The Effect of Task Performance and Partnership on Interpersonal Brain Synchrony during Cooperation
Abstract
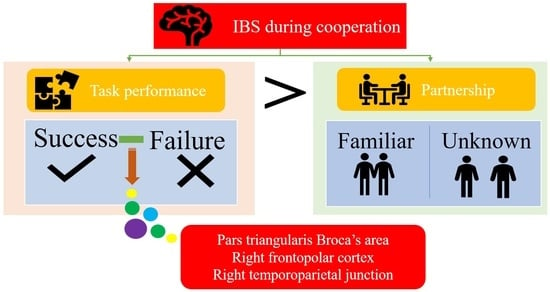
1. Introduction
2. Method
2.1. Participants
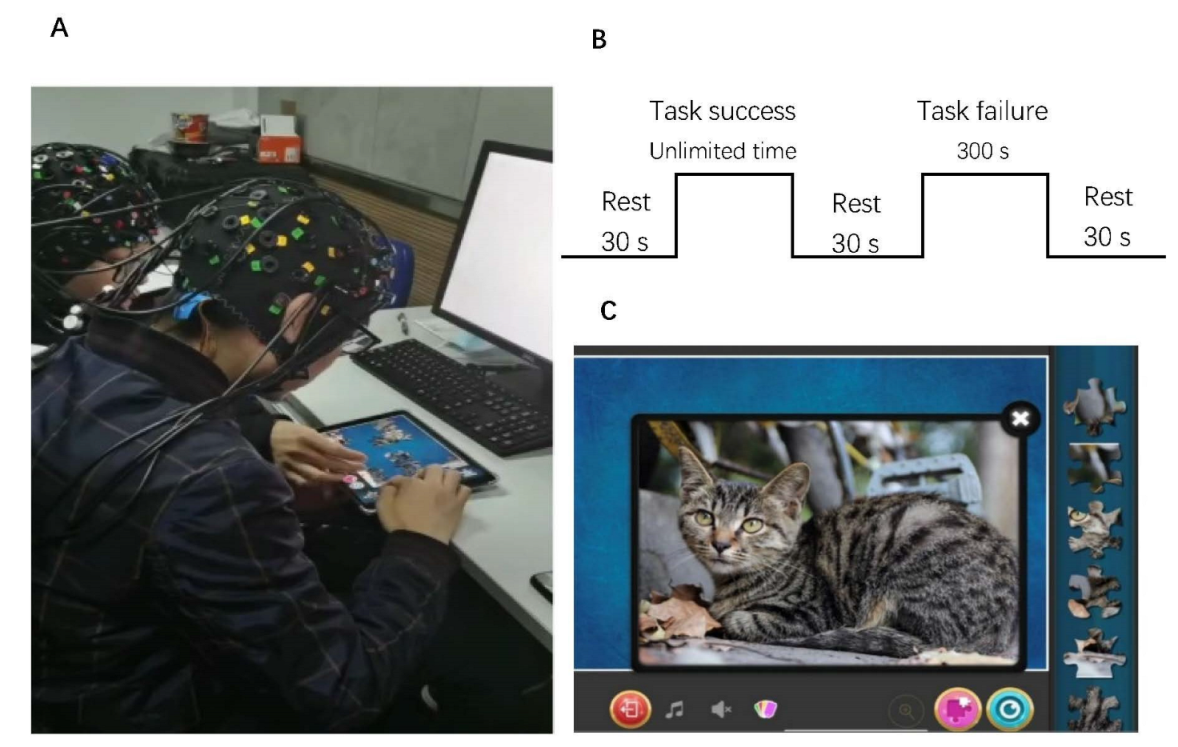
2.2. Experimental Tasks and Procedure
2.3. fNIRS Data Acquisition
2.4. Behavior Data Analysis
2.5. fNIRS Data Analysis
3. Results
3.1. Behavioral Data
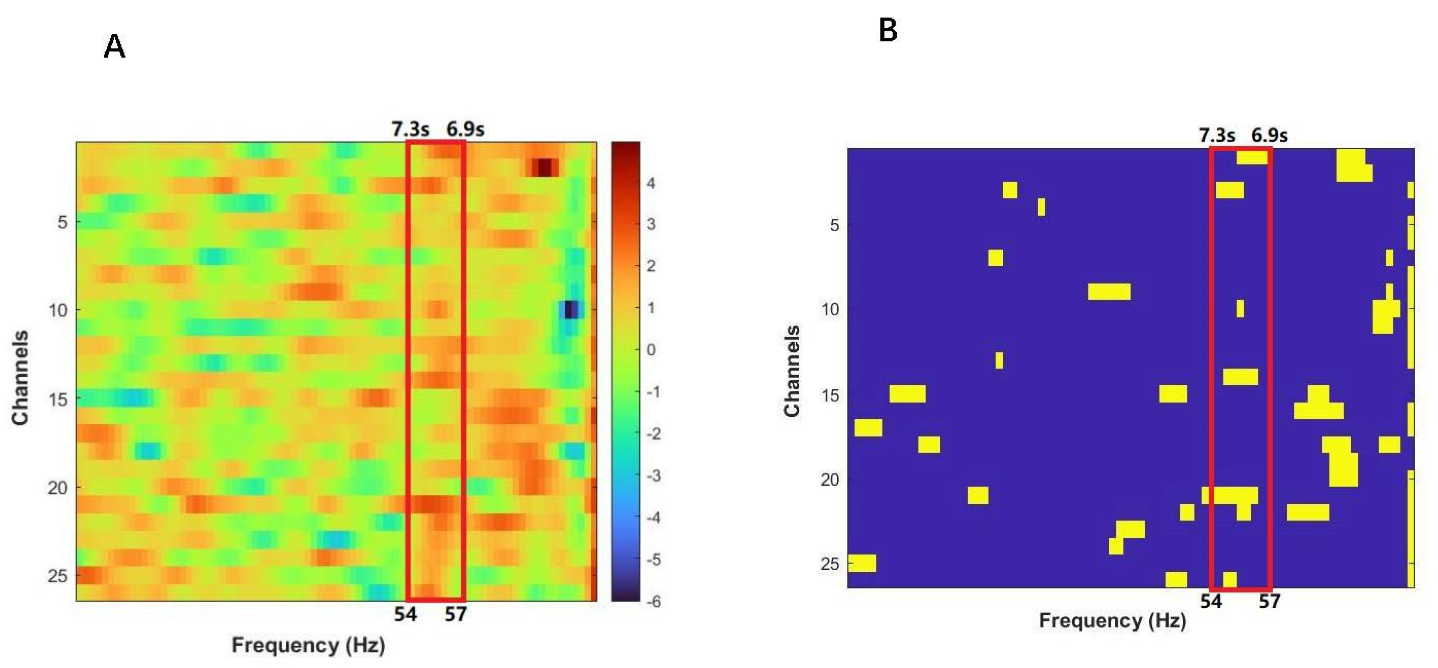
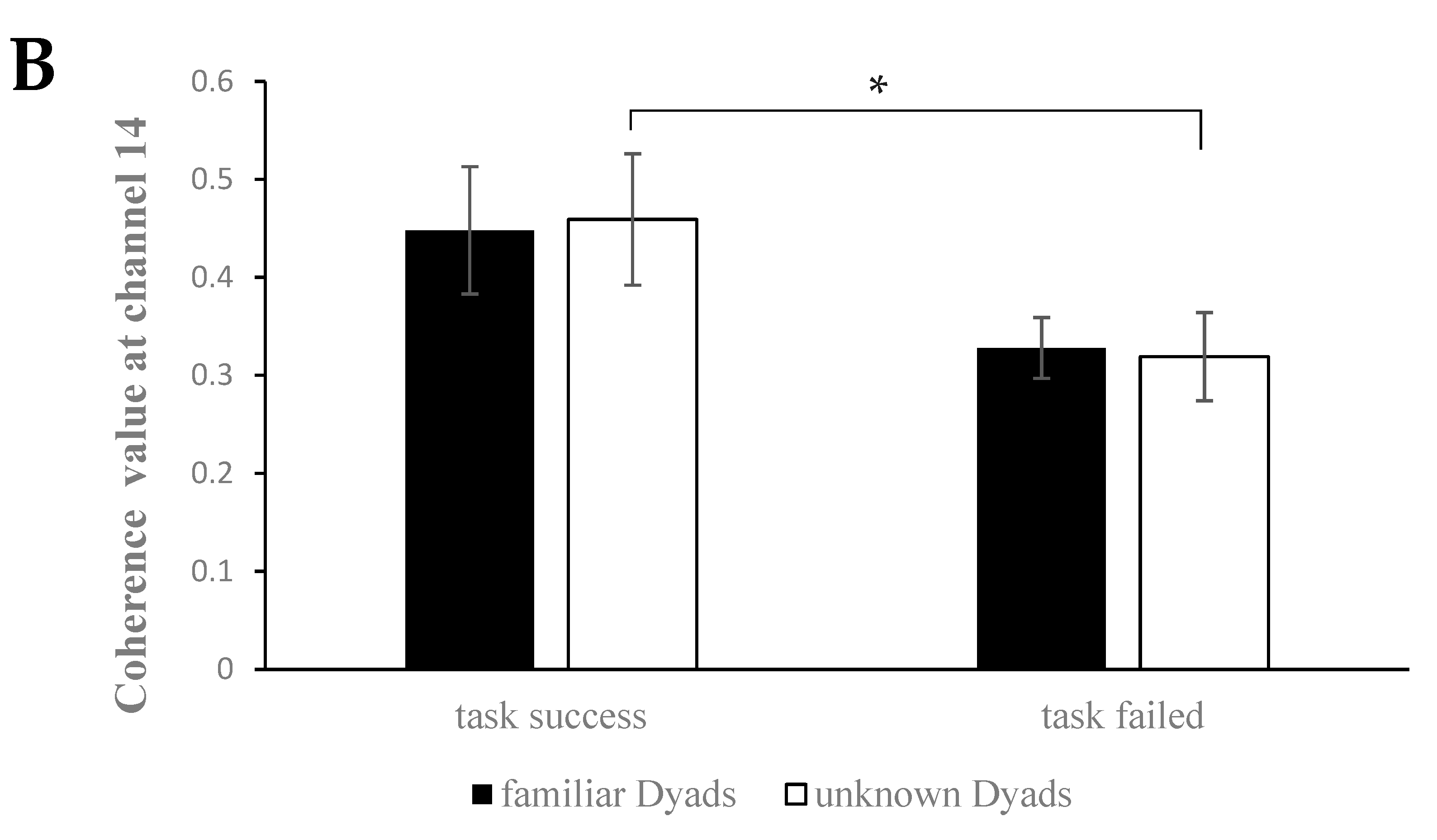
3.2. Interpersonal Brain Synchronization (IBS)
3.3. The IBS-Behavior Relation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, E.; Burger, J.; Field, C.B.; Norgaard, R.B.; Policansky, D. Revisiting the commons: Local lessons, global challenges. Science 1999, 284, 278–282. [Google Scholar] [CrossRef]
- Shinsuke, S.; Kazuhisa, N.; Syoken, F.; Eizo, A. Neural basis of conditional cooperation. Soc. Cogn. Affect. Neurosci. 2011, 6, 338–347. [Google Scholar] [CrossRef][Green Version]
- Rand, D.G. Cooperation, fast and slow: Meta-analytic evidence for a theory of social heuristics and self-interested deliberation. Psychol. Sci. 2016, 27, 1192–1206. [Google Scholar] [CrossRef]
- Gangl, K.; Pfabigan, D.M.; Lamm, C.; Kirchler, E.; Hofmann, E. Coercive and legitimate authority impact tax honesty: Evidence from behavioral and ERP experiments. Soc. Cogn. Affect. Neurosci. 2017, 12, 1108–1117. [Google Scholar] [CrossRef]
- Xue, H.; Lu, K.; Hao, N. Cooperation makes two less-creative individuals turn into a highly-creative pair. NeuroImage 2018, 172, 527–537. [Google Scholar] [CrossRef]
- Li, R.; Mayseless, N.; Balters, S.; Reiss, A.L. Dynamic inter-brain synchrony in real-life inter-personal cooperation: A functional near-infrared spectroscopy hyperscanning study. NeuroImage 2021, 238, 118–263. [Google Scholar] [CrossRef]
