Processing of an Audiobook in the Human Brain Is Shaped by Cultural Family Background
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimulus
2.3. Experimental Procedures
2.4. fMRI Acquisition
2.5. fMRI Reconstruction and Preprocessing
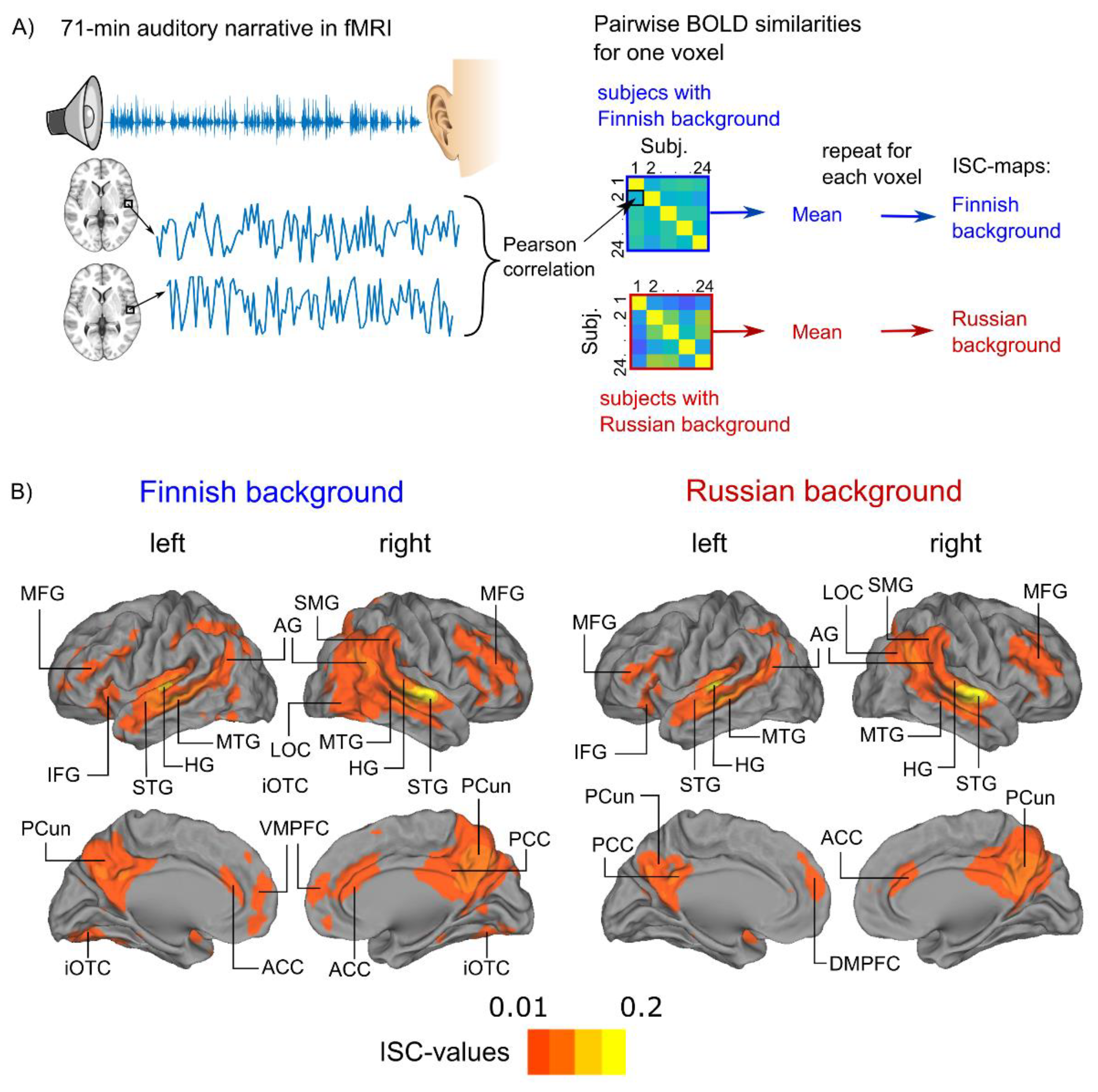
2.6. Inter-Subject Correlation (ISC) Analysis of Blood-Oxygen-Level-Dependent Responses
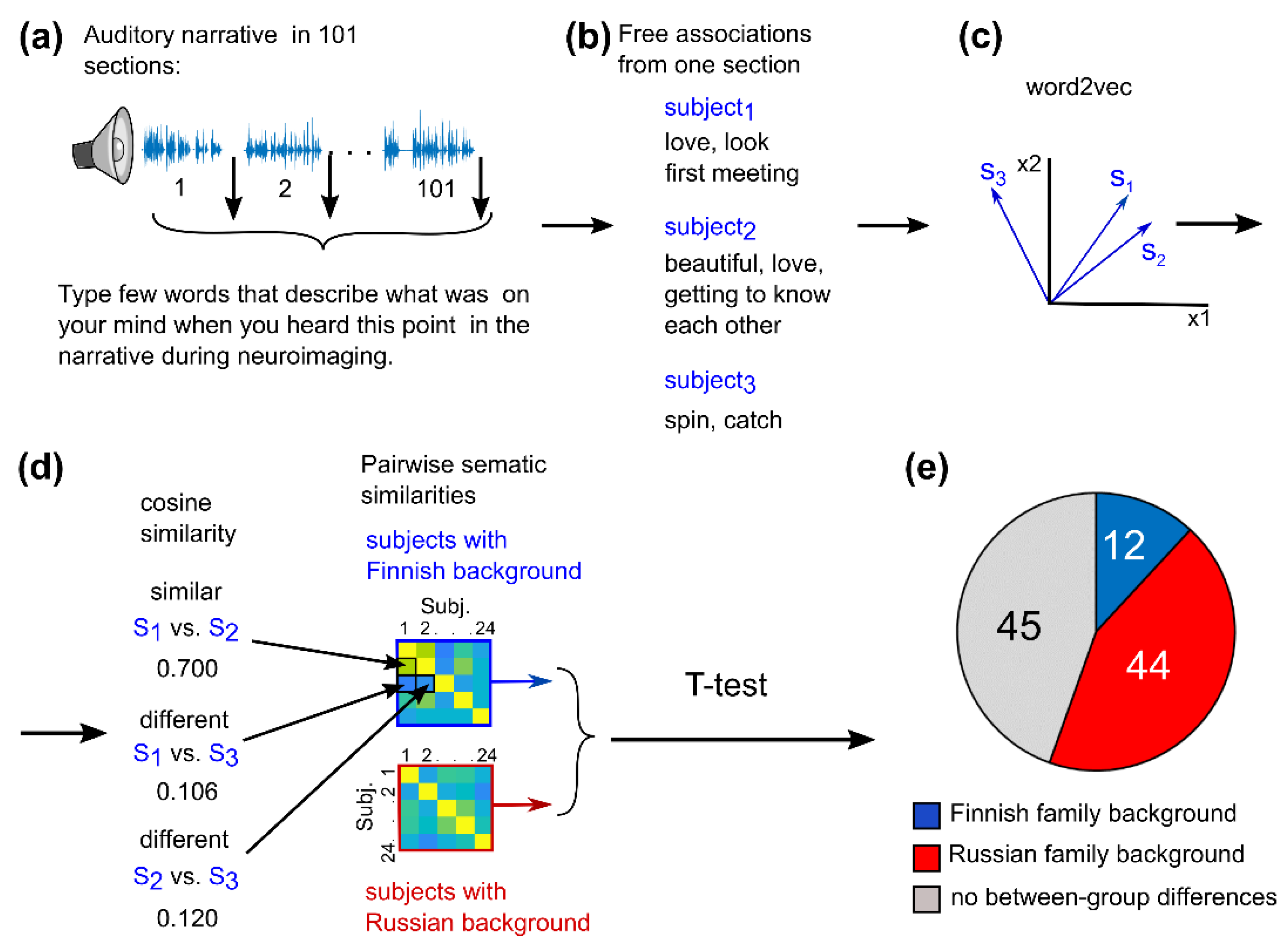
2.7. Semantic Similarity Analysis of the Self-Reported Word Lists
3. Results
3.1. Audiobook Listening Elicited Widespread ISC of BOLD Activity
3.2. Cultural Family Background Modulates Brain Activity during Audiobook Listening
3.3. Cultural Family Background Increased Similarity of Audiobook Interpretation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Language Learned First | Stronger Language | Language Used at Work | Language Used in Free Time | |
|---|---|---|---|---|
| Finnish | 3 | 19 | 18 | 8 |
| Russian | 10 | 3 | 0 | 1 |
| Finnish and Russian | 11 | 2 | 6 | 15 |
Appendix B. Implicit Association Test (IAT)
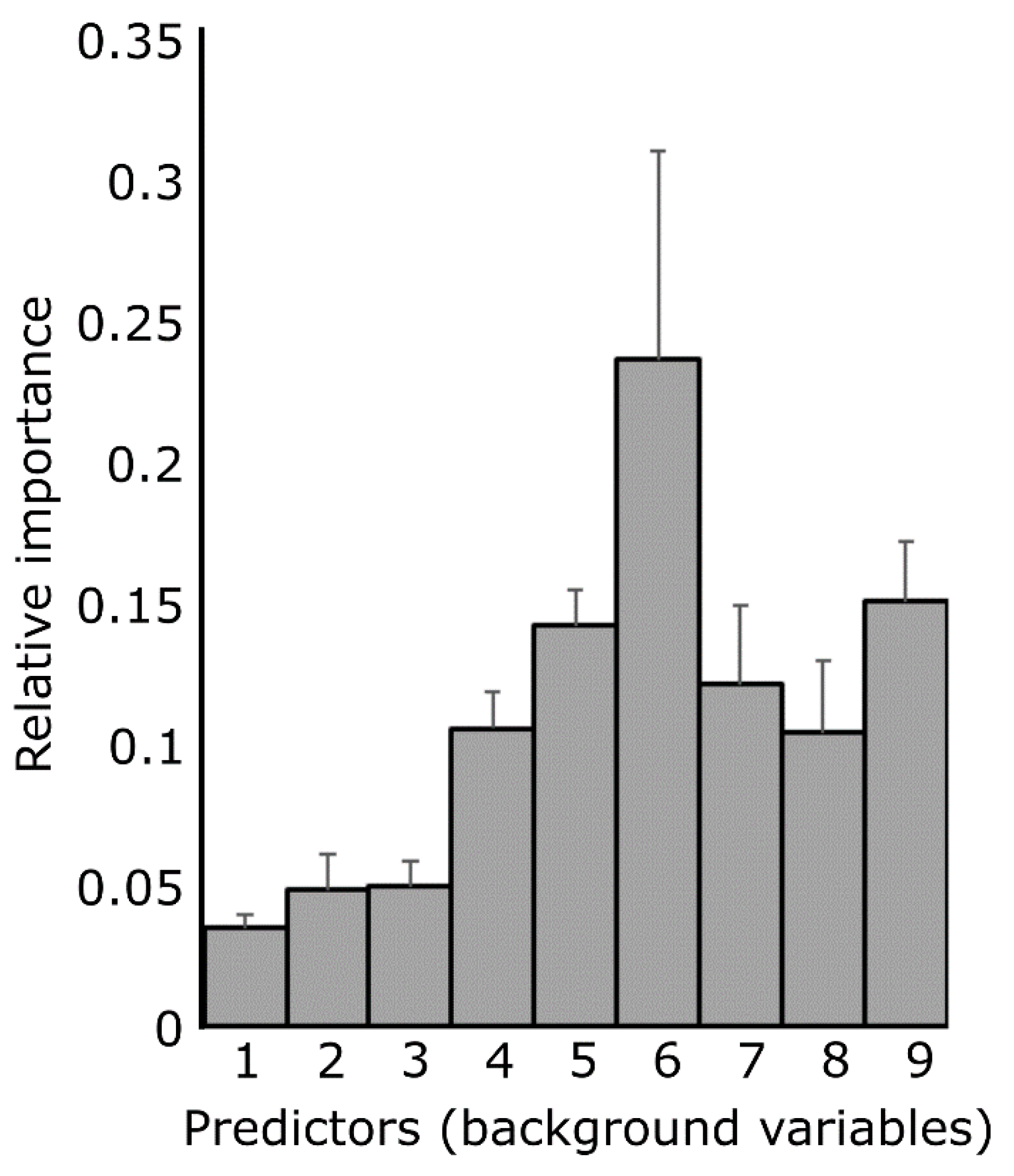
Appendix C. Background Variables Which Most Accurately Predict the Free Association Experiment Results
Appendix D
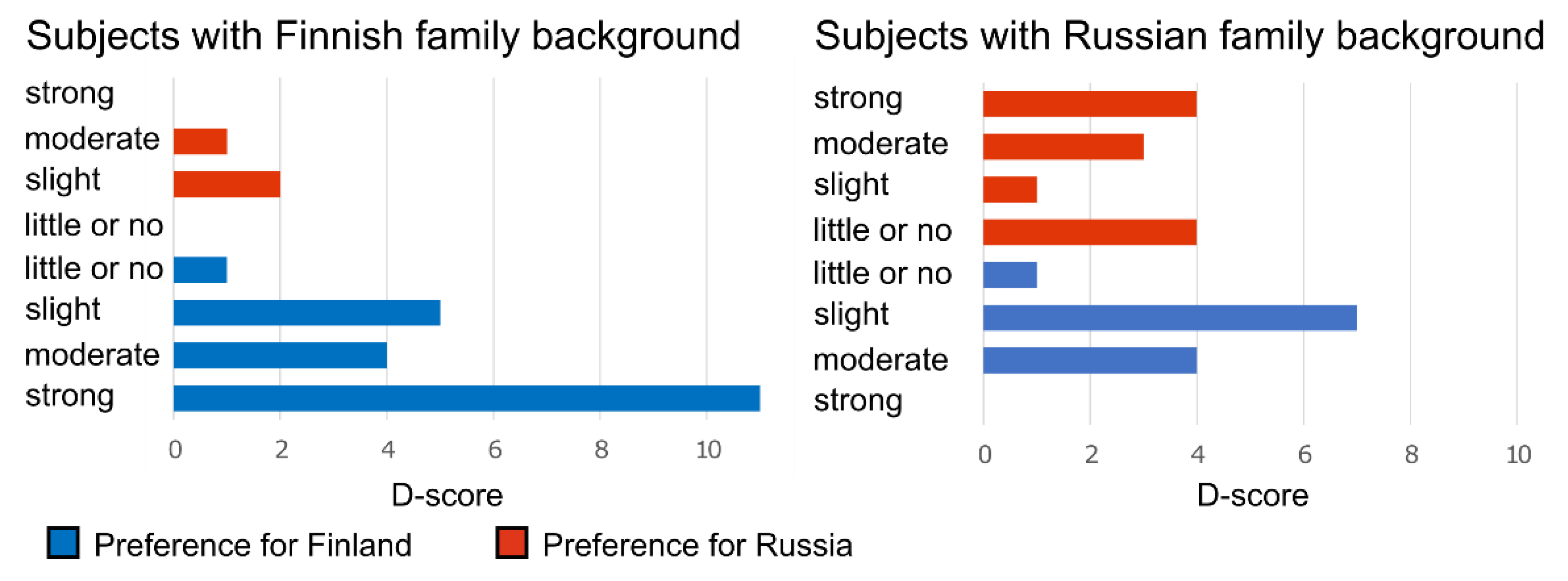
Appendix E
References
- McPherson, M.; Smith-Lovin, L.; Cook, J.M. Birds of a Feather: Homophily in Social Networks. Annu. Rev. Sociol. 2001, 27, 415–444. [Google Scholar] [CrossRef]
- Parkinson, C.; Kleinbaum, A.M.; Wheatley, T. Similar Neural Responses Predict Friendship. Nat. Commun. 2018, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Ames, D.L.; Fiske, S.T. Cultural Neuroscience. Asian J. Soc. Psychol. 2010, 13, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Chiao, J.Y.; Hariri, A.R.; Harada, T.; Mano, Y.; Sadato, N.; Parrish, T.B.; Iidaka, T. Theory and Methods in Cultural Neuroscience. Soc. Cogn. Affect. Neurosci. 2010, 5, 356. [Google Scholar] [CrossRef]
- Chiu, L.-H. A Cross-Cultural Comparison of Cognitive Styles in Chinese and American Children. Int. J. Psychol. 1972, 7, 235–242. [Google Scholar] [CrossRef]
- Ji, L.-J.; Zhang, Z.; Nisbett, R.E. Is It Culture or Is It Language? Examination of Language Effects in Cross-Cultural Research on Categorization. J. Pers. Soc. Psychol. 2004, 87, 57–65. [Google Scholar] [CrossRef]
- Nisbett, R.E.; Peng, K.; Choi, I.; Norenzayan, A.; Polytechnique, E. Culture and Systems of Thought: Holistic Versus Analytic Cognition. Psychol. Rev. 2001, 108, 291–310. [Google Scholar] [CrossRef]
