Prognosis of Guillain–Barré Syndrome Linked to COVID-19 Vaccination
Abstract
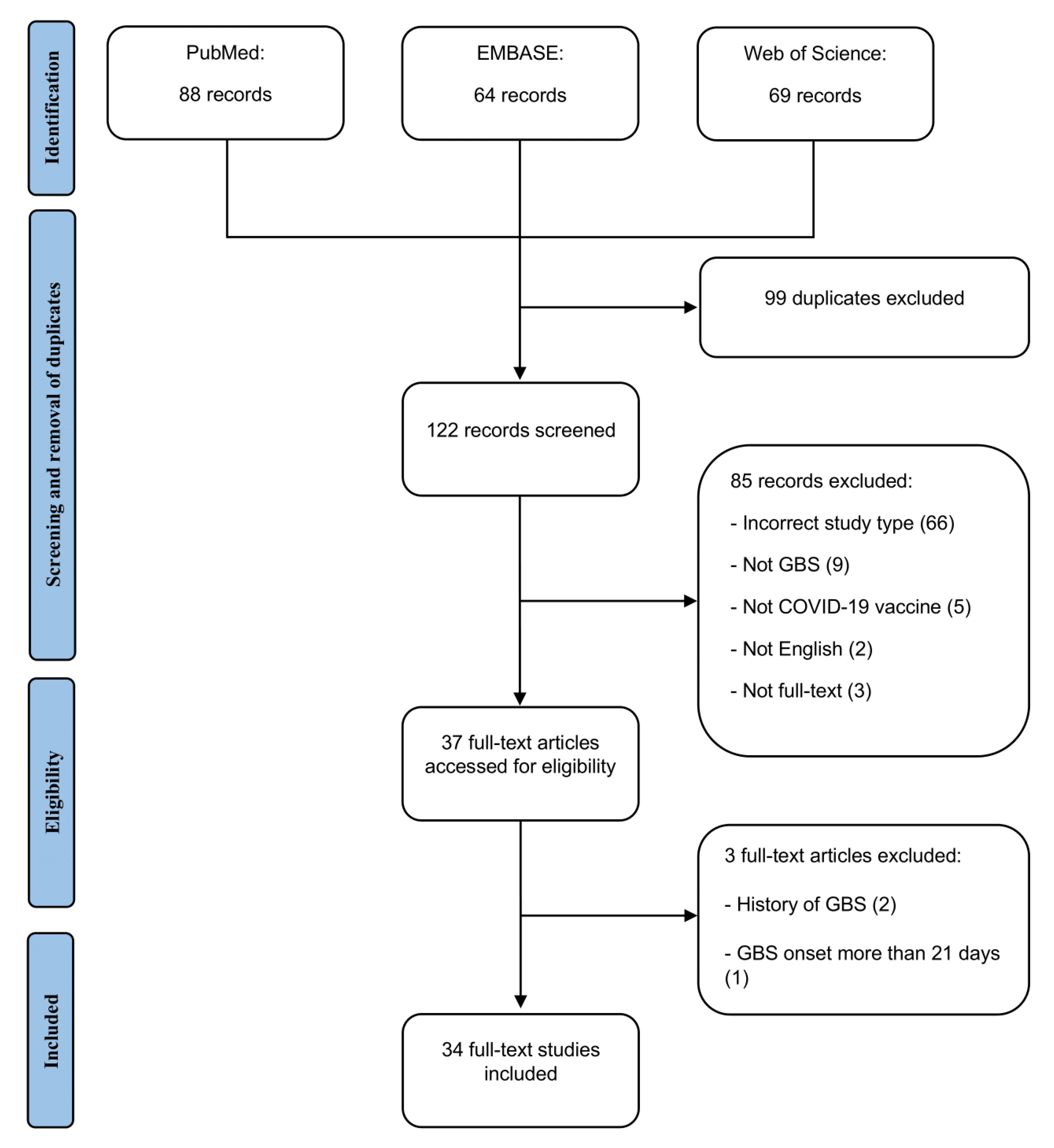
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Included Studies
3.2. Patient Demographics and Outcomes
3.3. Multivariate Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alter, M. The epidemiology of Guillain-Barré syndrome. Ann. Neurol. 1990, 27, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B.C.; van Doorn, P.A. Guillain–Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Asbury, A.K.; Cornblath, D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann. Neurol. 1990, 27, S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Wang, C.H.; Chang, K.C.; Hung, M.J.; Chen, H.Y.; Liao, S.C. Guillain-Barré Syndrome Associated with COVID-19 Vaccination. Emerg. Infect. Dis. 2021, 27, 3175–3178. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.E.; Goddard, K.; Lewis, N.; Fireman, B.; Myers, T.R.; Bakshi, N.; Weintraub, E.; Donahue, J.G.; Nelson, J.C.; Xu, S.; et al. Incidence of Guillain-Barré Syndrome After COVID-19 Vaccination in the Vaccine Safety Datalink. JAMA Netw. Open 2022, 5, e228879. [Google Scholar] [CrossRef] [PubMed]
- Lupica, A.; Di Stefano, V.; Iacono, S.; Pignolo, A.; Quartana, M.; Gagliardo, A.; Fierro, B.; Brighina, F. Impact of COVID-19 in AChR Myasthenia Gravis and the Safety of Vaccines: Data from an Italian Cohort. Neurol. Int. 2022, 14, 406–416. [Google Scholar] [CrossRef]
- Khan, F.; Pallant, J.; Ng, L.; Bhasker, A. Factors associated with long-term functional outcomes and psychological sequelae in Guillain–Barre syndrome. J. Neurol. 2010, 257, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakerley, B.; Uncini, A.; Yuki, N. Guillain–Barré and Miller Fisher syndromes—new diagnostic classification. Nat. Rev. Neurol. 2014, 10, 537–544. [Google Scholar] [CrossRef]
- Fokke, C.; van den Berg, B.; Drenthen, J.; Walgaard, C.; van Doorn, P.; Jacobs, B. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain 2013, 137, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oo, W.; Giri, P.; de Souza, A. AstraZeneca COVID-19 vaccine and Guillain- Barré Syndrome in Tasmania: A causal link? J. Neuroimmunol. 2021, 360, 577719. [Google Scholar] [CrossRef]
- García-Grimshaw, M.; Michel-Chávez, A.; Vera-Zertuche, J.; Galnares-Olalde, J.; Hernández-Vanegas, L.; Figueroa-Cucurachi, M.; Paredes-Ceballos, O.; Reyes-Terán, G.; Carbajal-Sandoval, G.; Ceballos-Liceaga, S.; et al. Guillain-Barré syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin. Immunol. 2021, 230, 108818. [Google Scholar] [CrossRef]
- Hasan, T.; Khan, M.; Khan, F.; Hamza, G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021, 14, e243629. [Google Scholar] [CrossRef] [PubMed]
