Evaluation of Approach to a Conspecific and Blood Biochemical Parameters in TAAR1 Knockout Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection and Storage
2.3. Measurement of Biochemical Parameters
2.4. Measurement of Testosterone
2.5. Interassay Repeatability
2.6. Sexual Incentive Motivation Test Protocol
- Hormone-induced estrous. WT female mice in estrous were used as a sexual incentive. To induce estrous, adult female mice were given 10 μg estrogen benzoate and 500 μg progesterone intraperitoneally 48 and 2 h before the experiment, respectively [42]. The stage of the cycle was checked 1 h before the experiment via an assessment of vaginal smears [43].
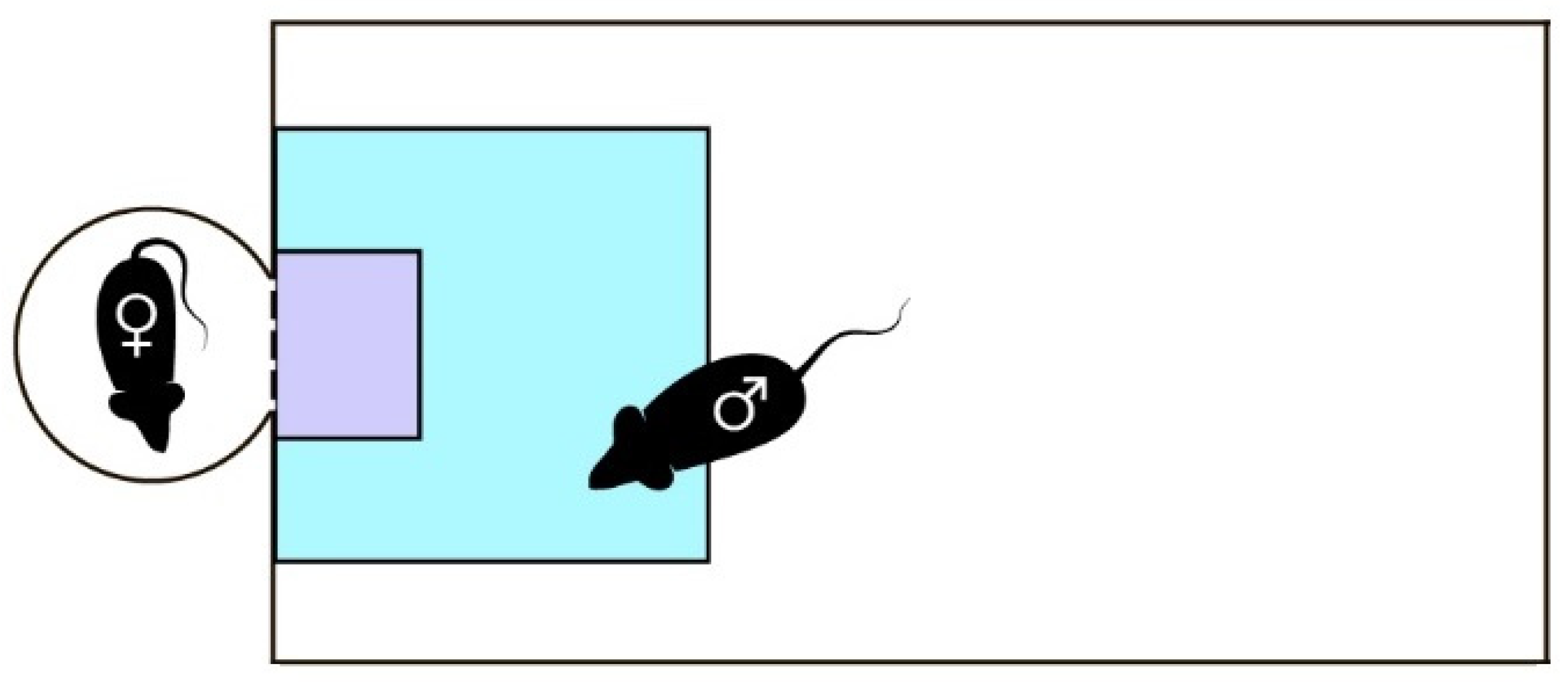
- Experimental setup and analysis. A modified sexual incentive motivation test (SIMT) was used to evaluate the sexual behavior of TAAR1-KO and WT male mice [44]. The setup consisted of 4 experimental chambers (15 × 30 cm2), each with an adjacent incentive cage separated by a permeable wall (Figure 1). A female mouse in estrous was placed in the incentive cage 20 min before the experiment. A male mouse was then placed into the experimental chamber for 20 min, while the behavior was recorded and then processed using Noldus EthoVision XT (Version 11.5; Noldus Information Technology, Wageningen, The Netherlands). To speed up the data collection and analysis, a set-up of 4 such independent cages was used simultaneously.
