehnet, J.; Park, R.; Schroff, S.; Dagliyan, G.; Conti, P.S.; Margulies, D.R.; Salim, A. The in vivo effect of propranolol on cerebral perfusion and hypoxia after traumatic brain injury. J. Trauma 2009, 66, 154–159. [Google Scholar] [CrossRef]
- Brooke, M.M.; Patterson, D.R.; Questad, K.A.; Cardenas, D.; Farrel-Roberts, L. The treatment of agitation during initial hospitalization after traumatic brain injury. Arch. Phys. Med. Rehabil. 1992, 73, 917–921. [Google Scholar]
- Fleminger, S.; Greenwood, R.J.; Oliver, D.L. Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database Syst. Rev. 2006, 4, CD003299. [Google Scholar] [CrossRef]
- Hinson, H.E.; Sheth, K.N. Manifestations of the hyperadrenergic state after acute brain injury. Curr. Opin. Crit. Care 2012, 18, 139–145. [Google Scholar] [CrossRef]
- Tran, T.Y.; Dunne, I.E.; German, J.W. Beta blockers exposure and traumatic brain injury: A literature review. Neurosurg. Focus 2008, 25, E8. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.A.; Stevens, R.D.; Lewin, J.J.; Misrki, M.A.; Bhardwaj, A. Glucocorticoid therapy in neurologic critical care. Crit. Care Med. 2005, 33, 1214–1224. [Google Scholar] [CrossRef]
- Moll, A.; Lara, M.; Pomar, J.; Orozco, M.; Frontera, G.; Llompart-Pou, J.A.; Moratinos, L.; Gonzalez, V.; Ibanez, J.; Perez-Barcena, J. Effects of dexamethasone in traumatic brain injury patients with pericontusional vasogenic edema: A prospective-observational DTI-MRI study. Medicine 2020, 23, e22879. [Google Scholar] [CrossRef]
- Roberts, I.; Yates, D.; Sandercock, P.; Farrell, B.; Wasserberg, J.; Lomas, G. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): Randomised placebo-controlled trial. Lancet 2004, 364, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- The CRASH-3 Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet 2019, 394, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Crash-3 Trial Results. Available online: https://crash3.lshtm.ac.uk/blog/crash-3-trial-results/ (accessed on 13 April 2022).
- Sharma, S.; Verma, S. Is positive publication bias really a bias, or an intentionally created discrimination toward negative results? Saudi J. Anaesth. 2019, 13, 352–355. [Google Scholar] [CrossRef]
- Mlinaric, A.; Horvat, M.; Smolcic, V.S. Dealing with the positive publication bias: Why you should really publish your negative results. Biomech. Med. 2017, 27, 030201. [Google Scholar] [CrossRef] [Green Version]
- The importance of no evidence. Nat. Hum. Behav. 2019, 3, 197. [CrossRef]
- Bespalov, A.; Steckler, T.; Skolnick, P. Be positive about negatives–recommendations for the publication of negative (or null) results. Eur. Neuropharmacol. 2019, 29, 1312–1320. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Jackson, T.C.; Jha, R.M.; Clark, R.S.B.; Okonkwo, D.O.; Bayir, H.; Poloyac, S.M.; Wagner, A.K.; Empey, P.E.; Conley, Y.P.; et al. Paths to Successful Translation of New Therapies for Severe Traumatic Brain Injury in the Golden Age of Traumatic Brain Injury Research: A Pittsburgh Vision. J. Neurotrauma 2020, 37, 2353–2371. [Google Scholar] [CrossRef]
- Huijben, J.A.; Volovici, V.; Cnossen, M.C.; Haitsma, I.K.; Stocchetti, N.; Maas, A.I.R.; Menon, D.K.; Ercole, A.; Citerio, G.; Nelson, D.; et al. Variation in general supportive and preventive intensive care management of traumatic brain injury: A survey in 66 neurotrauma centers participating in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. Crit. Care 2018, 22, 90. [Google Scholar] [CrossRef] [Green Version]