- Cui, X.; Bryant, D.M.; Reiss, A.L. Nirs-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage 2012, 59, 2430–2437. [Google Scholar] [CrossRef]
- Lu, K.; Xue, H.; Nozawa, T.; Hao, N. Cooperation makes a group be more creative. Cereb. Cortex 2019, 29, 3457–3470. [Google Scholar] [CrossRef]
- Astolfi, L.; Toppi, J.; Ciaramidaro, A.; Vogel, P.; Siniatchkin, M.F. Raising the bar: Can dual scanning improve our understanding of joint action? NeuroImage 2020, 216, 116813. [Google Scholar] [CrossRef]
- Holper, L.; Scholkmann, F.; Wolf, M. Between-brain connectivity during imitation measured by fNIRS. NeuroImage 2012, 63, 212–222. [Google Scholar] [CrossRef]
- Gvirts, H.Z.; Perlmutter, R. What guides us to neurally and behaviorally align with anyone specific? a neurobiological model based on fnirs hyperscanning studies. Neuroscientist 2019, 26, 1–9. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Hu, Y. Synchronous brain activity during cooperative exchange depends on gender of partner: A fnirs-based hyperscanning study. Hum. Brain Mapp. 2015, 36, 2039–2048. [Google Scholar] [CrossRef]
- Mayseless, N.; Hawthorne, G.; Reiss, A.L. Real-life creative problem solving in teams: fNIRS based hyperscanning study. Neuroimage 2019, 203, 116–161. [Google Scholar] [CrossRef]
- Jiang, J.; Dai, B.; Peng, D.; Zhu, C.; Liu, L.; Lu, C. Neural synchronization during face-to-face communication. J. Neurosci. 2012, 32, 16064–16069. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, X.; Zhang, Z.; Li, X.; Hu, Y. Cooperation in lovers: An fNIRS-based hyperscanning study. Hum. Brain Mapp. 2017, 38, 831–841. [Google Scholar] [CrossRef]
- Liu, N.; Charis, M.; Witt, E.E.; Pradhan, A.H.; Chen, J.E.; Reiss, A.L. Nirs-based hyperscanning reveals inter-brain neural synchronization during cooperative jenga game with face-to-face communication. Front. Hum. Neurosci. 2016, 10, 82. [Google Scholar] [CrossRef]