- McKean, N.; Chircop, C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021, 14, e244125. [Google Scholar] [CrossRef] [PubMed]
- Razok, A.; Shams, A.; Almeer, A.; Zahid, M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann. Med. Surg. 2021, 67, 102540. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Ju, W.; Ha, Y.; Ban, J.; Lee, S.; Sung, J.; Shin, J. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: Report of two cases and review of literature. J. Neuroimmunol. 2021, 359, 577691. [Google Scholar] [CrossRef] [PubMed]
- Scendoni, R.; Petrelli, C.; Scaloni, G.; Logullo, F. Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Hum. Vaccin. Immunother. 2021, 17, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Suri, V.; Pandey, S.; Singh, J.; Jena, A. Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021, 14, e245816. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Bayas, A.; Hindi, F.; Rizvi, Z.; Espinosa, P. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus 2021, 13, e13426. [Google Scholar] [CrossRef] [PubMed]
- Tutar, N.; Eyigürbüz, T.; Yildirim, Z.; Kale, N. A variant of Guillain-Barre syndrome after SARS-CoV-2 vaccination: AMSAN. Ideggyógy. Sz. 2021, 74, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Maramattom, B.; Krishnan, P.; Paul, R.; Vishnu, S.C. Guillain-Barré Syndrome following ChAdOx1-S / nCoV-19 Vaccine. Ann. Neurol. 2021, 90, 312–314. [Google Scholar] [CrossRef]
- Introna, A.; Caputo, F.; Santoro, C.; Guerra, T.; Ucci, M.; Mezzapesa, D.; Trojano, M. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: A causal or casual association? Clin. Neurol. Neurosurg. 2021, 208, 106887. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.; Gheihman, G.; O’Hare, M.; Kosowsky, J. Guillain-Barré Syndrome Presenting as Facial Diplegia after COVID-19 Vaccination: A Case Report. J. Emerg. Med. 2021, 61, e141–e145. [Google Scholar] [CrossRef] [PubMed]
- Nasuelli, N.; De Marchi, F.; Cecchin, M.; De Paoli, I.; Onorato, S.; Pettinaroli, R.; Savoini, G.; Godi, L. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol. Sci. 2021, 42, 4747–4749. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Brunn, J.; Jacobs, J.; Todd, P.; Askari, F.; Fontana, R. Guillain-Barré Syndrome After COVID-19 mRNA Vaccination in a Liver Transplantation Recipient With Favorable Treatment Response. Liver Transpl. 2021, 28, 134–137. [Google Scholar] [CrossRef]
- Prasad, A.; Hurlburt, G.; Podury, S.; Tandon, M.; Kingree, S.; Sriwastava, S. A Novel Case of Bifacial Diplegia Variant of Guillain-Barré Syndrome Following Janssen COVID-19 Vaccination. Neurol. Int. 2021, 13, 404–409. [Google Scholar] [CrossRef]