- Kurz, J.E.; Poloyac, S.M.; Abend, N.S.; Fabio, A.; Bell, M.J.; Wainwright, M.S. Variation in Anticonvulsant Selection and Electroencephalographic Monitoring Following Severe Traumatic Brain Injury in Children-Understanding Resource Availability in Sites Participating in a Comparative Effectiveness Study. Pediatr. Crit. Care Med. 2016, 17, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Talisa, V.B.; Yende, S.; Seymour, C.W.; Angus, D.C. Arguing for Adaptive Clinical Trials in Sepsis. Front. Immunol. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Shein, S.L.; Ferguson, N.M.; Kochanek, P.M.; Bayir, H.; Clark, R.S.; Fink, E.L.; Tyler-Kabara, E.C.; Wisniewski, S.R.; Tian, Y.; Balasubramani, G.K.; et al. Effectiveness of Pharmacological Therapies for Intracranial Hypertension in Children With Severe Traumatic Brain Injury—Results from an Automated Data Collection System Time-Synched to Drug Administration. Pediatr. Crit. Care Med. 2016, 17, 236–245. [Google Scholar] [CrossRef]
- Young, A.M.; Donnelly, J.; Czosnyka, M.; Jalloh, I.; Liu, X.; Aries, M.J.; Fernandes, H.M.; Garnett, M.R.; Smielewski, P.; Hutchinson, P.J.; et al. Continuous Multimodality Monitoring in Children after Traumatic Brain Injury-Preliminary Experience. PLoS ONE 2016, 11, e0148817. [Google Scholar] [CrossRef]




| NCT Number | Intervention | Age (Years) | Enrollment | Primary Outcome | Summary of Results |
|---|---|---|---|---|---|
| NCT01990768 | Tranexamic acid | 15 and older | 388 | Dichotomized Glasgow Outcome Scale Extended (GOS-E) at 6 months | No statistical difference between 1 g IV TXA followed by 1 g IV maintenance or 2 g TXA IV TXA only and placebo: 967 patients enrolled. |
| NCT00313716 | Recombinant human erythropoietin | 15 and older | 200 | Glasgow Outcome Scale at 6 months | No statistical differences between Epo treatment and placebo. All 200 patients enrolled. Results published. |
| NCT01322048 | IV Propranolol vs. clonidine | 16 to 64 | 63 | Ventilator-free days | No statistical change in ventilator-free days using Propanolol and clonidine. Secondary outcomes of plasma norepinephrine levels showed higher levels after treatment: 48 patients enrolled. Results published. |
| NCT01673828 | Allopregnanolone injection | 16 to 65 | 967 | Dichotomized Glasgow Outcome Scale Extended (GOS-E) at 6 months | No statistical difference between treatment and placebo in primary outcomes. Only 13 participants enrolled. |
| NCT00970944 | Amantadine Hydrochloride | 16 to 65 | 200 | Disability rating score (DRS) | No statistical difference in DRS scores over 6 weeks between treated and placebo. However, DRS score improved faster in the amantadine group in the first 4 weeks, then significantly slower than the normal group: 184 patients enrolled. |
| NCT01201863 | Androgel | 16 to 65 | 41 | Restricted functional independence measure (FIM) | No statistical difference between treated and placebo: 46 patients enrolled. |
| NCT00621751 | Carbamazepine | 16 to 65 | 157 | Neuropsychiatric Inventory Irritability-Aggression Domains Composite Measure—Observer | No statistical difference between treated and placebo: 70 patients enrolled. |
| NCT00779324 | Amantadine Hydrochloride | 16 to 75 | 52 | Proportion of Participants With >2-point increase on Neuropsychiatric Inventory—Irritability Domain rated by Observer day 28 | No statistical difference between treated and placebo: 168 patients enrolled. |
| NCT02957331 | Propranolol | 18 and older | 63 | 30-day mortality | In the treatment arm, 7.7% of patients died, whilst 33.3% of patients died in the non-treatment arm. No statistical data present: 26 patients enrolled. |