- Miller, J.G.; Vrtička, P.; Cui, X.; Shrestha, S.; Hosseini, S.M.H.; Baker, J.M.; Reiss, A.L. Inter-Brain Synchrony in Mother-Child Dyads During Cooperation: An fNIRS Hyperscanning Study. Neuropsychologia 2018, 124, 117–124. [Google Scholar] [CrossRef]
- Wang, Q.; Han, Z.; Hu, X.; Feng, S.; Wang, H.; Liu, T.; Yi, L. Autism symptoms modulate interpersonal neural synchronization in children with autism spectrum disorder in cooperative interactions. Brain Topogr. 2019, 33, 112–122. [Google Scholar] [CrossRef]
- McCabe, K.; Houser, D.; Ryan, L.; Smith, V.; Trouard, T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc. Natl. Acad. Sci. USA 2001, 98, 11832–11835. [Google Scholar] [CrossRef]
- Lu, K.; Hao, N. When do we fall in neural synchrony with others? Soc. Cogn. Affect. Neurosci. 2019, 14, 253–261. [Google Scholar] [CrossRef]
- Baker, J.M.; Liu, N.; Cui, X.; Vrticka, P.; Saggar, M.; Hosseini, S.M.; Reiss, A.L. Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Sci. Rep. 2016, 6, 26492. [Google Scholar] [CrossRef] [PubMed]
- Reindl, V.; Gerloff, C.; Scharke, W.; Konrad, K. Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. NeuroImage 2018, 178, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Anzolin, A.; Isenburg, K.; Grahl, A.; Toppi, J.; Napadow, V. Patient-Clinician Brain Response During Clinical Encounter and Pain Treatment. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) in Conjunction with the 43rd Annual Conference of the Canadian Medical and Biological Engineering Society, Montréal, QC, Canada, 1 July 2020; pp. 1512–1515. [Google Scholar] [CrossRef]