- Unsworth, S.J.; Sears, C.R.; Pexman, P.M. Cultural Influences on Categorization Processes. J. Cross. Cult. Psychol. 2005, 36, 662–688. [Google Scholar] [CrossRef]
- Gutchess, A.H.; Yoon, C.; Luo, T.; Feinberg, F.; Hedden, T.; Jing, Q.; Nisbett, R.E.; Park, D.C. Categorical Organization in Free Recall across Culture and Age. Gerontology 2006, 52, 314–323. [Google Scholar] [CrossRef]
- Yoon, C.; Feinberg, F.; Hu, P.; Gutchess, A.H.; Hedden, T.; Chen, H.-Y.M.; Jing, Q.; Cui, Y.; Park, D.C. Category Norms as a Function of Culture and Age: Comparisons of Item Responses to 105 Categories by American and Chinese Adults. Psychol. Aging 2004, 19, 379–393. [Google Scholar] [CrossRef]
- Gutchess, A.H.; Welsh, R.C.; Boduroglu, A.; Park, D.C. Cultural Differences in Neural Function Associated with Object Processing. Cogn. Affect. Behav. Neurosci. 2006, 6, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gutchess, A.H.; Indeck, A. Cultural Influences on Memory. Prog. Brain Res. 2009, 178, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ma, Y. Cultural Differences in Human Brain Activity: A Quantitative Meta-Analysis. Neuroimage 2014, 99, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Norenzayan, A.; Smith, E.E.; Kim, B.J.; Nisbett, R.E. Cultural Preferences for Formal versus Intuitive Reasoning. Cogn. Sci. 2002, 26, 653–684. [Google Scholar] [CrossRef]
- Zhang, L. Malleability of Cognitive Style and Its Implications for Management Practice. Doctoral Dissertation, University of Leeds, Leeds, UK, 2005. [Google Scholar]
- Dewitt, I.; Rauschecker, J.P. Phoneme and Word Recognition in the Auditory Ventral Stream. Proc. Natl. Acad. Sci. USA 2012, 109, 2709. [Google Scholar] [CrossRef]
- Lerner, Y.; Honey, C.J.; Silbert, L.J.; Hasson, U. Topographic Mapping of a Hierarchy of Temporal Receptive Windows Using a Narrated Story. J. Neurosci. 2011, 31, 2906–2915. [Google Scholar] [CrossRef]
- Huth, A.G.; de Heer, W.A.; Griffiths, T.L.; Theunissen, F.E.; Gallant, J.L. Natural Speech Reveals the Semantic Maps That Tile Human Cerebral Cortex. Nature 2016, 532, 453–458. [Google Scholar] [CrossRef]
- Whitney, C.; Huber, W.; Klann, J.; Weis, S.; Krach, S.; Kircher, T. Neural Correlates of Narrative Shifts during Auditory Story Comprehension. Neuroimage 2009, 47, 360–366. [Google Scholar] [CrossRef]
- Yarkoni, T.; Speer, N.K.; Zacks, J.M. Neural Substrates of Narrative Comprehension and Memory. Neuroimage 2008, 41, 1408–1425. [Google Scholar] [CrossRef]