- Allen, C.; Ramsamy, S.; Tarr, A.; Tighe, P.; Irving, P.; Tanasescu, R.; Evans, J. Guillain–Barré Syndrome Variant Occurring after SARS-CoV-2 Vaccination. Ann. Neurol. 2021, 90, 315–318. [Google Scholar] [CrossRef]
- Ogbebor, O.; Seth, H.; Min, Z.; Bhanot, N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: A temporal occurrence, not a causal association. Idcases 2021, 24, e01143. [Google Scholar] [CrossRef]
- Bonifacio, G.B.; Patel, D.; Cook, S.; Purcaru, E.; Couzins, M.; Domjan, J.; Ryan, S.; Alareed, A.; Tuohy, O.; Slaght, S.; et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J. Neurol. Neurosurg. 2021, 93, 341–342. [Google Scholar] [CrossRef]
- Bax, F.; Gigli, G.L.; Belgrado, E.; Brunelli, L.; Valente, M. Guillain–Barré syndrome following COVID-19 immunization: A report of two cases. Acta Neurol. Belg. 2021, 1–3. [Google Scholar] [CrossRef]
- Matarneh, A.; Al-battah, A.; Farooqui, K.; Ghamoodi, M.; Alhatou, M. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin. Case Rep. 2021, 9, e04756. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, M.; Zoleo, P.; Arabia, G.; Gambardella, A. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol. Sci. 2021, 42, 4401–4402. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Khurana, S.; Murthy, G.; Dawson, E.; Jazebi, N.; Haas, C. A case of Guillain–Barre syndrome following Pfizer COVID-19 vaccine. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 597–600. [Google Scholar] [CrossRef]
- James, J.; Jose, J.; Gafoor, V.; Smita, B.; Balaram, N. Guillain-Barré syndrome following ChAdOx1 nCoV-19 COVID-19 vaccination: A case series. Neurol. Clin. Neurosci. 2021, 9, 402–405. [Google Scholar] [CrossRef]
- Kripalani, Y.; Lakkappan, V.; Parulekar, L.; Shaikh, A.; Singh, R.; Vyas, P. A Rare Case of Guillain-Barré Syndrome following COVID-19 Vaccination. Eur. J. Case Rep. Intern. Med. 2021, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Khurram, R.; Lakhani, A.; Quirk, B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021, 14, e242956. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, Z.; Paulus, A.; Jasti, S.; Bing, X. A Rare Variant of Guillain-Barre Syndrome Following Ad26.COV2.S Vaccination. Cureus 2021, 13, e18153. [Google Scholar] [CrossRef] [PubMed]
- Kanabar, G.; Wilkinson, P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. 2021, 14, e244527. [Google Scholar] [CrossRef]
- Dalwadi, V.; Hancock, D.; Ballout, A.; Geraci, A. Axonal-Variant Guillian-Barre Syndrome Temporally Associated With mRNA-Based Moderna SARS-CoV-2 Vaccine. Cureus 2021, 13, e18291. [Google Scholar] [CrossRef]
