Delusional Severity Is Associated with Abnormal Texture in FLAIR MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Behavioral Analyses
2.3. APOE and CSF Markers
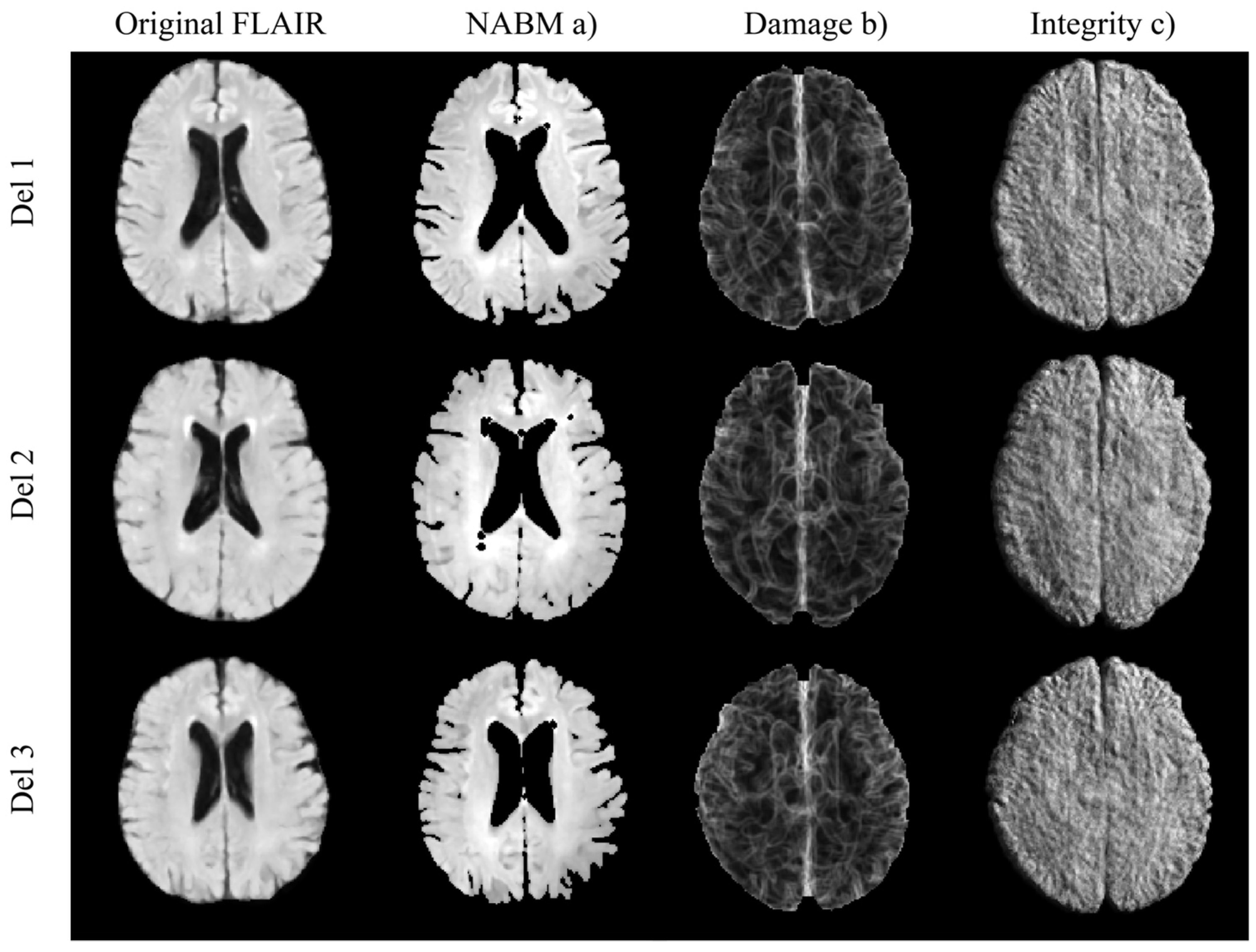
2.4. Imaging Analyses
2.5. Statistical Analyses
3. Results
3.1. Multivariable Regression
3.2. Post Hoc Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Vendors | TR (ms) | TE (ms) | TI (ms) | Pixel Size (mm) | Slice Thickness (mm) | Magnetic Field (T) |
|---|---|---|---|---|---|---|
| GE, Siemens, Philips | 9000–11002 | 90–153.978 | 2250–2500 | 0.8594–0.9375 | 5 | 3 |
| Diagnosis | MCI n = 24 | AD n = 17 | MCI → AD n = 1 |
|---|---|---|---|
| Age: MEAN ± SD | 71.4 ± 7.52 | 80.2 ± 6.72 | 76.42 ± 0.49 |
| Sex [nM:nF] | 15:10 | 10:7 | 1:0 |
| Delusional Severity: MEAN ± SD | 1.40 ± 0.69 | 1.53 ± 0.74 | 2.0 ± 0 |
| Abbreviation | Definition |
|---|---|
| NABM | Normal Appearing Brain Matter |
| GM | Grey-Matter |
| WM | White-Matter |
| WML | White-Matter Lesion |
| APOE4 | Apolipoprotein epsilon4 allele |
| CSFTAU | Cerebro-Spinal Fluid (CSF) tau |
| AD | Alzheimer’s Disease |
| MCI | Mild Cognitive Impairment |
| NPI-Q | Neuropsychiatric Inventory Questionnaire |
| NPS | Neuropsychiatric Symptoms |
| FLAIR MRI | Fluid-attenuated inversion recovery (FLAIR) Magnetic resonance imaging (MRI) |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative |
References
- Fischer, C.E.; Agüera-Ortiz, L. Psychosis and dementia: Risk factor, prodrome, or cause? Int. Psychogeriatr. 2018, 30, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.E.; Sweet, R.A. Psychosis in Alzheimer’s Disease: A Review of Recent Research Findings. Curr. Behav. Neurosci. Rep. 2016, 3, 308–317. [Google Scholar] [CrossRef]
- Anor, C.J.; Dadar, M.; Collins, D.L.; Tartaglia, M.C. The Longitudinal Assessment of Neuropsychiatric Symptoms in Mild Cognitive Impairment and Alzheimer’s Disease and Their Association with White Matter Hyperintensities in the National Alzheimer’s Coordinating Center’s Uniform Data Set. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.C.; Lyketsos, C.G.; Sweet, R.A.; Ballard, C. Psychosis in Alzheimer disease—Mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 2022, 18, 131–144. [Google Scholar] [CrossRef]
- Rootes-Murdy, K.; Goldsmith, D.R.; Turner, J.A. Clinical and Structural Differences in Delusions Across Diagnoses: A Systematic Review. Front. Integr. Neurosci. 2022, 15, 726321. [Google Scholar] [CrossRef]