- Jung-Beeman, M. Bilateral Brain Processes for Comprehending Natural Language. Trends Cogn. Sci. 2005, 9, 512–518. [Google Scholar] [CrossRef]
- Saalasti, S.; Alho, J.; Bar, M.; Glerean, E.; Honkela, T.; Kauppila, M.; Sams, M.; Jääskeläinen, I.P. Inferior Parietal Lobule and Early Visual Areas Support Elicitation of Individualized Meanings during Narrative Listening. Brain Behav. 2019, 9, e01288. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, M.; Kurimo, M.; Gross, J.; Hyvärinen, A.; Hari, R. Brain Activity Reflects the Predictability of Word Sequences in Listened Continuous Speech: Brain Activity Predicts Word Sequences. Neuroimage 2020, 219, 116936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lauer, G. How Culture Shapes the Reading of Fairy Tales: A Cross-Cultural Approach. Comp. Lit. Stud. 2015, 52, 663–681. [Google Scholar] [CrossRef]
- Anderson, B.V.; Barnitz, J.G. Cultural Schemata and Reading Comprehension. J. Read. 1984, 28, 102–108. [Google Scholar] [CrossRef]
- Ellis, C.; Kuipers, J.R.; Thierry, G.; Lovett, V.; Turnbull, O.; Jones, M.W. Language and Culture Modulate Online Semantic Processing. Soc. Cogn. Affect. Neurosci. 2015, 10, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Marks, G.; Miller, N. Ten Years of Research on the False-Consensus Effect: An Empirical and Theoretical Review. Psychol. Bull. 1987, 102, 72–90. [Google Scholar] [CrossRef]
- Crinion, J.T.; Lambon-Ralph, M.A.; Warburton, E.A.; Howard, D.; Wise, R.J.S. Temporal Lobe Regions Engaged during Normal Speech Comprehension. Brain 2003, 126, 1193–1201. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Swanson, S.; Simony, E.; Chen, J.; Lazaridi, C.; Honey, C.J.; Hasson, U. Same Story, Different Story. Psychol. Sci. 2017, 28, 307–319. [Google Scholar] [CrossRef]
- Nguyen, M.; Vanderwal, T.; Hasson, U. Shared Understanding of Narratives Is Correlated with Shared Neural Responses. Neuroimage 2019, 184, 161–170. [Google Scholar] [CrossRef]
- Finn, E.S.; Corlett, P.R.; Chen, G.; Bandettini, P.A.; Constable, R.T. Trait Paranoia Shapes Inter-Subject Synchrony in Brain Activity during an Ambiguous Social Narrative. Nat. Commun. 2018, 9, 2043. [Google Scholar] [CrossRef]
- Kauppi, J.P.; Jääskeläinen, I.P.; Sams, M.; Tohka, J. Inter-Subject Correlation of Brain Hemodynamic Responses during Watching a Movie: Localization in Space and Frequency. Front. Neuroinform. 2010, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Mikolov, T.; Chen, K.; Corrado, G.; Dean, J. Efficient Estimation of Word Representations in Vector Space. arXiv 2013, arXiv:1301.3781. [Google Scholar]