- Aomar-Millán, I.; Martínez de Victoria-Carazo, J.; Peregrina-Rivas, J.; Villegas-Rodríguez, I. COVID-19, Guillain-Barré syndrome, and the vaccine. A dangerous combination. Rev. Clin. Esp. 2021, 221, 555–557. [Google Scholar] [CrossRef]
- Jain, E.; Pandav, K.; Regmi, P.; Michel, G.; Altshuler, I. Facial Diplegia: A Rare, Atypical Variant of Guillain-Barré Syndrome and Ad26.COV2.S Vaccine. Cureus 2021, 13, e16612. [Google Scholar] [CrossRef] [PubMed]
- Márquez Loza, A.; Holroyd, K.; Johnson, S.; Pilgrim, D.; Amato, A. Guillain-Barré Syndrome in the Placebo and Active Arms of a COVID-19 Vaccine Clinical Trial. Neurology 2021, 96, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.F.; da Silva, C.F.; Oliveira, R.E.N.d.; Romancini, F.; Mendes, R.M.; Locks, A.; Longo, M.F.M.; Moro, C.H.C.; Longo, A.L.; Braatz, V.L. Guillain–Barré syndrome after coronavirus disease 2019 vaccine: A temporal association. Clin. Exp. Neuroimmunol. 2021, 18, 215–218. [Google Scholar] [CrossRef]
- Lunn, M.P.; Cornblath, D.R.; Jacobs, B.C.; Querol, L.; van Doorn, P.A.; Hughes, R.A.; Willison, H.J. COVID-19 vaccine and Guillain-Barré syndrome: Let’s not leap to associations. Brain 2021, 144, 357–360. [Google Scholar] [CrossRef]
- Woo, E.; Mba-Jonas, A.; Dimova, R.; Alimchandani, M.; Zinderman, C.; Nair, N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February–July 2021. JAMA 2021, 326, 1606. [Google Scholar] [CrossRef] [PubMed]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.P.; Attia, S. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef]
- Klein, S.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Iftikhar, H.; Noor, S.M.U.; Masood, M.; Bashir, K. Bell’s Palsy After 24 Hours of mRNA-1273 SARS-CoV-2 Vaccine. Cureus 2021, 13, e15935. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Chui, C.S.L.; Lai, F.T.T.; Chan, E.W.Y.; Li, X.; Yan, V.K.C.; Gao, L.; Yu, Q.; Lam, I.C.H.; Chun, R.K.C.; et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: A case series and nested case-control study. Lancet Infect. Dis. 2022, 22, 64–72. [Google Scholar] [CrossRef]
- Goodman, J.L.; Grabenstein, J.D.; Braun, M.M. Answering key questions about COVID-19 vaccines. JAMA 2020, 324, 2027–2028. [Google Scholar] [CrossRef]
| Variable | Mean (SD)/No. (%) |
|---|---|
| Demographics | |
| Age (year) | 59.5 (14.8) |
| Age (range) | 25, 90 |
| Male gender | 31 (54) |
| Vaccine Type | |
| Viral Vector | |
| AstraZeneca | 34 (60) |
| Janssen/Johnson & Johnson | 5 (9) |
| mRNA | |
| Pfizer | 15 (26) |
| Moderna | 2 (4) |
| Inactivated (CoronaVac-SinoVac) | 1 (2) |
| Vaccine Dose Number | |
| First dose | 46 (81) |
| Second dose | 8 (14) |
| Not Reported | 3 (6) |
| Duration of onset (days) | |