- Ismail, Z.; Nguyen, M.Q.; Fischer, C.E.; Schweizer, T.A.; Mulsant, B.H. Neuroimaging of delusions in Alzheimer’s disease. Psychiatry Res. 2012, 202, 89–95. [Google Scholar] [CrossRef]
- Ting, W.K.; Fischer, C.E.; Millikin, C.P.; Ismail, Z.; Chow, T.W.; Schweizer, T.A. Grey matter atrophy in mild cognitive impairment/early Alzheimer disease associated with delusions: A voxel-based morphometry study. Curr. Alzheimer Res. 2015, 12, 165–172. [Google Scholar] [CrossRef]
- Qian, W.; Schweizer, T.A.; Churchill, N.W.; Millikin, C.; Ismail, Z.; Smith, E.E.; Lix, L.M.; Munoz, D.G.; Barfett, J.J.; Rajji, T.K.; et al. Gray Matter Changes Associated with the Development of Delusions in Alzheimer Disease. Am. J. Geriatr. Psychiatry 2019, 27, 490–498. [Google Scholar] [CrossRef]
- Nakaaki, S.; Sato, J.; Torii, K.; Oka, M.; Negi, A.; Nakamae, T.; Narumoto, J.; Miyata, J.; Furukawa, T.A.; Mimura, M. Decreased white matter integrity before the onset of delusions in patients with Alzheimer’s disease: Diffusion tensor imaging. Neuropsychiatr. Dis Treat. 2013, 9, 25–29. [Google Scholar]
- Lee, N.Y.; Choo, I.H.; Kim, K.W.; Jhoo, J.H.; Youn, J.C.; Lee, U.Y.; Woo, J.I. White Matter Changes Associated with Psychotic Symptoms in Alzheimer’s Disease Patients. J. Neuropsychiatry Clin. Neurosci. 2006, 18, 191–198. [Google Scholar] [CrossRef]
- Ochi, R.; Noda, Y.; Tsuchimoto, S.; Tarumi, R.; Honda, S.; Matsushita, K.; Tsugawa, S.; Plitman, E.; Masuda, F.; Ogyu, K.; et al. White matter microstructural organizations in patients with severe treatment-resistant schizophrenia: A diffusion tensor imaging study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 100, 109871. [Google Scholar] [CrossRef] [PubMed]
- Koppel, J.; Sunday, S.; Buthorn, J.; Goldberg, T.; Davies, P.; Greenwald, B.; Alzheimer’s Disease Neuroimaging Initiative. Elevated CSF Tau is Associated with Psychosis in Alzheimer’s Disease. Am. J. Psychiatry 2013, 170, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.F.; Smith, R.X.; Beaumont, H.; Roe, C.M.; Gordon, B.A.; Mishra, S.; Adeyemo, B.; Christensen, J.J.; Su, Y.; Morris, J.C.; et al. Loss of white matter integrity reflects tau accumulation in Alzheimer disease defined regions. Neurology 2018, 91, e313–e318. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Liu, C.; Zhang, J.; Relkin, N.; Xing, Y.; Li, Y. Cerebrospinal fluid Aβ42, t-tau, and p-tau levels in the differential diagnosis of idiopathic normal-pressure hydrocephalus: A systematic review and meta-analysis. Fluids Barriers CNS 2017, 14, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Fischer, C.E.; Schweizer, T.A.; Munoz, D.G. Gender and Pathology-Specific Effect of Apolipoprotein E Genotype on Psychosis in Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 834–840. [Google Scholar] [CrossRef] [Green Version]
