Selective Probiotic Treatment Positively Modulates the Microbiota–Gut–Brain Axis in the BTBR Mouse Model of Autism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Probiotic Administration
2.3. Behavioural Assessments
2.4. Fecal Microbial Analysis
2.5. Cytokine Analysis
2.6. Metabolic Assessment
2.7. Mitochondrial Energetics
2.8. Statistical Analysis
3. Results
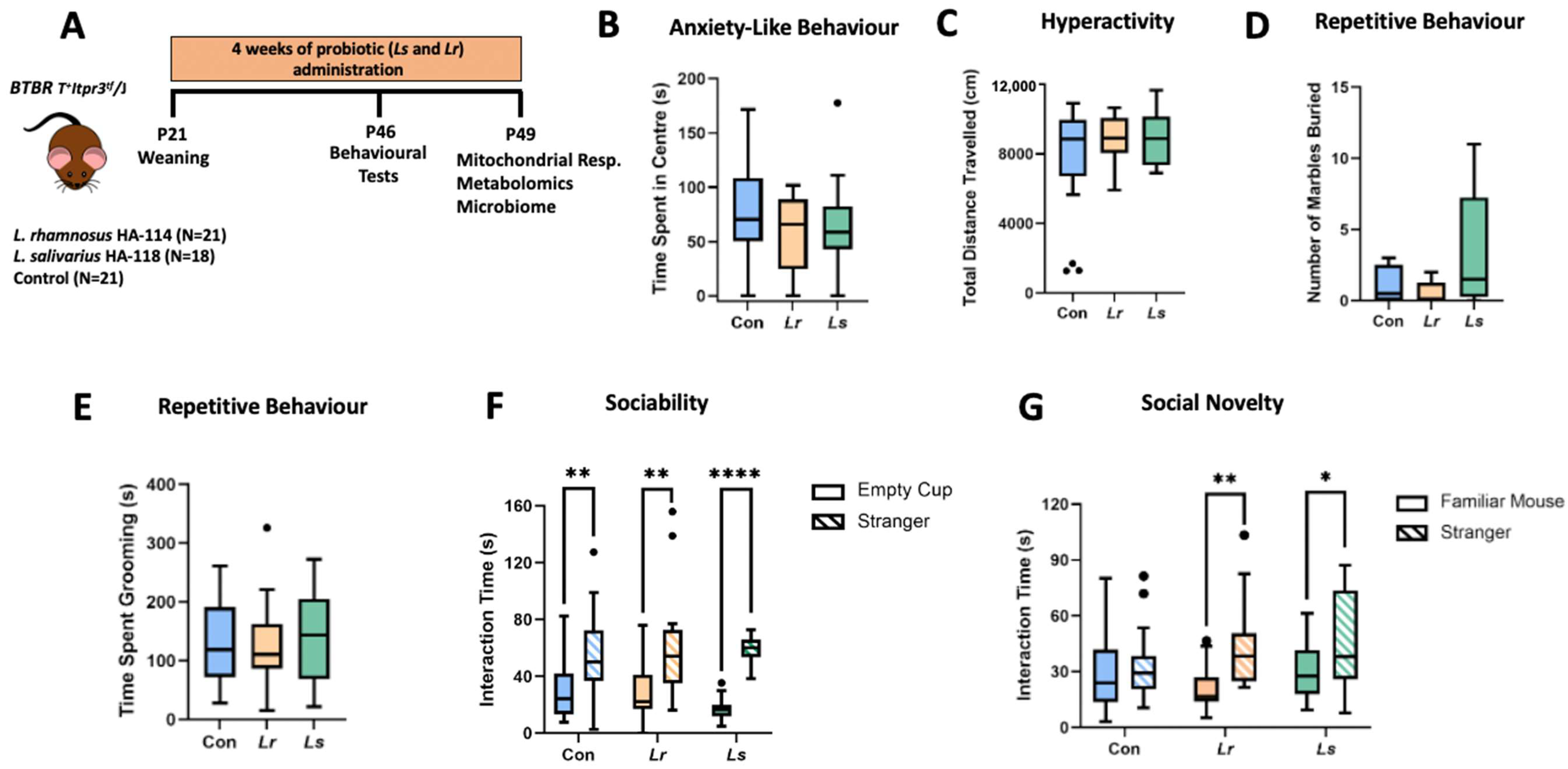
3.1. Effects of Probiotics on ASD-Related Behaviours
3.2. Effects of Probiotics on Fecal Microbiota Composition and Function
3.3. Cytokine Profiles
3.4. Blood and Serum Metabolic Alterations following Probiotic Treatment
3.5. Brain Metabolomic Responses to Probiotic Treatment
3.6. Brain Mitochondrial Respiration in Response to Probiotics
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIMH Autism Spectrum Disorder. Available online: https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd/index.shtml (accessed on 21 September 2020).