- Nguyen, T.; Schleihauf, H.; Kungl, M.T.; Kayhan, E.; Vrtika, P. Interpersonal neural synchrony during father-child problem solving: An fNIRS hyperscanning study. Child Dev. 2021, 92, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.M.; Kenrick, D.T. Cooperative courtship: Helping friends raise and raze relationship barriers. Personal. Soc. Psychol. Bull. 2009, 35, 1285–1300. [Google Scholar] [CrossRef]
- Dommer, L.; Jäger, N.; Scholkmann, F.; Wolf, M.; Holper, L. Between-brain coherence during joint n-back task performance: A two-person functional near-infrared spectroscopy study. Behav. Brain Res. 2012, 234, 212–222. [Google Scholar] [CrossRef]
- Assad, K.K.; Donnellan, M.B.; Conger, R.D. Optimism: An enduring resource for romantic relationships. J. Personal. Soc. Psychol. 2007, 93, 285–297. [Google Scholar] [CrossRef]
- Randall, A.; Post, J.; Reed, R.; Butler, E. Cooperating with your romantic partner: Associations with interpersonal emotion coordination. J. Soc. Personal. Relatsh. 2013, 30, 1072–1095. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Brains in competition: Improved cognitive performance and inter-brain coupling by hyperscanning paradigm with functional near-infrared spectroscopy. Front. Behav. Neurosci. 2017, 11, 163. [Google Scholar] [CrossRef]
- Hoehl, S.; Fairhurst, M.; Schirmer, A. Interactional synchrony: Signals, mechanisms, and benefits. Soc. Cogn. Affect. Neurosci. 2020, 16, 5–18. [Google Scholar] [CrossRef]
- Aron, A.; Aron, E.N.; Smollan, D. Inclusion of other in the self scale and the structure of interpersonal closeness. J. Personal. Soc. Psychol. 1992, 63, 596–612. [Google Scholar] [CrossRef]
- Victorino, L. Structured and Unstructured Regulation Priming Induction and Effects on Task Performance. 2019. Available online: https://doi.org/10.31219/osf.io/v5yf4 (accessed on 26 December 2019).
