Omitting Hyperventilation in Electroencephalogram during the COVID-19 Pandemic May Reduce Interictal Epileptiform Discharges in Patients with Juvenile Myoclonic Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
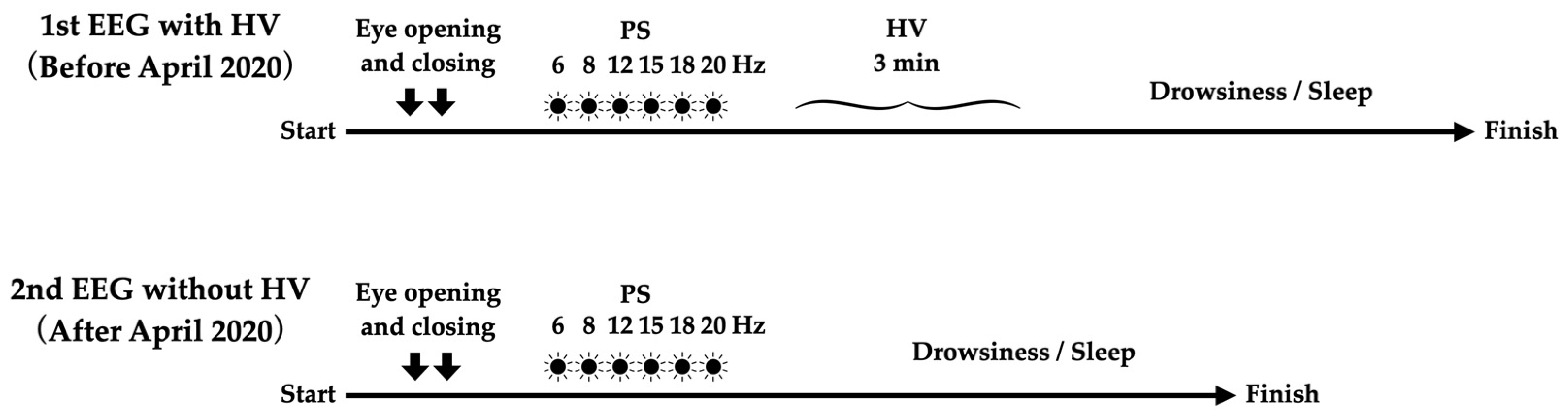
2.1. Participants and Study Design
2.2. EEG Measurement
2.3. Outome Measurement
2.4. Statistical Analysis
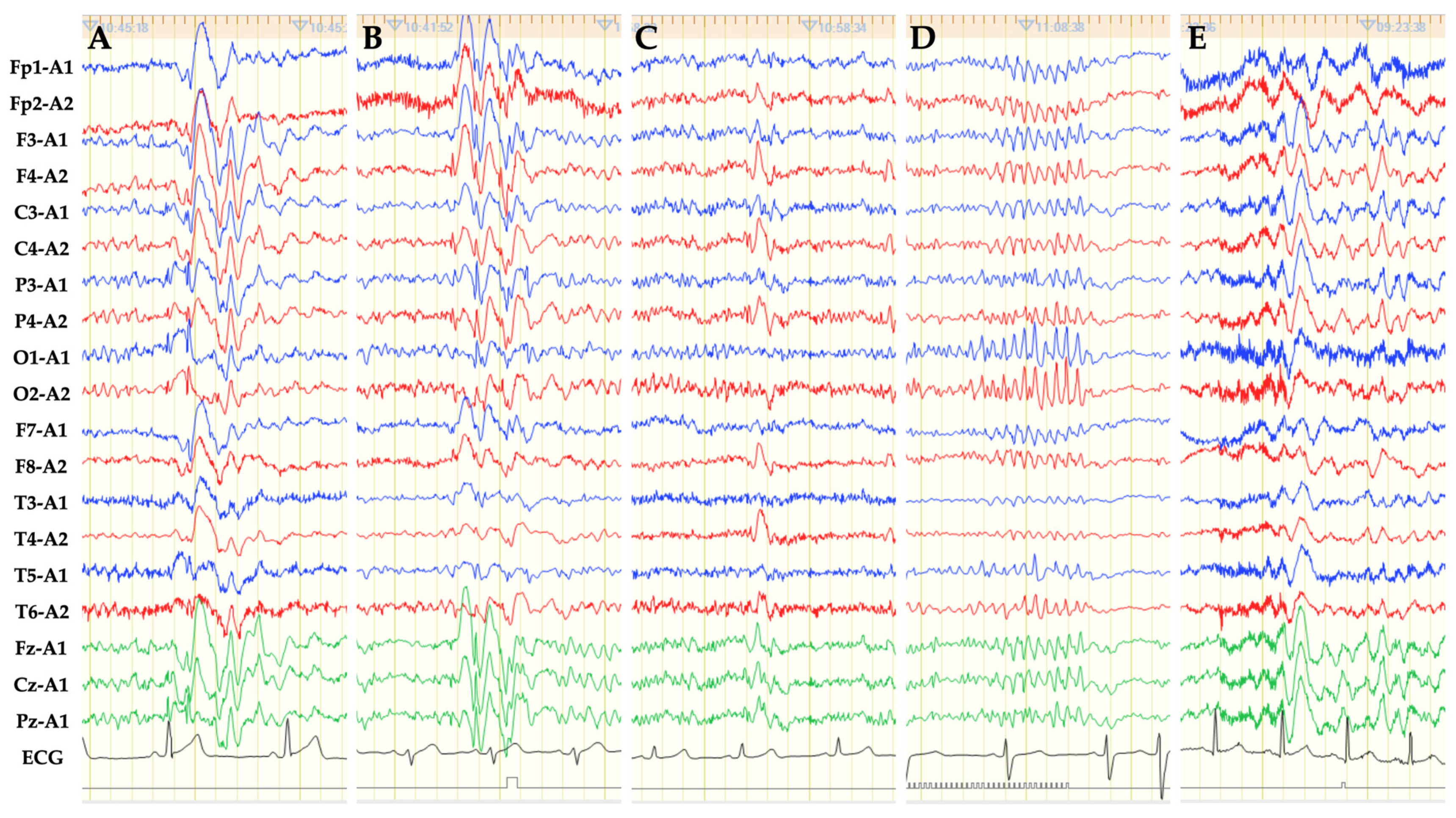
3. Results
3.1. Patient Characteristics
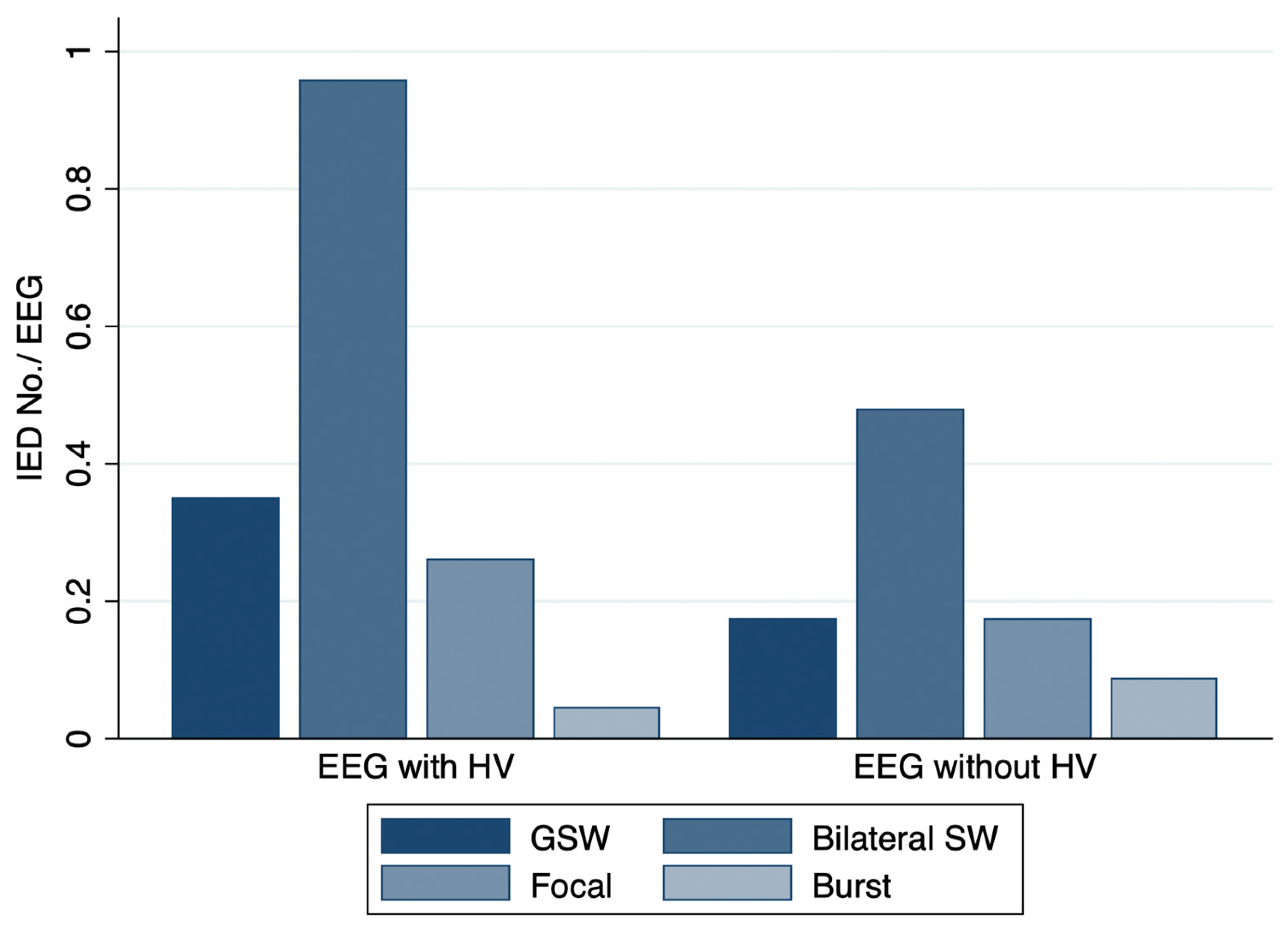
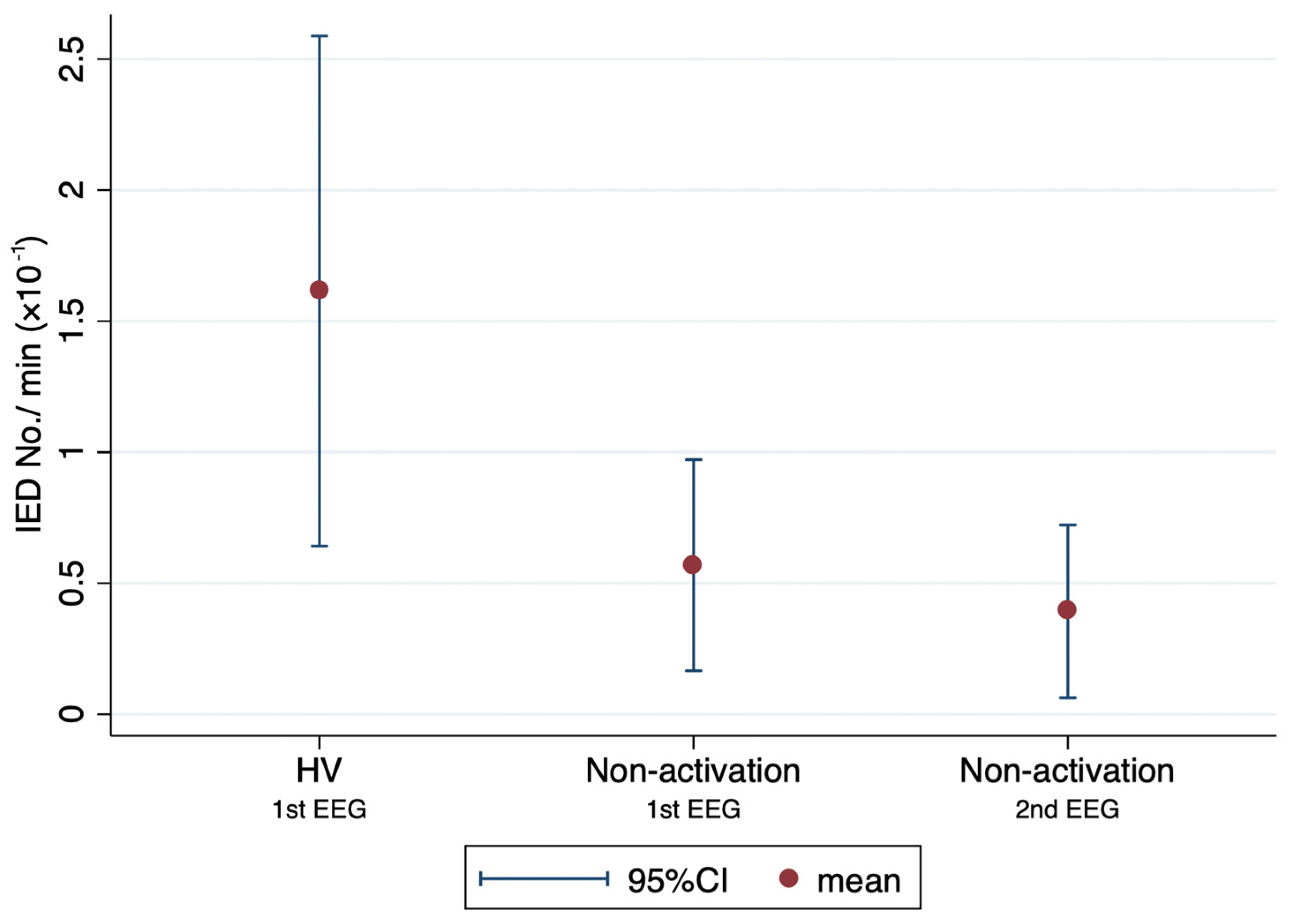
3.2. Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krysl, D.; Beniczky, S.; Franceschetti, S.; Arzimanoglou, A. The COVID-19 outbreak and approaches to performing EEG in Europe. Epileptic Disord. 2020, 22, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Assenza, G.; Lanzone, J.; Ricci, L.; Boscarino, M.; Tombini, M.; Galimberti, C.A.; Alvisi, L.; Tassi, L.; Broglia, L.; Di Lazzaro, V.; et al. Electroencephalography at the time of COVID-19 pandemic in Italy. Neurol. Sci. 2020, 41, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, D.; Jimenez, C.R.; Camilli, C.X.; de la Cruz Reyes, L.A.; Galindo, E.G.A.; Burbano, G.E.R.; Penela, M.M.; Perassolo, M.B.; Valdez, A.T.; Godoy, J.G.; et al. Guidance for clinical neurophysiology examination throughout the COVID-19 pandemic. Latin American chapter of the IFCN task force—COVID-19. Clin. Neurophysiol. 2020, 131, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Rios-Pohl, L.; Franco, M.; Gonzalez, M. Hyperventilation maneuver during EEG in children with epilepsy after the COVID-19 pandemic. Is a routine procedure necessary? Epilepsia Open 2021, 6, 437–442. [Google Scholar] [CrossRef]