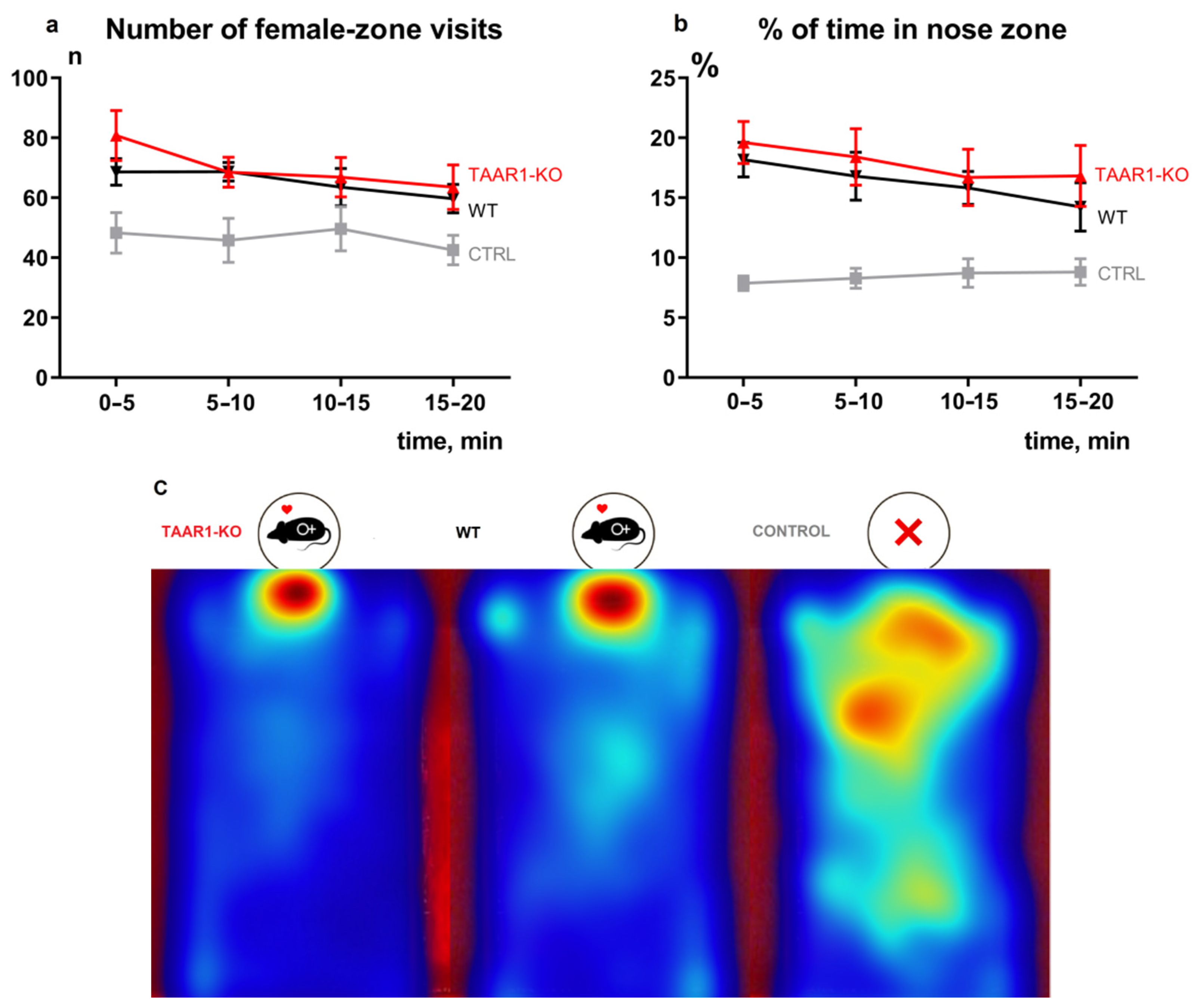
- To assess the recognition of sexually relevant stimuli, two zones were differentiated for the analysis: a 10 × 10 cm2 zone adjacent to the incentive cage called “female”, and a 4 × 3 cm2 zone closest to the cage called “nose”. The following parameters potentially descriptive of sexual behavior were analyzed: percentage of time spent in the “female” zone by the center-point of the animal’s body, number of visits to the “female” zone by the center-point, percentage of time spent in the “nose” zone by the nose-point, number of visits to the “nose” zone by the nose-point.
- Experimental design. Firstly, to assess the validity of the suggested method, 15 WT male mice were tested in two conditions: in the presence of a sexual incentive and with empty incentive cages. The parameters described in the previous paragraph were compared to assess the validity of individual parameters and the method itself for the evaluation of sexual behavior. 16 TAAR1-KO and 15 WT male mice were tested in SIMT.
2.7. Statistical Analysis
3. Results
3.1. Lack of TAAR1 Does Not Affect Sexual Motivation
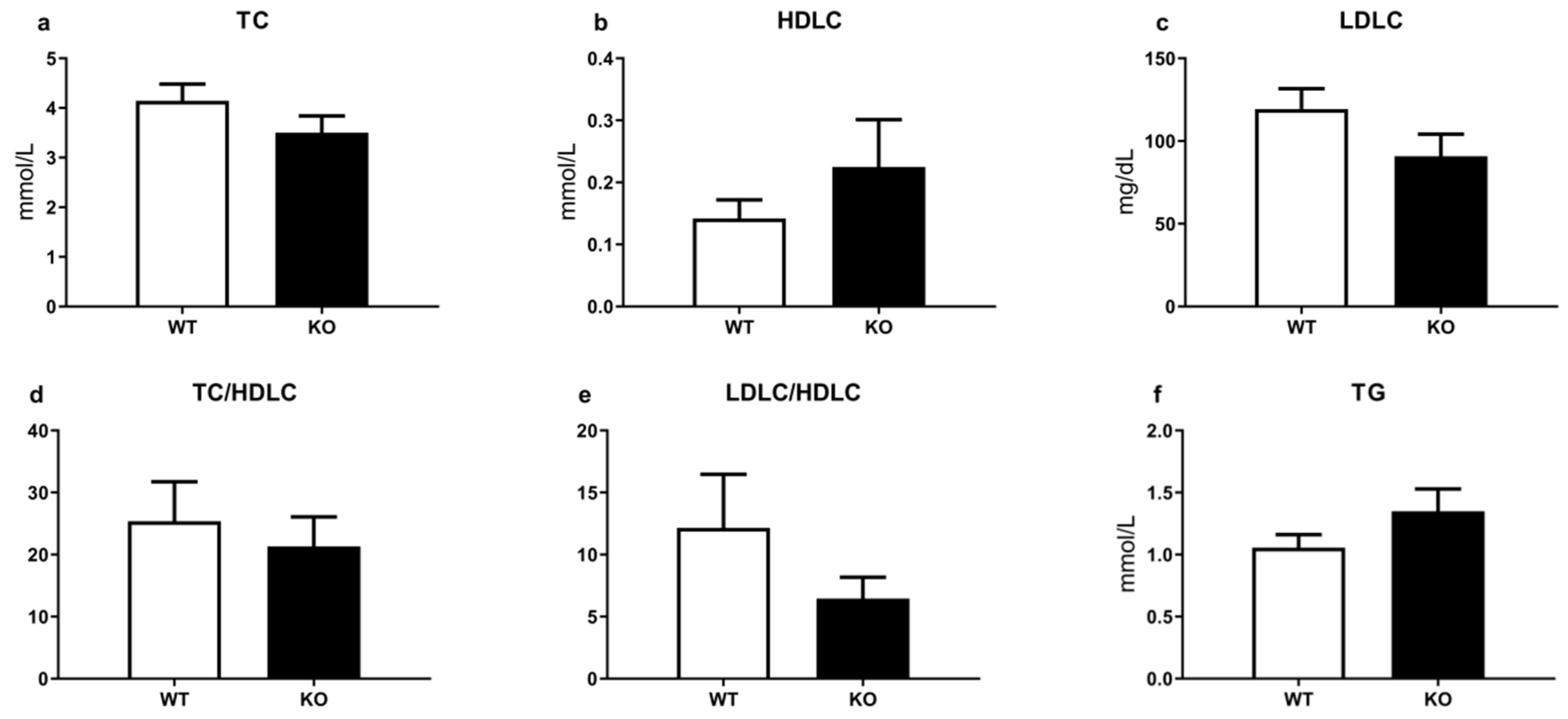
3.2. TAAR1 Gene Knockout Does Not Significantly Affect Biochemical Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premont, R.T.; Gainetdinov, R.R.; Caron, M.G. Following the trace of elusive amines. Proc. Natl. Acad. Sci. USA 2001, 98, 9474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [Green Version]
- Boulton, A.A. Letter: Amines and theories in psychiatry. Lancet 1974, 2, 52–53. [Google Scholar] [CrossRef]
- Koblan, K.S.; Kent, J.; Hopkins, S.C.; Krystal, J.H.; Cheng, H.; Goldman, R.; Loebel, A. A Non–D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N. Engl. J. Med. 2020, 382, 1497–1506. [Google Scholar] [CrossRef]
- Everitt, B.J. Sexual motivation: A neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 1990, 14, 217–232. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Phillips, A.G. Role of Dopamine in Anticipatory and Consummatory Aspects of Sexual Behavior in the Male Rat. Behav. Neurosci. 1991, 105, 727–743. [Google Scholar] [CrossRef]