- Zhang, D.; Zhou, Y.; Yuan, J. Speech prosodies of different emotional categories activate different brain regions in adult cortex: An fnirs study. Sci. Rep. 2018, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- De Witte, S.; Klooster, D.; Dedoncker, J.; Duprat, R.; Remue, J.; Baeken, C. Left prefrontal neuronavigated electrode localization in tDCS: 10–20 EEG system versus MRI-guided neuronavigation. Psychiatry Res. Neuroimaging 2018, 274, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morais, G.Z.; Balardin, J.B.; Sato, J.R. Fnirs optodes’ location decider (fold): A toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.R.; Robinson, J.L.; McMillan, K.M.; Tordesillas-Guterrez, D.; Moran, S.T.; Gonzales, S.M.; Ray, K.L.; Franklin, C.; Glahn, D.C.; Fox, P.T.; et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. Neuroimage 2010, 51, 677–683. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Chang, C.; Glover, G.H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage 2010, 50, 81–98. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Wang, X.; Kan, Y.; Xu, S.; Duan, H. Inter-brain neural mechanism underlying turn-based interaction under acute stress in women: A hyperscanning study using functional near-infrared spectroscopy. Soc. Cogn. Affect. Neurosci. 2022, nsac005. [Google Scholar] [CrossRef]
- Nozawa, T.; Sasaki, Y.; Sakaki, K.; Yokoyama, R.; Kawashima, R. Interpersonal frontopolar neural synchronization in group communication: An exploration toward fNIRS hyperscanning of natural interactions. Neuroimage 2016, 133, 484–497. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; Yong, H.; Peter, C. Brainnet viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Roseth, C.J.; Johnson, D.W.; Johnson, R.T. Promoting early adolescents’ achievement and peer relationships: The effects of cooperative, competitive, and individualistic goal structures. Psychol. Bull. 2008, 134, 223–246. [Google Scholar] [CrossRef]
- Baron, R.; Moore, D.; Sanders, G. Distraction as a source of drive in social facilitation research. J. Personal. Soc. Psychol. 1978, 36, 816–824. [Google Scholar] [CrossRef]