- Spalletta, G.; Bernardini, S.; Bellincampi, L.; Federici, G.; Trequattrini, A.; Caltagirone, C. Delusion symptoms are associated with Apo, E epsilon4 allelic variant at the early stage of Alzheimer’s disease with late onset. Eur. J. Neurol. 2006, 13, 176–182. [Google Scholar] [CrossRef]
- Emrani, S.; Arain, H.A.; De Marshall, C.; Nuriel, T. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: A systematic review. Alzheimer’s Res. Ther. 2020, 12, 141. [Google Scholar] [CrossRef]
- Sudre, C.H.; Cardoso, M.J.; Frost, C.; Barnes, J.; Barkhof, F.; Fox, N.; Ourselin, S. APOE ε4 status is associated with white matter hyperintensities volume accumulation rate independent of AD diagnosis. Neurobiol. Aging 2017, 53, 67–75. [Google Scholar] [CrossRef]
- Bahsoun, M.-A.; Khan, M.; Mitha, S.; Ghazvanchahi, A.; Khosravani, H.; Maralani, P.J.; Tardif, J.-C.; Moody, A.; Tyrrell, P.; Khademi, A. FLAIR MRI biomarkers of the normal appearing brain matter are related to cognition. Neuroimage Clin. 2022, 34, 102955. [Google Scholar] [CrossRef] [PubMed]
- Reiche, B.; Moody, A.; Khademi, A. Pathology-preserving intensity standardization framework for multi-institutional FLAIR MRI datasets. Magn. Reson. Imaging 2019, 62, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iaboni, A.; Fischer, C.E.; Gallagher, D. Neuropsychiatric symptoms in mild cognitive impairment: An update on prevalence, mechanisms and clinical significance. Can. J. Psychiatry 2017, 62, 161–169. [Google Scholar]
- Di Gregorio, J.; Arezza, G.; Gibicar, A.; Moody, A.R.; Tyrrell, P.N.; Khademi, A. Intracranial volume segmentation for neurodegenerative populations using multicentre FLAIR MRI. Neuroimage Rep. 2021, 1, 100006. [Google Scholar] [CrossRef]
- d’Arbeloff, T.; Elliott, M.L.; Knodt, A.R.; Melzer, T.R.; Keenan, P.; Ireland, D.; Ramrakha, S.; Poulton, R.; Anderson, T.; Caspi, A.; et al. White matter hyperintensities are common in midlife and already associated with cognitive decline. Brain Commun. 2019, 1, fcz041. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, P.; Parslow, R.; Wen, W.; Anstey, K.; Easteal, S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol. Aging 2009, 30, 946–956. [Google Scholar] [CrossRef]