- Hull, E.M.; Du, J.; Lorrain, D.S.; Matuszewich, L. Extracellular dopamine in the medial preoptic area: Implications for sexual motivation and hormonal control of copulation. J. Neurosci. 1995, 15, 7465–7471. [Google Scholar] [CrossRef] [Green Version]
- Argiolas, A.; Melis, M.R. Central control of penile erection: Role of the paraventricular nucleus of the hypothalamus. Prog. Neurobiol. 2005, 76, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Argiolas, A. Central control of penile erection: A re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci. Biobehav. Rev. 2011, 35, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Argiolas, A.; Melis, M.R. Neuropeptides and central control of sexual behaviour from the past to the present: A review. Prog. Neurobiol. 2013, 108, 80–107. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G. Dopamine: Helping Males Copulate for at Least 200 Million Years: Theoretical Comment on Kleitz-Nelson et al. (2010). Behav. Neurosci. 2010, 124, 877–880. [Google Scholar] [CrossRef]
- Sanna, F.; Bratzu, J.; Piludu, M.A.; Corda, M.G.; Melis, M.R.; Giorgi, O.; Argiolas, A. Dopamine, noradrenaline and differences in sexual behavior between Roman high and low avoidance male rats: A microdialysis study in the medial prefrontal cortex. Front. Behav. Neurosci. 2017, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Hull, E.M.; Dominguez, J.M. Male Sexual Behavior. In Knobil and Neill’s Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 2015; pp. 2211–2285. [Google Scholar] [CrossRef]
- Sanna, F.; Bratzu, J.; Serra, M.P.; Leo, D.; Quartu, M.; Boi, M.; Espinoza, S.; Gainetdinov, R.R.; Melis, M.R.; Argiolas, A. Altered Sexual Behavior in Dopamine Transporter (DAT) Knockout Male Rats: A Behavioral, Neurochemical and Intracerebral Microdialysis Study. Front. Behav. Neurosci. 2020, 14, 58. [Google Scholar] [CrossRef]
- Horowitz, L.F.; Saraiva, L.R.; Kuang, D.; Yoon, K.H.; Buck, L.B.; Horowitz, L.F.; Saraiva, L.R.; Yoon, K.H. Olfactory receptor patterning in a higher primate. J. Neurosci. 2014, 34, 12241–12252. [Google Scholar] [CrossRef] [Green Version]
- Liberles, S.D.; Buck, L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature 2006, 442, 645–650. [Google Scholar] [CrossRef]