- Nisbett, R.E.; Miyamoto, Y. The Influence of Culture: Holistic versus Analytic Perception. Trends Cogn. Sci. 2005, 9, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Greenwald, A.G.; Mcghee, D.E.; Schwartz, J.L.K. Measuring Individual Differences in Implicit Cognition: The Implicit Association Test. J. Personal. Soc. Psychol. 1998, 74, 1464. [Google Scholar] [CrossRef]
- Greenwald, A.G.; Nosek, B.A.; Banaji, M.R. Understanding and Using the Implicit Association Test: I. An Improved Scoring Algorithm. J. Personal. Soc. Psychol. 1998, 85, 197. [Google Scholar] [CrossRef]
- Ottaway, S.A.; Hayden, D.C.; Oakes, M.A. Implicit Attitudes and Racism: Effects of Word Familiarity and Frequency on the Implicit Association Test. Soc. Cogn. 2005, 19, 97–144. [Google Scholar] [CrossRef]
- Rothermund, K.; Wentura, D. Underlying Processes in the Implicit Association Test: Dissociating Salience from Associations. J. Exp. Psychol. Gen. 2004, 133, 139–165. [Google Scholar] [CrossRef]
- Schimmack, U. Invalid Claims About the Validity of Implicit Association Tests by Prisoners of the Implicit Social-Cognition Paradigm. Perspect. Psychol. Sci. 2021, 16, 435. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Chu, Y.-H.; Tsai, S.-Y.; Kuo, W.-J.; Chang, C.-Y.; Lin, F.-H. Simultaneous Multi-Slice Inverse Imaging of the Human Brain. Sci. Rep. 2017, 7, 17019. [Google Scholar] [CrossRef]
- Feinberg, D.A.; Setsompop, K. Ultra-Fast MRI of the Human Brain with Simultaneous Multi-Slice Imaging. J. Magn. Reson. 2013, 229, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Setsompop, K.; Gagoski, B.A.; Polimeni, J.R.; Witzel, T.; Wedeen, V.J.; Wald, L.L. Blipped-Controlled Aliasing in Parallel Imaging for Simultaneous Multislice Echo Planar Imaging with Reduced g-Factor Penalty. Magn. Reson. Med. 2011, 67, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-H.; Tsai, K.W.K.; Chu, Y.-H.; Witzel, T.; Nummenmaa, A.; Raij, T.; Ahveninen, J.; Kuo, W.-J.; Belliveau, J.W. Ultrafast Inverse Imaging Techniques for FMRI. Neuroimage 2012, 62, 699–705. [Google Scholar] [CrossRef]
- Lewis, L.D.; Setsompop, K.; Rosen, B.R.; Polimeni, J.R. Fast FMRI Can Detect Oscillatory Neural Activity in Humans. Proc. Natl. Acad. Sci. USA 2016, 113, E6679–E6685. [Google Scholar] [CrossRef] [PubMed]