- Ganeshan, B.; Miles, K.A.; Young, R.C.D.; Chatwin, C.; Gurling, H.M.D.; Critchley, H. Three-dimensional textural analysis of brain images reveals distributed grey-matter abnormalities in schizophrenia. Eur. Radiol. 2010, 20, 941–948. [Google Scholar] [CrossRef]
- Koizumi, K.; Hattori, Y.; Ahn, S.J.; Buendia, I.; Ciacciarelli, A.; Uekawa, K.; Wang, G.; Hiller, A.; Zhao, L.; Voss, H.U.; et al. Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat. Commun. 2018, 9, 3618. [Google Scholar] [CrossRef] [Green Version]
- Haller, S.; Montandon, M.-L.; Rodriguez, C.; Ackermann, M.; Herrmann, F.; Giannakopoulos, P. APOE*E4Is Associated with Gray Matter Loss in the Posterior Cingulate Cortex in Healthy Elderly Controls Subsequently Developing Subtle Cognitive Decline. Am. J. Neuroradiol. 2017, 38, 1335–1342. [Google Scholar] [CrossRef] [Green Version]
- Pelkmans, W.; Ossenkoppele, R.; Dicks, E.; Strandberg, O.; Barkhof, F.; Tijms, B.M.; Pereira, J.B.; Hansson, O. Tau-related grey matter network breakdown across the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2021, 13, 138. [Google Scholar] [CrossRef]
- Hampel, H.; Blennow, K. CSF tau and β-amyloid as biomarkers for mild cognitive impairment. Dialog-Clin. Neurosci. 2004, 6, 379–390. [Google Scholar] [CrossRef]
- Visser, P.J.; Reus, L.M.; Gobom, J.; Jansen, I.; Dicks, E.; Tsolaki, M.; Verhey, F.R.J.; Popp, J.; Martinez-Lage, P.; Vandenberghe, R.; et al. Cerebrospinal fluid total tau levels indicate aberrant neuronal plasticity in Alzheimer’s disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Kheirbek, R.E.; Fokar, A.; Little, J.T.; Balish, M.; Shara, N.M.; Boustani, M.A.; Llorente, M. Association between Antipsychotics and All-Cause Mortality among Community-Dwelling Older Adults. J. Gerontol. Ser. A 2019, 74, 1916–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.-J.; Kim, H.-G.; Rhee, H.Y.; Ryu, C.-W.; Liu, T.; Wang, Y.; Jahng, G.-H. Texture analyses of quantitative susceptibility maps to differentiate Alzheimer’s disease from cognitive normal and mild cognitive impairment. Med. Phys. 2016, 43, 4718–4728. [Google Scholar] [CrossRef]
- Tozer, D.J.; Zeestraten, E.; Lawrence, A.J.; Barrick, T.R.; Markus, H.S. Texture Analysis of T1-Weighted and Fluid-Attenuated Inversion Recovery Images Detects Abnormalities That Correlate with Cognitive Decline in Small Vessel Disease. Stroke 2018, 49, 1656–1661. [Google Scholar] [CrossRef]


| NABM Volume (%) | Integrity | Damage | ||||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Delusion 2 | −0.98% | 0.52 | −1.84 | 0.03 | 298.50 | 0.30 |