| Mean | 10.7 (5.2) |
| Median (IQR) | 11 (7, 14) |
| Early-onset (≤7 days) | 17 (30) |
| Late-onset (≥8 days) | 40 (70) |
| Severity of Symptoms | |
| ICU admission | 16 (28) |
| Respiratory failure | 14 (25) |
| Mechanical ventilation | 13 (23) |
| Death | 1 (2) |
| Clinical Classification | |
| Classic GBS | 35 (61) |
| Paraparetic GBS | 9 (16) |
| Facial diplegia | 12 (21) |
| Cervicobrachial weakness | 1 (2) |
| Electrodiagnosis | |
| Not done | 5 (9) |
| AIDP | 37/52 (65) |
| AMAN | 4/52 (8) |
| AMSAN | 8/52 (15) |
| Equivocal | 3/52 (6) |
| CSF | |
| Not done | 5 (9) |
| Normal | 5/52 (10) |
| Albuminocytological dissociation | 47/52 (90) |
| Protein levels (mean, mg/dL) | 211 ± 325 |
| Treatment | |
| IVIG | 49 (86) |
| Plasmapheresis | 1 (2) |
| IVIG and plasmapheresis | 2 (4) |
| Gabapentin | 1 (2) |
| Oral prednisolone | 1 (2) |
| Not done | 3 (5) |
| Treatment Course | |
| Not reported | 1 (2) |
| No improvement | 4 (7) |
| Minimal improvement | 16 (28) |
| Definite improvement | 34 (60) |
| Recovery | 2 (4) |
| GBS Disability Score Post-treatment | |
| NA | 6 (11) |
| 0 | 5/51 (10) |
| 1 | 14/51 (28) |
| 2 | 7/51 (14) |
| 3 | 8/51 (16) |
| 4 | 11/51 (22) |
| 5 | 5/51 (10) |
| 6 | 1/51 (2) |
| Variable | Event vs. Reference * | Vaccine (mRNA vs. Viral Vector) (n = 53) ‡ | Clinical Severity † (n = 54) § | GBS Disability Score (n = 48) || | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | β (95% CI) | p Value | ||
| Sex | Male vs. Female | 0.89 (0.19, 4.12) | 0.879 | 0.26 (0.07, 0.91) | 0.035 | −1.41 (−2.27, −0.55) | 0.002 |
| Dose number | 2 vs. 1 | 43 (1.43, >999) | 0.030 | 0.08 (0.003, 1.86) | 0.115 | −1.11 (−2.31, 0.09) | 0.070 |
| Onset | Early vs Late | 11.1 (2.35, 52.1) | 0.0020 | 1.41 (0.35, 5.70) | 0.629 | 0.17 (−0.80. 1.14) | 0.727 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, S.K.K.; Soh, Q.Y.; Saffari, S.E.; Tan, E.-K. Prognosis of Guillain–Barré Syndrome Linked to COVID-19 Vaccination. Brain Sci. 2022, 12, 711. https://doi.org/10.3390/brainsci12060711
Chua SKK, Soh QY, Saffari SE, Tan E-K. Prognosis of Guillain–Barré Syndrome Linked to COVID-19 Vaccination. Brain Sciences. 2022; 12(6):711. https://doi.org/10.3390/brainsci12060711
Chicago/Turabian StyleChua, Shaun Kai Kiat, Qian Ying Soh, Seyed Ehsan Saffari, and Eng-King Tan. 2022. "Prognosis of Guillain–Barré Syndrome Linked to COVID-19 Vaccination" Brain Sciences 12, no. 6: 711. https://doi.org/10.3390/brainsci12060711
APA StyleChua, S. K. K., Soh, Q. Y., Saffari, S. E., & Tan, E.-K. (2022). Prognosis of Guillain–Barré Syndrome Linked to COVID-19 Vaccination. Brain Sciences, 12(6), 711. https://doi.org/10.3390/brainsci12060711