- Guaranha, M.S.; Garzon, E.; Buchpiguel, C.A.; Tazima, S.; Yacubian, E.M.; Sakamoto, A.C. Hyperventilation revisited: Physiological effects and efficacy on focal seizure activation in the era of video-EEG monitoring. Epilepsia 2005, 46, 69–75. [Google Scholar] [CrossRef]
- Blume, W.T.; David, R.B.; Gomez, M.R. Generalized sharp and slow wave complexes. Associated clinical features and long-term follow-up. Brain 1973, 96, 289–306. [Google Scholar] [CrossRef]
- Markand, O.N. Slow spike-wave activity in EEG and associated clinical features: Often called ‘Lennox’ or ‘Lennox-Gastaut’ syndrome. Neurology 1977, 27, 746–757. [Google Scholar] [CrossRef]
- Miley, C.E.; Forster, F.M. Activation of partial complex seizures by hyperventilation. Arch. Neurol. 1977, 34, 371–373. [Google Scholar] [CrossRef]
- Holmes, M.D.; Dewaraja, A.S.; Vanhatalo, S. Does hyperventilation elicit epileptic seizures? Epilepsia 2004, 45, 618–620. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Hashemzehi, Z.; Emami, M. Epidemiology and clinical manifestations of juvenile myoclonic epilepsy (JME) in Iran. Neurol. Sci. 2015, 36, 713–716. [Google Scholar] [CrossRef]