- Syed, A.S.; Sansone, A.; Röner, S.; Bozorg Nia, S.; Manzini, I.; Korsching, S.I. Different expression domains for two closely related amphibian TAARs generate a bimodal distribution similar to neuronal responses to amine odors. Sci. Rep. 2015, 5, 13935. [Google Scholar] [CrossRef]
- Espinoza, S.; Sukhanov, I.; Efimova, E.V.; Kozlova, A.; Antonova, K.A.; Illiano, P.; Leo, D.; Merkulyeva, N.; Kalinina, D.; Musienko, P.; et al. Trace Amine-Associated Receptor 5 Provides Olfactory Input Into Limbic Brain Areas and Modulates Emotional Behaviors and Serotonin Transmission. Front. Mol. Neurosci. 2020, 13, 18. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kozlova, A.A.; Razenkova, V.; Katolikova, N.V.; Antonova, K.A.; Sotnikova, T.D.; Merkulyeva, N.S.; Veshchitskii, A.S.; Kalinina, D.S.; Korzhevskii, D.E.; et al. Increased dopamine transmission and adult neurogenesis in trace amine-associated receptor 5 (TAAR5) knockout mice. Neuropharmacology 2021, 182, 108373. [Google Scholar] [CrossRef] [PubMed]
- Efimova, E.V.; Katolikova, N.V.; Kanov, E.V.; Gainetdinov, R.R. Trace amine-associated receptors at the cross-road between innate olfaction of amines, emotions, and adult neurogenesis. Neural Regen. Res. 2022, 17, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Vignozzi, L.; Corona, G.; Petrone, L.; Filippi, S.; Morelli, A.M.; Forti, G.; Maggi, M. Testosterone and sexual activity. J. Endocrinol. Investig. 2005, 28, 39–44. [Google Scholar] [PubMed]
- Sukhanov, I.; Espinoza, S.; Yakovlev, D.S.; Hoener, M.C.; Sotnikova, T.D.; Gainetdinov, R.R. TAAR1-dependent effects of apomorphine in mice. Int. J. Neuropsychopharmacol. 2014, 17, 1683–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, S.; Lignani, G.; Caffino, L.; Maggi, S.; Sukhanov, I.; Leo, D.; Mus, L.; Emanuele, M.; Ronzitti, G.; Harmeier, A.; et al. TAAR1 Modulates Cortical Glutamate NMDA Receptor Function. Neuropsychopharmacology 2015, 40, 2217–2227. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, G.; Terrazzino, S.; Fortin, D.; Farruggio, A.; Rinaldi, L.; Leon, A. HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci. Lett. 2003, 346, 89–92. [Google Scholar] [CrossRef]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef]