- Kühn, S.; Brass, M.; Gallinat, J. Imitation and speech: Commonalities within broca’s area. Brain Struc. Funct. 2013, 218, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Bohsali, A.A.; Triplett, W.; Sudhyadhom, A.; Gullett, J.M.; Mcgregor, K.; Fitzgerald, D.B.; Mareci, T.; White, K.; Crosson, B. Broca’s area-thalamic connectivity. Brain Lang. 2015, 141, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Heim, S.; Eickhoff, S.B.; Amunts, K. Specialisation in broca’s region for semantic, phonological, and syntactic fluency? Neuroimage 2008, 40, 1362–1368. [Google Scholar] [CrossRef]
- Gold, B.T.; Buckner, R.L. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron 2002, 35, 803–812. [Google Scholar] [CrossRef]
- Kohler, E.; Keysers, C.; Umilta, M.A.; Fogassi, L.; Gallese, V.; Rizzolatti, G. Hearing sounds, understanding actions: Action representation in mirror neurons. Science 2002, 297, 846–848. [Google Scholar] [CrossRef]
- Hasson, U.; Frith, C.D. Mirroring and beyond: Coupled dynamics as a generalized framework for modelling social interactions. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150366. [Google Scholar] [CrossRef]
- Ikeda, S.; Nozawa, T.; Yokoyama, R.; Miyazaki, A.; Sasaki, Y.; Sakaki, K.; Kawashima, R. Steady beat sound facilitates both coordinated group walking and inter-subject neural synchrony. Front. Hum. Neurosci. 2017, 11, 147. [Google Scholar] [CrossRef]
- Sanfey, A.G.; Rilling, J.K.; Aronson, J.A.; Nystrom, L.E.; Cohen, J.D. The neural basis of economic decision-making in the ultimatum game. Science 2003, 300, 1755–1758. [Google Scholar] [CrossRef]
- Li, Y.; Chen, R.; Turel, O.; Feng, T.; Zhu, C.Z.; He, Q. Dyad sex composition effect on inter-brain synchronization in face-to-face cooperation. Brain Imaging Behav. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Koster-Hale, J.; Saxe, R. Functional neuroimaging of theory of mind. In Understanding Other Minds; Baron-Cohen, S., Lombardo, M., Tager-Flusberg, H., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 132–163. [Google Scholar] [CrossRef]