- Camfield, C.S.; Striano, P.; Camfield, P.R. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013, 28 (Suppl. S1), S15–S17. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Ichikawa, N.; Sato, K.; Nishimura, M.; Enoki, H.; Okanishi, T. Inclusion of general physicians in the multidisciplinary treatment team for epilepsy may lead to an economic benefit. Epilepsy Behav. 2019, 95, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Petersen, K.A. Juvenile myoclonic epilepsy: Clinical and EEG features. Acta Neurol. Scand. 1998, 97, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef]
- Beniczky, S.; Aurlien, H.; Brogger, J.C.; Hirsch, L.J.; Schomer, D.L.; Trinka, E.; Pressler, R.M.; Wennberg, R.; Visser, G.H.; Eisermann, M.; et al. Standardized computer-based organized reporting of EEG: SCORE—Second version. Clin. Neurophysiol. 2017, 128, 2334–2346. [Google Scholar] [CrossRef]
- Panayiotopoulos, C.P.; Obeid, T.; Tahan, A.R. Juvenile myoclonic epilepsy: A 5-year prospective study. Epilepsia 1994, 35, 285–296. [Google Scholar] [CrossRef]
- Shahnaz; Sher, K.; Abdul Sattar, R. Clinical and EEG characteristics of juvenile myoclonic epilepsy. Pak. J. Med. Sci. 2014, 30, 12–15. [Google Scholar] [CrossRef]
- Montalenti, E.; Imperiale, D.; Rovera, A.; Bergamasco, B.; Benna, P. Clinical features, EEG findings and diagnostic pitfalls in juvenile myoclonic epilepsy: A series of 63 patients. J. Neurol. Sci. 2001, 184, 65–70. [Google Scholar] [CrossRef]