- Zhukov, I.S.; Kubarskaya, L.G.; Tissen, I.Y.; Kozlova, A.A.; Dagayev, S.G.; Kashuro, V.A.; Vlasova, O.L.; Sinitca, E.L.; Karpova, I.V.; Gainetdinov, R.R. Minimal Age-Related Alterations in Behavioral and Hematological Parameters in Trace Amine-Associated Receptor 1 (TAAR1) Knockout Mice. Cell. Mol. Neurobiol. 2020, 40, 273–282. [Google Scholar] [CrossRef]
- Zhukov, I.S.; Kubarskaya, L.G.; Karpova, I.V.; Vaganova, A.N.; Karpenko, M.N.; Gainetdinov, R.R. Minor changes in erythrocyte osmotic fragility in trace amine-associated receptor 5 (Taar5) knockout mice. Int. J. Mol. Sci. 2021, 22, 7307. [Google Scholar] [CrossRef]
- Barnes, D.A.; Galloway, D.A.; Hoener, M.C.; Berry, M.D.; Moore, C.S. Taar1 expression in human macrophages and brain tissue: A potential novel facet of ms neuroinflammation. Int. J. Mol. Sci. 2021, 22, 11576. [Google Scholar] [CrossRef]
- Lee, J.; Sul, H.J.; Kim, K.H.; Chang, J.Y.; Shong, M. Primary Cilia Mediate TSH-Regulated Thyroglobulin Endocytic Pathways. Front. Endocrinol. 2021, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Szumska, J.; Qatato, M.; Rehders, M.; Führer, D.; Biebermann, H.; Grandy, D.K.; Köhrle, J.; Brix, K. Trace Amine-Associated Receptor 1 Localization at the Apical Plasma Membrane Domain of Fisher Rat Thyroid Epithelial Cells Is Confined to Cilia. Eur. Thyroid J. 2015, 4, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qatato, M.; Szumska, J.; Skripnik, V.; Rijntjes, E.; Köhrle, J.; Brix, K. Canonical TSH Regulation of Cathepsin-Mediated Thyroglobulin Processing in the Thyroid Gland of Male Mice Requires Taar1 Expression. Front. Pharmacol. 2018, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qatato, M.; Venugopalan, V.; Al-Hashimi, A.; Rehders, M.; Valentine, A.D.; Hein, Z.; Dallto, U.; Springer, S.; Brix, K. Trace Amine-Associated Receptor 1 Trafficking to Cilia of Thyroid Epithelial Cells. Cells 2021, 10, 1518. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, T.L.R.; Vattai, A.; Schmoeckel, E.; Kaltofen, T.; Chelariu-Raicu, A.; Zheng, M.; Mahner, S.; Mayr, D.; Jeschke, U.; Trillsch, F. Trace amine-associated receptor 1 (Taar1) is a positive prognosticator for epithelial ovarian cancer. Int. J. Mol. Sci. 2021, 22, 8479. [Google Scholar] [CrossRef] [PubMed]