- LeVan, P.; Akin, B.; Hennig, J.; LeVan, P.; Akin, B.; Hennig, J. Fast Imaging for Mapping Dynamic Networks. Neuroimage 2018, 180, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-H.; Huang, T.-Y.; Chen, N.-K.; Wang, F.-N.; Stufflebeam, S.M.; Belliveau, J.W.; Wald, L.L.; Kwong, K.K. Functional MRI Using Regularized Parallel Imaging Acquisition. Magn. Reson. Med. 2005, 54, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-H.; Kwong, K.K.; Belliveau, J.W.; Wald, L.L. Parallel Imaging Reconstruction Using Automatic Regularization. Magn. Reson. Med. 2004, 51, 559–567. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A Global Optimisation Method for Robust Affine Registration of Brain Images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Pamilo, S.; Malinen, S.; Hotta, J.; Seppä, M. A Correlation-Based Method for Extracting Subject-Specific Components and Artifacts from Group-FMRI Data. Eur. J. Neurosci. 2015, 42, 2726–2741. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but Systematic Correlations in Functional Connectivity MRI Networks Arise from Subject Motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, J.-P.; Pajula, J.; Tohka, J. A Versatile Software Package for Inter-Subject Correlation Based Analyses of FMRI. Front. Neuroinform. 2014, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.E.; Holmes, A.P. Nonparametric Permutation Tests for Functional Neuroimaging Experiments: A Primer with Examples. Hum. Brain Mapp. 2001, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation Inference for the General Linear Model. Neuroimage 2014, 92, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Glerean, E.; Pan, R.K.; Salmi, J.; Kujala, R.; Lahnakoski, J.M.; Roine, U.; Nummenmaa, L.; Leppämäki, S.; Nieminen-von Wendt, T.; Tani, P.; et al. Reorganization of Functionally Connected Brain Subnetworks in High-Functioning Autism. Hum. Brain Mapp. 2016, 37, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Bacha-Trams, M.; Glerean, E.; Dunbar, R.; Lahnakoski, J.M.; Ryyppö, E.; Sams, M.; Jääskeläinen, I.P. Differential Inter-Subject Correlation of Brain Activity When Kinship Is a Variable in Moral Dilemma. Sci. Rep. 2017, 7, 14244. [Google Scholar] [CrossRef]
- Bacha-Trams, M.; Alexandrov, Y.I.; Broman, E.; Glerean, E.; Kauppila, M.; Kauttonen, J.; Ryyppö, E.; Sams, M.; Jääskeläinen, I.P. A Drama Movie Activates Brains of Holistic and Analytical Thinkers Differentially. Soc. Cogn. Affect. Neurosci. 2018, 13, 1293–1304. [Google Scholar] [CrossRef]