- Saxe, R.; Kanwisher, N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. NeuroImage 2003, 19, 1835–1842. [Google Scholar] [CrossRef]
- Dimitrov, M.; Grafman, J.; Hollnagel, C. The effects of frontal lobe damage on everyday problem solving. Cortex 1996, 32, 357. [Google Scholar] [CrossRef]
- Tomasello, M. Joint attention as social cognition. In Joint Attention: Its Origins and Role in Development; Moore, C., Dunham, P.J., Eds.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1995; pp. 103–130. [Google Scholar] [CrossRef]
- Pourtois, G.; Schwartz, S.; Seghier, M.L.; Lazeyras, F.; Vuilleumier, P. Neural systems for orienting attention to the location of threat signals: An event-related fMRI study. Neuroimage 2006, 31, 920–933. [Google Scholar] [CrossRef] [PubMed]
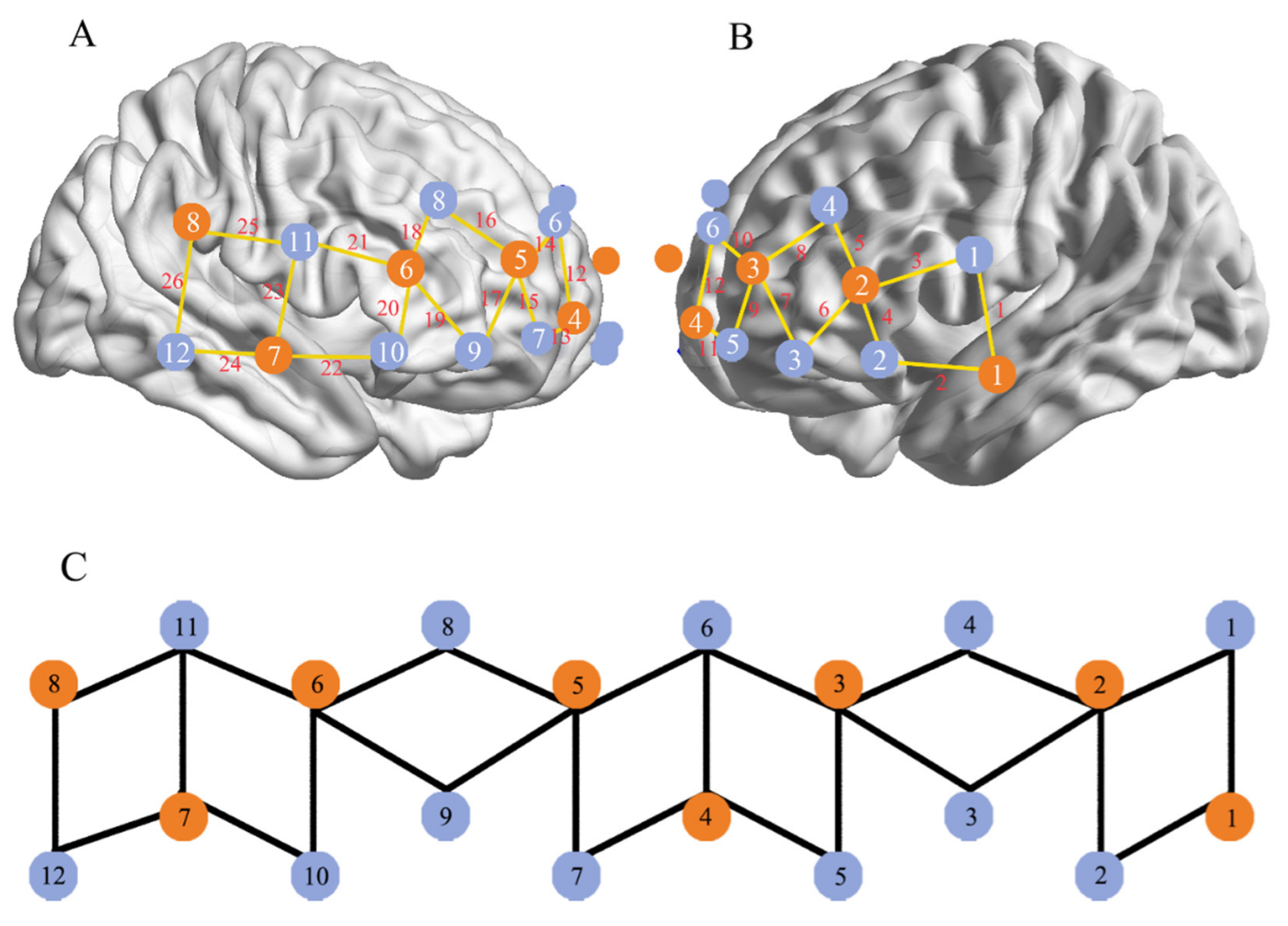
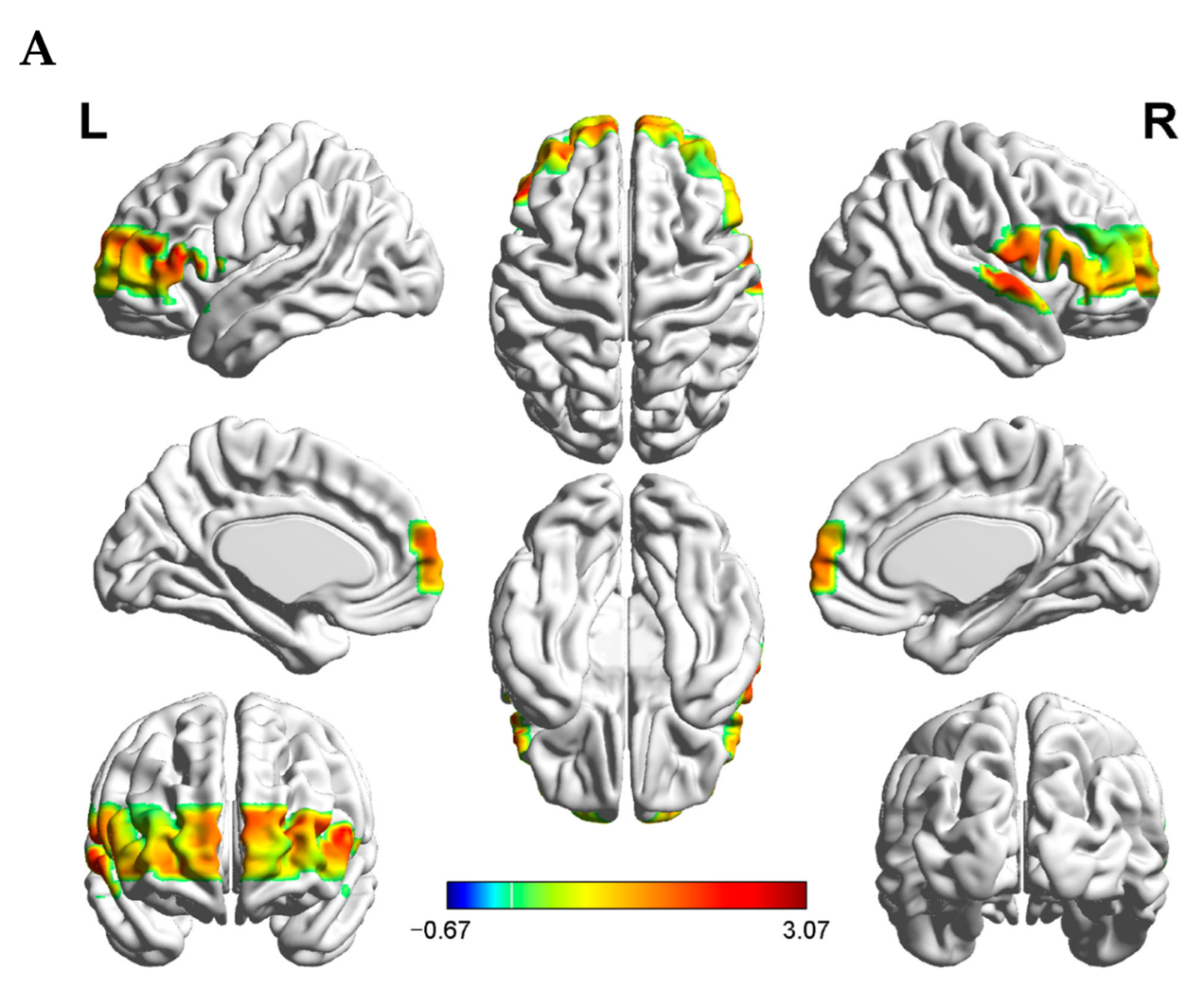
| Channels | MNI Coordinates | Brodmann’s Areas | Percentage of Overlap | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 FT7-FC5 | −62 | 8 | 5 | 48—Retrosubicular area 6—Pre-Motor and Supplementary Motor Cortex | 0.620 0.295 |
| 2 FT7-F7 | −55 | 17 | −13 | 38—Temporopolar area | 0.986 |
| 3 F5-FC5 | −57 | 28 | 16 | 45—pars triangularis Broca’s area 44—pars opercularis, part of Broca’s area | 0.845 0.155 |
| 4 F5-F7 | −54 | 40 | 0 | 45—pars triangularis Broca’s area 46—Dorsolateral prefrontal cortex | 0.717 0.257 |
| 5 F5-F3 | −47 | 45 | 24 | 45—pars triangularis Broca’s area 46—Dorsolateral prefrontal cortex | 0.751 0.249 |
| 6 F5-AF7 | −48 | 51 | 0 | 46—Dorsolateral prefrontal cortex | 0.929 |
| 7 AF3-AF7 | −36 | 64 | 3 | 10—Frontopolar area 11—Orbitofrontal area | 0.820 0.132 |
| 8 AF3-F3 | −33 | 57 | 26 | 46—Dorsolateral prefrontal cortex | 0.966 |
| 9 AF3-Fp1 | −24 | 70 | 5 | 10—Frontopolar area 11—Orbitofrontal area | 0.698 0.302 |
| 10 AF3-Afz | −13 | 68 | 24 | 10—Frontopolar area | 0.997 |
| 11 Fpz-Fp1 | −12 | 73 | −4 | 11—Orbitofrontal area 10—Frontopolar area | 0.505 0.495 |
| 12 Fpz-Afz | 2 | 68 | 13 | 10—Frontopolar area | 1 |
| 13 Fpz-Fp2 | 14 | 73 | −4 | 11—Orbitofrontal area 10—Frontopolar area | 0.516 0.484 |
| 14 AF4-Afz | 16 | 69 | 24 | 10—Frontopolar area | 1 |