- Shinderman-Maman, E.; Cohen, K.; Moskovich, D.; Hercbergs, A.; Werner, H.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. Thyroid hormones derivatives reduce proliferation and induce cell death and DNA damage in ovarian cancer. Sci. Rep. 2017, 7, 16475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, S.A.; Lin, H.Y.; Tang, H.Y.; Hercbergs, A.; Luidens, M.K.; Davis, P.J. Modulation of angiogenesis by thyroid hormone and hormone analogues: Implications for cancer management. Angiogenesis 2014, 17, 463–469. [Google Scholar] [CrossRef]
- Revel, F.G.; Moreau, J.-L.; Gainetdinov, R.R.; Ferragud, A.; Velázquez-Sánchez, C.; Sotnikova, T.D.; Morairty, S.R.; Harmeier, A.; Groebke Zbinden, K.; Norcross, R.D.; et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol. Psychiatry 2012, 72, 934–942. [Google Scholar] [CrossRef]
- Raab, S.; Wang, H.; Uhles, S.; Cole, N.; Alvarez-Sanchez, R.; Künnecke, B.; Ullmer, C.; Matile, H.; Bedoucha, M.; Norcross, R.D.; et al. Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol. Metab. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Murtazina, R.Z.; Zhukov, I.S.; Korenkova, O.M.; Popova, E.A.; Kuvarzin, S.R.; Efimova, E.V.; Kubarskaya, L.G.; Batotsyrenova, E.G.; Zolotoverkhaya, E.A.; Vaganova, A.N.; et al. Genetic deletion of trace-amine associated receptor 9 (TAAR9) in rats leads to decreased blood cholesterol levels. Int. J. Mol. Sci. 2021, 22, 2942. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y. Progesterone Intramuscularly or Vaginally Administration May Not Change Live Birth Rate or Neonatal Outcomes in Artificial Frozen-Thawed Embryo Transfer Cycles. Front. Endocrinol. 2020, 11, 539427. [Google Scholar] [CrossRef] [PubMed]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Li, Y.; Lv, Y.; Liu, Z.; Zheng, X. Complex motivated behaviors for natural rewards following a binge-like regimen of morphine administration: Mixed phenotypes of anhedonia and craving after short-term withdrawal. Front. Behav. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ågmo, A. Unconditioned Sexual Incentive Motivation in the Male Norway Rat (Rattus norvegicus). J. Comp. Psychol. 