- Haverinen, K.; Nyblom, J.; Viljanen, T.; Laippala, V.; Kohonen, S.; Missilä, A.; Ojala, S.; Salakoski, T.; Ginter, F. Building the Essential Resources for Finnish: The Turku Dependency Treebank. Lang. Resour. Eval. 2014, 48, 493–531. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An Automated Labeling System for Subdividing the Human Cerebral Cortex on MRI Scans into Gyral Based Regions of Interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Van Essen, D.C. Cortical Cartography and Caret Software. Neuroimage 2012, 62, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Regev, M.; Honey, C.J.; Simony, E.; Hasson, U. Selective and Invariant Neural Responses to Spoken and Written Narratives. J. Neurosci. 2013, 33, 15978–15988. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G.; Poeppel, D. The Cortical Organization of Speech Processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J. A Review and Synthesis of the First 20years of PET and FMRI Studies of Heard Speech, Spoken Language and Reading. Neuroimage 2012, 62, 816–847. [Google Scholar] [CrossRef]
- Bruneau, E.G.; Dufour, N.; Saxe, R. Social Cognition in Members of Conflict Groups: Behavioural and Neural Responses in Arabs, Israelis and South Americans to Each Other’s Misfortunes. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 717–730. [Google Scholar] [CrossRef][Green Version]
- Cavanna, A.E.; Trimble, M.R. The Precuneus: A Review of Its Functional Anatomy and Behavioural Correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Khalighinejad, B.; Herrero, J.; Bickel, S.; Mehta, A.; Mesgarani, N. Functional Characterization of Human Heschl’s Gyrus in Response to Natural Speech. bioRxiv 2020. bioRxiv:2020.06.29.178178. [Google Scholar] [CrossRef]
- Sussman, E.; Winkler, I.; Huotilainen, M.; Ritter, W.; Näätänen, R. Top-down Effects Can Modify the Initially Stimulus-Driven Auditory Organization. Cogn. Brain Res. 2002, 13, 393–405. [Google Scholar] [CrossRef]
- Asilador, A.; Llano, D.A. Top-Down Inference in the Auditory System: Potential Roles for Corticofugal Projections. Front. Neural Circuits 2021, 14, 89. [Google Scholar] [CrossRef]
- Sohoglu, E.; Peelle, J.E.; Carlyon, R.P.; Davis, M.H. Predictive Top-Down Integration of Prior Knowledge during Speech Perception. J. Neurosci. 2012, 32, 8443–8453. [Google Scholar] [CrossRef]
- Kitayama, S.; Park, J. Cultural Neuroscience of the Self: Understanding the Social Grounding of the Brain. Soc. Cogn. Affect. Neurosci. 2010, 5, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Borst, G.; Kosslyn, S.M. Visual Mental Imagery and Visual Perception: Structural Equivalence Revealed by Scanning Processes. Mem. Cognit. 2008, 36, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, P. The Relationship Between Visual Perception and Visual Mental Imagery: A Reappraisal of the Neuropsychological Evidence. Cortex 2002, 38, 357–378. [Google Scholar] [CrossRef]