| 15 AF4-Fp2 | 27 | 70 | 6 | 10—Frontopolar area 11—Orbitofrontal area | 0.721 0.279 |
| 16 AF4-F4 | 36 | 57 | 27 | 46—Dorsolateral prefrontal cortex | 0.956 |
| 17 AF4-AF8 | 40 | 64 | 4 | 10—Frontopolar area 46—Dorsolateral prefrontal cortex | 0.798 0.107 |
| 18 F6-F4 | 49 | 44 | 25 | 45—pars triangularis Broca’s area 46—Dorsolateral prefrontal cortex | 0.809 0.191 |
| 19 F6-AF8 | 50 | 51 | 1 | 46—Dorsolateral prefrontal cortex | 0.906 |
| 20 F6-F8 | 57 | 38 | 1 | 45—pars triangularis Broca’s area 46—Dorsolateral prefrontal cortex | 0.803 0.188 |
| 21 F6-FC6 | 60 | 27 | 18 | 45—pars triangularis Broca’s area 44—pars opercularis, part of Broca’s area | 0.825 0.175 |
| 22 FT8-F8 | 59 | 15 | −13 | 38—Temporopolar area 21—Middle Temporal gyrus | 0.881 0.119 |
| 23 FT8-FC6 | 64 | 7 | 6 | 48—Retrosubicular area 6—Pre-Motor and Supplementary Motor Cortex | 0.627 0.300 |
| 24 FT8-T8 | 71 | −10 | −12 | 21—Middle Temporal gyrus | 0.990 |
| 25 C6-FC6 | 69 | −5 | 25 | 43—Subcentral area | 0.949 |
| 26 C6-T8 | 73 | −23 | 7 | 22—Superior Temporal Gyrus 21—Middle Temporal gyrus | 0.717 0.283 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhang, Y.; Fu, Y.; Wu, L.; Li, X.; Zhu, N.; Li, D.; Zhang, M. The Effect of Task Performance and Partnership on Interpersonal Brain Synchrony during Cooperation. Brain Sci. 2022, 12, 635. https://doi.org/10.3390/brainsci12050635
Zhou S, Zhang Y, Fu Y, Wu L, Li X, Zhu N, Li D, Zhang M. The Effect of Task Performance and Partnership on Interpersonal Brain Synchrony during Cooperation. Brain Sciences. 2022; 12(5):635. https://doi.org/10.3390/brainsci12050635
Chicago/Turabian StyleZhou, Shujin, Yuxuan Zhang, Yiwen Fu, Lingling Wu, Xiaodie Li, Ningning Zhu, Dan Li, and Mingming Zhang. 2022. "The Effect of Task Performance and Partnership on Interpersonal Brain Synchrony during Cooperation" Brain Sciences 12, no. 5: 635. https://doi.org/10.3390/brainsci12050635
APA StyleZhou, S., Zhang, Y., Fu, Y., Wu, L., Li, X., Zhu, N., Li, D., & Zhang, M. (2022). The Effect of Task Performance and Partnership on Interpersonal Brain Synchrony during Cooperation. Brain Sciences, 12(5), 635. https://doi.org/10.3390/brainsci12050635