- Arntsen, V.; Sand, T.; Syvertsen, M.R.; Brodtkorb, E. Prolonged epileptiform EEG runs are associated with persistent seizures in juvenile myoclonic epilepsy. Epilepsy Res. 2017, 134, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sadleir, L.G.; Farrell, K.; Smith, S.; Connolly, M.B.; Scheffer, I.E. Electroclinical features of absence seizures in childhood absence epilepsy. Neurology 2006, 67, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, U.; Cook, M.; D’Souza, W. The electroencephalogram of idiopathic generalized epilepsy. Epilepsia 2012, 53, 234–248. [Google Scholar] [CrossRef]
- Granados, J.; Gillum, T.L.; Castillo, W.; Christmas, K.M.; Kuennen, M.R. “Functional” respiratory muscle training during endurance exercise causes modest hypoxemia but overall is well tolerated. J. Strength Cond. Res. 2016, 30, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Cao, D.; Li, S.; Wang, R.; Wang, Y.; Lin, H. Effects of hyperventilation with face mask on brain network in patients with epilepsy. Epilepsy Res. 2021, 176, 106741. [Google Scholar] [CrossRef]
| EEG with HV (n = 23) | EEG without HV (n = 23) | p Value | |
|---|---|---|---|
| Age at EEG, years * | 29.8 ± 13.6 | 31.1 ± 13.5 | 0.75 |
| Seizure-free > 2 years, % | 91.3% | 91.3% | 1 |
| Time in EEG, min * | 21.4 ± 2.9 | 16.7 ± 3.0 | <0.0001 |
| Drowsiness, % | 87.0% | 91.3% | 1 |
| Sleep, % | 60.9% | 60.9% | 1 |
| EEG with HV | EEG without HV | p Value | |
|---|---|---|---|
| IED-yes, % (n) | 65.2% (15/23) | 34.8% (8/23) | 0.016 |
| during PS, % (n) | 21.7% (5/23) | 17.4% (4/23) | 1 |
| during HV, % (n) | 39.1% (9/23) | - | |
| during drowsiness, % (n) | 10.0% (2/20) | 9.5% (2/21) | 1 |