| NCT01014403 | Enoxaparin | 18 and older | 184 | Percentage of participants with worsening TBI hemorrhage | No statistical difference between treated and placebo: 62 patients enrolled. |
| NCT01847755 | Oxygen at 1.5 ATA | 18 and older | 71 | Number of participants with improved cerebral perfusion | Single group allocation and so no comparisons possible: 18 patients enrolled. |
| NCT00795366 | Arginine vasopressin vs. catecholamine | 18 and older | 60 | Time ICP > 20 | No statistical difference between treated and standard catecholamine: 96 patients enrolled. |
| NCT00618436 | Levetiracetam vs. phenytoin | 18 and older | 47 | Seizure incidence | No statistical difference between Levetiracetam or phenytoin: 52 patients enrolled. |
| NCT00233103 | Sertraline | 18 and older | 30 | Depression at baseline | No statistical difference between treatment and placebo: 52 patients enrolled. |
| NCT01368432 | Escitalopram | 18 and older | 30 | Montgomery–Asberg depression rating scale (MADRS) at baseline | No statistical difference between treatment and placebo: 16 patients enrolled. |
| NCT00766038 | Recombinant human growth hormone | 18 to 50 | 336 | Functional outcome 6 months after injury, as measured by the processing speed index | No statistical difference between treatment and placebo: 63 patients enrolled. |
| NCT00973674 | Premarin IV | 18 to 50 | 256 | Percent passing the Galveston orientation amnesia test (GOAT) within 28 days post injury | No statistical difference between treatment and placebo: 50 patients enrolled. |
| NCT00623506 | Pregnenolone | 18 to 55 | 50 | Brief assessment of cognition in affective disorders (BAC-A) | No statistical difference between treatment and placebo: 30 patients enrolled. |
| NCT02225106 | Methylphenidate | 18 to 55 | 34 | Perceptual organization and processing speed index | Single group allocation and so no comparisons possible: 11 patients enrolled. |
| NCT00727246 | CDP-Choline | 18 to 55 | 13 | Cognitive composite score for group of subjects with TBI and healthy controls matched by age, education and treatment group | No statistical difference between treatment and placebo. Low group numbers: 19 patients enrolled. |
| NCT01856270 | Amitriptyline | 18 to 60 | 12,737 | Frequency and severity of headaches | No statistical difference between treatment and placebo: 50 patients enrolled. |
| NCT01342549 | Valproate and Naltrexone | 18 to 60 | 15 | Time to relapse to heavy drinking | Patients on Naltrexone relapsed to heavy drinking at 15.99 weeks compared to those on Valproate who relapsed after 8.78 weeks: 62 patients enrolled. |
| NCT02025439 | Amantadine | 18 to 64 | 41 | Intensity of adverse event | Patients receiving repetitive transcranial magnetic stimulation (rTMS) combined with amantadine (TMS + amantadine) had significantly higher intensity of adverse events. Patient numbers very low: 4 patients enrolled. |
| NCT01760785 | Divalproex sodium | 18 to 65 | 606 | Severity of affective lability based on shortened agitated behavior scale | No statistical difference between treatment and placebo: 50 patients enrolled. |
| NCT02240589 | Memantine | 18 to 65 | 238 | California Verbal Learning Test—Second Edition (CVLT-II)—Long Delay Free Recall | Memantine group had a higher z-score (−2.000), i.e., worse performance, compared to placebo (−1.375): 11 patients enrolled. |