2003, 117, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.W. Sniffing Behavior Communicates Social Hierarchy. Curr. Biol. 2013, 23, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Caramaschi, D.; de Boer, S.F.; de Vries, H.; Koolhaas, J.M. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav. Brain Res. 2008, 189, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Ishii, K.K.; Touhara, K. Neural circuits regulating sexual behaviors via the olfactory system in mice. Neurosci. Res. 2019, 140, 59–76. [Google Scholar] [CrossRef]
- Wallrabenstein, I.; Kuklan, J.; Weber, L.; Zborala, S.; Werner, M.; Altmüller, J.; Becker, C.; Schmidt, A.; Hatt, H.; Hummel, T.; et al. Human trace amine-associated receptor TAAR5 can be activated by trimethylamine. PLoS ONE 2013, 8, e54950. [Google Scholar] [CrossRef] [Green Version]
- Sukhanov, I.; Dorofeikova, M.; Dolgorukova, A.; Dorotenko, A.; Gainetdinov, R.R. Trace amine-associated receptor 1 modulates the locomotor and sensitization effects of nicotine. Front. Pharmacol. 2018, 9, 329. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukov, I.S.; Ptukha, M.A.; Zolotoverkhaja, E.A.; Sinitca, E.L.; Tissen, I.Y.; Karpova, I.V.; Volnova, A.B.; Gainetdinov, R.R. Evaluation of Approach to a Conspecific and Blood Biochemical Parameters in TAAR1 Knockout Mice. Brain Sci. 2022, 12, 614. https://doi.org/10.3390/brainsci12050614
Zhukov IS, Ptukha MA, Zolotoverkhaja EA, Sinitca EL, Tissen IY, Karpova IV, Volnova AB, Gainetdinov RR. Evaluation of Approach to a Conspecific and Blood Biochemical Parameters in TAAR1 Knockout Mice. Brain Sciences. 2022; 12(5):614. https://doi.org/10.3390/brainsci12050614
Chicago/Turabian StyleZhukov, Ilya S., Maria A. Ptukha, Ekaterina A. Zolotoverkhaja, Ekaterina L. Sinitca, Ilya Y. Tissen, Inessa V. Karpova, Anna B. Volnova, and Raul R. Gainetdinov. 2022. "Evaluation of Approach to a Conspecific and Blood Biochemical Parameters in TAAR1 Knockout Mice" Brain Sciences 12, no. 5: 614. https://doi.org/10.3390/brainsci12050614
APA StyleZhukov, I. S., Ptukha, M. A., Zolotoverkhaja, E. A., Sinitca, E. L., Tissen, I. Y., Karpova, I. V., Volnova, A. B., & Gainetdinov, R. R. (2022). Evaluation of Approach to a Conspecific and Blood Biochemical Parameters in TAAR1 Knockout Mice. Brain Sciences, 12(5), 614. https://doi.org/10.3390/brainsci12050614