| during sleep, % (n) | 6.7% (1/15) | 0% (0/14) | 1 |
| Number of IEDs/EEG * | 1.6 ± 1.8 | 0.9 ± 1.5 | 0.073 |
| Number of IED/min × 10−1 * | 7.9 ± 9.2 | 5.5 ± 9.5 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatano, K.; Fujimoto, A.; Sato, K.; Yamamoto, T.; Enoki, H. Omitting Hyperventilation in Electroencephalogram during the COVID-19 Pandemic May Reduce Interictal Epileptiform Discharges in Patients with Juvenile Myoclonic Epilepsy. Brain Sci. 2022, 12, 769. https://doi.org/10.3390/brainsci12060769
Hatano K, Fujimoto A, Sato K, Yamamoto T, Enoki H. Omitting Hyperventilation in Electroencephalogram during the COVID-19 Pandemic May Reduce Interictal Epileptiform Discharges in Patients with Juvenile Myoclonic Epilepsy. Brain Sciences. 2022; 12(6):769. https://doi.org/10.3390/brainsci12060769
Chicago/Turabian StyleHatano, Keisuke, Ayataka Fujimoto, Keishiro Sato, Takamichi Yamamoto, and Hideo Enoki. 2022. "Omitting Hyperventilation in Electroencephalogram during the COVID-19 Pandemic May Reduce Interictal Epileptiform Discharges in Patients with Juvenile Myoclonic Epilepsy" Brain Sciences 12, no. 6: 769. https://doi.org/10.3390/brainsci12060769
APA StyleHatano, K., Fujimoto, A., Sato, K., Yamamoto, T., & Enoki, H. (2022). Omitting Hyperventilation in Electroencephalogram during the COVID-19 Pandemic May Reduce Interictal Epileptiform Discharges in Patients with Juvenile Myoclonic Epilepsy. Brain Sciences, 12(6), 769. https://doi.org/10.3390/brainsci12060769