| NCT00702364 | Atomoxetine | 18 to 65 | 179 | CDR power of attention and Stroop test interference T-score | No statistical differences between treatment and placebo: 60 patients enrolled. |
| NCT02012582 | VAS203 | 18 to 65 | 100 | Effects on intracranial pressure (ICP), cerebral perfusion pressure (CPP), brain metabolism using microdialysis and the therapy intensity level (TIL). Extended Glasgow outcome score (eGOS) at 6 months was exploratory | No statistical differences between treatment and placebo for ICP, CPP and brain metabolism. Scores for eGOS were significantly higher in treated groups: 32 patients enrolled. Results published. |
| NCT01750268 | Topiramate | 18 to 65 | 60 | Change in the number of drinking days per week as assessed by the timeline followback (TLFB) | No statistical difference between treatment arm and placebo. Topiramate transiently impaired verbal fluency and working memory. No statistical difference in processing speed, cognitive inhibition and mental flexibility: 32 patients enrolled. Results published. |
| NCT01611194 | Hyperbaric oxygen | 18 to 65 | 50 | Summary of treatment-emergent adverse events | Higher incidence of Barotitis media, upper respiratory tract infection and eye disorders in treated group compared to control groups: 71 patients enrolled. |
| NCT01306968 | Hyperbaric oxygen | 18 to 65 | 47 | Post-intervention post-concussion symptom scores using RPQ | No statistical differences between treatment and placebo: 79 patients enrolled. |
| NCT02791945 | N-acetylcysteine | 18 to 65 | 32 | Change in percent of heavy drinking days per week as assessed by the timeline followback (TFLB) | No statistical differences between treatment and placebo: 30 patients enrolled. |
| NCT00453921 | Methylphenidate | 18 to 65 | 31 | Neuropsychological assessment, CVLT-II and CPT, distractibility condition (reaction time) | No statistical differences between treatment and placebo in CVLT-II or CPT: 76 patients enrolled. |
| NCT01854385 | Sumatriptan | 18 to 65 | 16 | Change in headache relief | Single arm study so comparisons not possible: 40 patients enrolled. |
| NCT01249404 | Botulinum toxin type A | 18 to 80 | 18 | Least squares mean change from baseline to week 4 in the mas score in the gastrocnemius-soleus complex (GSC) (knee extended) | No statistical differences between treatment and placebo at 1000 U of Dysport, but statistically significant at 1500 U Dysport (p = 0.0091): 388 patients enrolled. |
| NCT01313299 | Botulinum toxin type A | 18 to 80 | 16 | Change from baseline in mas score in the primary targeted muscle group (PTMG) | Treatment 500 and 1000 U of Dysport caused worse outcomes compared to placebo groups: 243 patients enrolled. |
| NCT00704379 | Sertraline | 18 to 85 | 200 | Time to onset of diagnostic and statistical manual (DSM) IV defined mood and anxiety disorders associated with TBI | DSM appeared significantly earlier in treated groups (mean 15.78 weeks) compared to 21.42 weeks in placebo treated controls (p < 0.05): 94 patients enrolled. |
| NCT01670526 | Rivastigmine transdermal patch | 19 to 65 | 40 | Improvements from baseline on the Hopkins verbal learning test-revised (HVLT-R) total recall | No statistical differences between treatment and placebo: 94 patients enrolled. |
| NCT02270736 | NT 201 | 2 to 17 | 17 | Change from baseline in unstimulated salivary flow rate (uSFR) at week 4 | Superiority of NT201 shown with a significant uSFR decrease with NT201 compared to placebo (p = 0.0012). Secondary outcome of Carer’s GICS rating was also significant and favored treatment (p = 0.032): 256 patients enrolled. Results published. |