- Kaas, A.; Weigelt, S.; Roebroeck, A.; Kohler, A.; Muckli, L. Imagery of a Moving Object: The Role of Occipital Cortex and Human MT/V5+. Neuroimage 2010, 49, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, J. Language and Perceptual Categorisation. Trends Cogn. Sci. 2001, 5, 382–387. [Google Scholar] [CrossRef]
- McClure, S.M.; Li, J.; Tomlin, D.; Cypert, K.S.; Montague, L.M.; Montague, P.R. Neural Correlates of Behavioral Preference for Culturally Familiar Drinks. Neuron 2004, 44, 379–387. [Google Scholar] [CrossRef]
- Steffensen, M.S.; Joag-Dev, C.; Anderson, R.C. A Cross-Cultural Perspective on Reading Comprehension. Read. Res. Q. 1979, 15, 10. [Google Scholar] [CrossRef]
- Tulviste, T.; Mizera, L.; De Geer, B.; Tryggvason, M.-T. A Silent Finn, a Silent Finno–Ugric, or a Silent Nordic? A Comparative Study of Estonian, Finnish, and Swedish Mother–Adolescent Interactions. Appl. Psycholinguist. 2003, 24, 249–265. [Google Scholar] [CrossRef]
- Jääskeläinen, I.P.; Ahveninen, J.; Klucharev, V.; Shestakova, A.N.; Levy, J. Behavioral Experience-Sampling Methods in Neuroimaging Studies With Movie and Narrative Stimuli. Front. Hum. Neurosci. 2022, 16, 10. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy Function Approximation: A Gradient Boosting Machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]

|
Cultural Background | Level of Command of Russian Language *** | Lifetime Visits to Russia *** | How Finnish Do I Feel Myself? (Scale 1–100) ** | How Russian Do I Feel Myself? (Scale 1–100) *** | How Positively/Negatively Do I See Finns (Scale 1–100) n.s. | How Positively/Negatively Do I See Russians (Scale 1–100) n.s. |
|---|---|---|---|---|---|---|
| Finnish background | 1.2 ± 0.1 | 1.4 ± 0.1 | 93.3 ± 1.9 | 1.6 ± 0.3 | 86.3 ± 2.2 | 59.7 ± 2.7 |
| Russian | 4.7 ± 0.1 | 3.9 ± 0.1 | 69.6 ± 3.1 | 55.8 ± 3.2 | 77.3 ± 2.6 | 67.3 ± 2.8 |
| Cluster Label | Cluster Extent (Voxels) | x MNI (mm) | y MNI (mm) | z MNI (mm) | Max ISC Value |
|---|---|---|---|---|---|
| ISC Finnish background | |||||
| Planum temporale (R) | 1730 | 60 | −12 | 0 | 0.14 |
| Heschl’s gyrus (L) | 1568 | −48 | −18 | 3 | 0.14 |
| Frontal Orbital cortex (L) | 82 | −39 | 27 | −9 | 0.02 |
| Frontal pole (R) | 69 | 48 | 42 | 3 | 0.02 |