| Delusion 3 | −9.36% | <0.01 | −3.71 | <0.01 | 927.90 | 0.01 |
| Tau | −0.01% | 0.03 | −0.006 | <0.01 | 0.96 | 0.06 |
| Age | −0.33% | <0.01 | 0.11 | <0.01 | 39.89 | <0.01 |
| Gender | −2.37% | <0.01 | −1.08 | <0.01 | 403.00 | <0.01 |
| Education | −0.21% | 0.07 | −0.04 | 0.52 | 2.51 | 0.91 |
| ApoE4 | 0.46% | 0.46 | 0.43 | 0.25 | −243.60 | 0.04 |
| ApoE44 | 1.69% | 0.04 | 1.18 | 0.01 | −384.70 | 0.01 |
| Delusion 2*Tau | 0.001% | 0.78 | 0.004 | 0.22 | −0.63 | 0.48 |
| Delusion 3*Tau | 0.02% | <0.01 | 0.01 | <0.01 | −1.77 | 0.03 |
| NABM Volume (%) | Integrity | Damage | ||||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Severe Delusions | −3.72% | <0.01 | −2.50 | <0.01 | 485.40 | 0.05 |
| Tau | −0.01% | 0.02 | −0.56 | <0.01 | 1.03 | 0.04 |
| Age | −0.30% | <0.01 | −0.09 | <0.01 | 37.90 | <0.01 |
| Gender | −1.79% | <0.01 | −0.94 | <0.01 | 363.60 | <0.01 |
| Education | −0.09% | 0.42 | −0.02 | 0.75 | −7.04 | 0.91 |
| ApoE4 | 0.40% | 0.54 | 0.38 | 0.29 | −244.10 | 0.04 |
| ApoE44 | 2.26% | 0.01 | 1.30 | <0.01 | −427.70 | <0.01 |
| Severe Delusions * Tau | 0.01% | 0.01 | 0.56 | <0.01 | −1.04 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoury, M.A.; Bahsoun, M.-A.; Fadhel, A.; Shunbuli, S.; Venkatesh, S.; Ghazvanchahi, A.; Mitha, S.; Chan, K.; Fornazzari, L.R.; Churchill, N.W.; et al. Delusional Severity Is Associated with Abnormal Texture in FLAIR MRI. Brain Sci. 2022, 12, 600. https://doi.org/10.3390/brainsci12050600
Khoury MA, Bahsoun M-A, Fadhel A, Shunbuli S, Venkatesh S, Ghazvanchahi A, Mitha S, Chan K, Fornazzari LR, Churchill NW, et al. Delusional Severity Is Associated with Abnormal Texture in FLAIR MRI. Brain Sciences. 2022; 12(5):600. https://doi.org/10.3390/brainsci12050600
Chicago/Turabian StyleKhoury, Marc A., Mohamad-Ali Bahsoun, Ayad Fadhel, Shukrullah Shunbuli, Saanika Venkatesh, Abdollah Ghazvanchahi, Samir Mitha, Karissa Chan, Luis R. Fornazzari, Nathan W. Churchill, and et al. 2022. "Delusional Severity Is Associated with Abnormal Texture in FLAIR MRI" Brain Sciences 12, no. 5: 600. https://doi.org/10.3390/brainsci12050600
APA StyleKhoury, M. A., Bahsoun, M.-A., Fadhel, A., Shunbuli, S., Venkatesh, S., Ghazvanchahi, A., Mitha, S., Chan, K., Fornazzari, L. R., Churchill, N. W., Ismail, Z., Munoz, D. G., Schweizer, T. A., Moody, A. R., Fischer, C. E., & Khademi, A. (2022). Delusional Severity Is Associated with Abnormal Texture in FLAIR MRI. Brain Sciences, 12(5), 600. https://doi.org/10.3390/brainsci12050600