| NCT01322009 | Probenecid and N-acetyl cysteine | 2 to 18 | 17 | Number of participants who experienced adverse events | None in the treatment arm experienced adverse events; 2 patients in the placebo arm experienced adverse events: 14 patients enrolled. |
| NCT00957671 | Recombinant human growth hormone | 21 and older | 210 | Maximum oxygen uptake at baseline and maximum oxygen uptake after one year | Single arm study so comparisons not possible: 15 patients enrolled. |
| NCT01336413 | Pregnenolone | 21 to 55 | 34 | CAPS (Cluster D symptoms)—primary behavioral outcome measure and Tower of London (Subscale Test of BAC)—primary cognitive outcome measure | No significant differences in CAPS between treatment and placebo. Tower of London score slightly better at 4 weeks but worse at 8 weeks compared to placebo: 53 patients enrolled. |
| NCT01463033 | Levetiracetam | 6 and older | 15 | Post-traumatic epilepsy | No significant difference in post-traumatic epilepsy rate in treated versus control arms: 126 patients enrolled. Results published. |
| NCT02712996 | Lisdexamfetamine | 6 to 16 | 150 | Assessing severity of symptoms associated with attention-deficit/hyperactivity disorder (ADHD) using the Conners-3 parent form and executive using the behavior rating inventory of executive function (BRIEF) | Better overall scores in the Conners-3 and BRIEF outcomes. No statistical data present: 20 patients enrolled. |
| NCT01933217 | Methylphenidate | 6 to 17 | 300 | Parent outcome-Vanderbilt ADHD parent rating scales (VADPRS) and parent outcome-behavior rating inventory of executive functioning (BRIEF) | Slightly better overall scores in VADPRS and BRIEF outcomes. No statistical data present: 26 patients enrolled. |
| NCT Number | Intervention | Age (Years) | Enrollment | Phase of Trial | Date Last Updated |
|---|---|---|---|---|---|
| NCT04833218 | Propanolol | 18 to 60 | 90 | Early Phase I | 6 April 2021 |
| NCT03554265 | Somatropin | 18 to 70 | 54 | Phase III | 30 November 2021 |
| NCT02255799 | Donepezil | 18 to 60 | 160 | Phase III | 26 April 2021 |
| NCT Number | Intervention | Age (Years) | Enrollment | Phase of Trial | Date Last Updated |
|---|---|---|---|---|---|
| NCT04487275 | MLC901 | 15 to 65 | 80 | Phase IV | 27 July 2020 |
| NCT05033444 | PRV-002 | 18 to 55 | 24 | Phase I | 24 September 2021 |
| NCT01048138 | Biperiden Lactate | 18 to 75 | 132 | Phase III | 18 June 2021 |
| NCT02404779 | Cisatracurium besilate | 18 and older | 34 | Phase IV | 7 July 2020 |
| NCT04550377 | Cannabidiol | 18 to 70 | 120 | Phase II | 2 June 2021 |
| NCT04303065 | Dexamethasone | 18 to 85 | 600 | Phase III | 23 November 2020 |
| NCT03559114 | Dalteparin | 18 and older | 1100 | Phase III | 15 June 2021 |
| NCT04489160 | C1 Inhibitor | 18 to 64 | 106 | Phase II | 13 September 2021 |
| NCT04006054 | Dexmedetomidine vs. Midazolam | 18 to 70 | 82 | Phase IV | 2 July 2019 |
| NCT01821690 | Buspirone | 18 to 70 | 74 | NA | 2 April 2021 |
| NCT03061565 | Erythropoietin | 15 to 75 | 603 | Not specified | 25 August 2021 |
| NCT03992404 | NT 201 | 18 to 85 | 600 | Phase III | 22 November 2021 |
| NCT04521881 | Tranexamic acid | 50 and older | 10,000 | Phase III | 20 October 2021 |
| NCT02990091 | Omega 3 fatty acid/Safflower seed oil | 18 to 55 | 45 | Phase II | 23 September 2021 |
| NCT04974060 | Remifentanil injection | 18 and older | 30 | NA | 29 July 2021 |
| NCT04280965 | Quetiapine Fumarate | 18 and older | 20 | Early Phase I | 1 March 2021 |