| Precuneus cortex (R) | 1338 | 6 | −63 | 33 | 0.06 |
| Frontal pole (L) | 68 | −36 | 36 | 9 | 0.02 |
| Cingulate gyrus (R) | 71 | 6 | 33 | 21 | 0.02 |
| ISC Russian background | |||||
| Cerebellar crus II (R) | 38 | 30 | −75 | −39 | 0.04 |
| Superior temporal gyrus, posterior division (R) | 1055 | 63 | −12 | 0 | 0.14 |
| Heschl’s gyrus (L) | 880 | −48 | −21 | 3 | 0.16 |
| Inferior frontal gyrus (R) | 72 | 45 | 18 | 24 | 0.03 |
| Precuneus cortex (R) | 920 | 6 | −63 | 30 | 0.06 |
| Cluster Label | Cluster Extent (Voxels) | x MNI (mm) | y MNI (mm) | z MNI (mm) | Max t-Value | p-Value |
|---|---|---|---|---|---|---|
| ISC Finnish > ISC Russian | ||||||
| Temporal pole (superior, R) | 87 | 54 | 21 | −24 | 6.79 | p < 0.03 |
| Middle occipital gyrus (L) | 468 | −42 | −66 | 3 | 6.81 | p < 0.002 |
| Middle temporal gyrus (R) | 481 | 57 | −63 | 12 | 6.92 | p < 0.002 |
| Middle temporal gyrus (R) | 143 | 51 | −42 | 3 | 5.89 | p < 0.02 |
| Superior temporal gyrus (L) | 217 | −57 | −6 | 0 | 6.32 | p < 0.01 |
| Precuneus cortex (R) | 401 | 18 | −60 | 33 | 7.74 | p < 0.002 |
| ISC Russian > ISC Finnish | ||||||
| Superior temporal gyrus (L) | 210 | −48 | −24 | 6 | −6.25 | p < 0.01 |
| Calcarine cortex (L) | 85 | −9 | −69 | 18 | −5.23 | p < 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakonen, M.; Ikäheimonen, A.; Hultèn, A.; Kauttonen, J.; Koskinen, M.; Lin, F.-H.; Lowe, A.; Sams, M.; Jääskeläinen, I.P. Processing of an Audiobook in the Human Brain Is Shaped by Cultural Family Background. Brain Sci. 2022, 12, 649. https://doi.org/10.3390/brainsci12050649
Hakonen M, Ikäheimonen A, Hultèn A, Kauttonen J, Koskinen M, Lin F-H, Lowe A, Sams M, Jääskeläinen IP. Processing of an Audiobook in the Human Brain Is Shaped by Cultural Family Background. Brain Sciences. 2022; 12(5):649. https://doi.org/10.3390/brainsci12050649
Chicago/Turabian StyleHakonen, Maria, Arsi Ikäheimonen, Annika Hultèn, Janne Kauttonen, Miika Koskinen, Fa-Hsuan Lin, Anastasia Lowe, Mikko Sams, and Iiro P. Jääskeläinen. 2022. "Processing of an Audiobook in the Human Brain Is Shaped by Cultural Family Background" Brain Sciences 12, no. 5: 649. https://doi.org/10.3390/brainsci12050649
APA StyleHakonen, M., Ikäheimonen, A., Hultèn, A., Kauttonen, J., Koskinen, M., Lin, F.-H., Lowe, A., Sams, M., & Jääskeläinen, I. P. (2022). Processing of an Audiobook in the Human Brain Is Shaped by Cultural Family Background. Brain Sciences, 12(5), 649. https://doi.org/10.3390/brainsci12050649