| NCT03982602 | Ketogenic diet | 18 and older | 10 | Early Phase I | 12 October 2021 |
| NCT03260569 | Inhaled nitric oxide | 18 and older | 38 | Phase III | 3 November 2020 |
| NCT04527289 | Amantadine | 18 to 75 | 50 | Phase IV | 5 October 2021 |
| NCT04426487 | Progesterone | 20 to 65 | 200 | Early Phase I | 11 June 2020 |
| NCT04508244 | Propranolol | 18 to 65 | 771 | Phase IV | 21 February 2021 |
| NCT04718155 | Atorvastatin | 18 to 40 | 30 | NA | 25 March 2021 |
| NCT04244058 | Amantadine + L-DOPA | 18 to 75 | 30 | Early Phase I | 4 October 2021 |
| NCT05097261 | Ketamine | 18 and older | 100 | Phase IV | 28 October 2021 |
| NCT03095066 | AVP-786 | 18 to 75 | 150 | Phase II | 29 November 2021 |
| NCT04499755 | Nucleo CMP Forte | up to 18 | 100 | Phase III | 5 August 2020 |
| NCT04558346 | Ghrelin (OXE-103) | 18 to 60 | 40 | Phase II | 28 July 2021 |
| NCT03417492 | Sildenafil Citrate | 40 to 65 | 30 | Phase I | 23 September 2021 |
| NCT04673240 | Botulinum toxin type A injection | 18 and older | 70 | Not specified | 17 December 2020 |
| NCT02407028 | Hyperbaric oxygen | 16 to 65 Years | 200 | Phase II | 27 August 2021 |
| NCT03814356 | Methylphenidate | 18 and older | 22 | Phase I | 2 July 2021 |
| NCT04117672 | Salovum (dietary supplement) | 10 to 70 | 20 | Phase II | 24 March 2021 |
| NCT04588311 | Epoetin Alfa 40000 UNT/ML | 18 to 75 | 2500 | Phase III | 25 August 2021 |
| NCT04631484 | Cytoflavin | 18 to 60 | 320 | Phase III | 18 August 2021 |
| NCT04099667 | Rimabotulinumtoxin B | 18 to 80 | 272 | Phase II/III | 15 July 2021 |
| NCT04710550 | F18-3F4AP | 18 to 90 | 66 | Phase I | 24 February 2021 |
| NCT04744051 | Adipose Derived Stem Cell Infusion | 18 to 65 | 20 | Phase I | 9 February 2021 |
| NCT Number | Intervention | Age (Years) | Enrollment | Phase of Trial | Date Last Updated |
|---|---|---|---|---|---|
| NCT05049057 | Erenumab | 18 to 50 | 404 | Phase II | 17 September 2021 |
| NCT04573803 | Phenytoin Sodium | 10 and older | 1649 | Phase III | 3 November 2020 |
| NCT04945213 | Biperiden | 18 to 75 | 312 | Phase III | 22 October 2021 |
| NCT04003285 | Allopregnanolone | 21 to 62 | 132 | Phase II | 23 September 2021 |
| NCT05058677 | Aerosolized 2% lidocaine | up to 16 | 12 | Phase IV | 11 October 2021 |
| NCT04400266 | B + MEL | 18 to 64 | 10 | Phase IV | 22 May 2020 |
| NCT04427241 | Amantadine Sulfate + Cerebrolysin | 19 to 64 | 12 | Phase IV | 11 June 2020 |
| NCT04867317 | Somatropin | 21 to 55 | 172 | Phase III | 15 October 2021 |
| NCT05095857 | S-ketamine | 18 and older | 400 | Phase IV | 27 October 2021 |
| NCT04815967 | Rimabotulinumtoxin B | 18 to 80 | 272 | Phase II/III | 15 July 2021 |
| NCT05008926 | Naloxegol | 18 and older | 370 | Phase III | 23 August 2021 |
| NCT04387305 | Tranexamic acid injection | up to 17 | 2000 | Phase III | 12 July 2021 |
| NCT04515420 | Noradrenaline | 18 to 80 | 60 | Not specified | 17 August 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, Z. Current Clinical Trials in Traumatic Brain Injury. Brain Sci. 2022, 12, 527. https://doi.org/10.3390/brainsci12050527
Ahmed Z. Current Clinical Trials in Traumatic Brain Injury. Brain Sciences. 2022; 12(5):527. https://doi.org/10.3390/brainsci12050527
Chicago/Turabian StyleAhmed, Zubair. 2022. "Current Clinical Trials in Traumatic Brain Injury" Brain Sciences 12, no. 5: 527. https://doi.org/10.3390/brainsci12050527
APA StyleAhmed, Z. (2022). Current Clinical Trials in Traumatic Brain Injury. Brain Sciences, 12(5), 527. https://doi.org/10.3390/brainsci12050527