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Autistic Spectrum Disorders: A Review of Clinical Features, Theories and Diagnosis. Int. J. Dev. Neurosci. 2015, 43, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. A Review of Research Trends in Physiological Abnormalities in Autism Spectrum Disorders: Immune Dysregulation, Inflammation, Oxidative Stress, Mitochondrial Dysfunction and Environmental Toxicant Exposures. Mol. Psychiatry 2011, 17, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, C.; Corley, M.J.; Lee, R.W.Y.; Wong, M.; Pang, A.; Arakaki, G.; Miyamoto, R.; Rho, J.M.; Mickiewicz, B.; Dowlatabadi, R.; et al. Metabolic Framework for the Improvement of Autism Spectrum Disorders by a Modified Ketogenic Diet: A Pilot Study. J. Proteome Res. 2020, 19, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M. Autism Medical Comorbidities. World J. Clin. Pediatrics 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal Flora and Gastrointestinal Status in Children with Autism-Comparisons to Typical Children and Correlation with Autism Severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, C. Intestinal Permeability. In Textbook of Natural Medicine; Churchill Livingstone: London, UK, 2020; pp. 166–177.e4. [Google Scholar] [CrossRef]
- Saffouri, G.B.; Shields-Cutler, R.R.; Chen, J.; Yang, Y.; Lekatz, H.R.; Hale, V.L.; Cho, J.M.; Battaglioli, E.J.; Bhattarai, Y.; Thompson, K.J.; et al. Small Intestinal Microbial Dysbiosis Underlies Symptoms Associated with Functional Gastrointestinal Disorders. Nat. Commun. 2019, 10, 2012. [Google Scholar] [CrossRef] [Green Version]
- Fattorusso, A.; di Genova, L.; Dell’isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [Green Version]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.A.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-Related Dietary Preferences Mediate Autism-Gut Microbiome Associations. Cell 2021, 184, 5916–5931.e17. [Google Scholar] [CrossRef]
- Tochitani, S.; Ikeno, T.; Ito, T.; Sakurai, A.; Yamauchi, T.; Matsuzaki, H. Administration of Non-Absorbable Antibiotics to Pregnant Mice to Perturb the Maternal Gut Microbiota Is Associated with Alterations in Offspring Behavior. PLoS ONE 2016, 11, e0138293. [Google Scholar] [CrossRef]
- Connolly, N.; Anixt, J.; Manning, P.; Ping-I Lin, D.; Marsolo, K.A.; Bowers, K. Maternal Metabolic Risk Factors for Autism Spectrum Disorder—An Analysis of Electronic Medical Records and Linked Birth Data. Autism Res. 2016, 9, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verna, E.C.; Lucak, S. Use of Probiotics in Gastrointestinal Disorders: What to Recommend? Ther. Adv. Gastroenterol. 2010, 3, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Trudeau, M.S.; Madden, R.F.; Parnell, J.A.; Gibbard, W.B.; Shearer, J. Dietary and Supplement-Based Complementary and Alternative Medicine Use in Pediatric Autism Spectrum Disorder. Nutrients 2019, 11, 1783. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A Human-Origin Probiotic Cocktail Ameliorates Aging-Related Leaky Gut and Inflammation via Modulating the Microbiota/Taurine/Tight Junction Axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Macfabe, D.F. Short-Chain Fatty Acid Fermentation Products of the Gut Microbiome: Implications in Autism Spectrum Disorders. Microb. Ecol. Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef]
- Laviola, G.; Macrì, S.; Morley-Fletcher, S.; Adriani, W. Risk-Taking Behavior in Adolescent Mice: Psychobiological Determinants and Early Epigenetic Influence. Neurosci. Biobehav. Rev. 2003, 27, 19–31. [Google Scholar] [CrossRef]
- Martin, L.; Sample, H.; Gregg, M.; Wood, C. Validation of Operant Social Motivation Paradigms Using BTBR T+tf/J and C57BL/6J Inbred Mouse Strains. Brain Behav. 2014, 4, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.; Bailey, K. Anxiety-Related Behaviors in Mice. In Models of Behavior Analysis in Neuroscience; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2008; pp. 77–101. ISBN 9781420052343. [Google Scholar]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Cheng, N.; Shearer, J.; Borgland, S.L.; Rho, J.M.; Reimer, R.A. Prebiotic, Probiotic, and Synbiotic Consumption Alter Behavioral Variables and Intestinal Permeability and Microbiota in BTBR Mice. Microorganisms 2021, 9, 1833. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble Burying and Nestlet Shredding as Tests of Repetitive, Compulsive-like Behaviors in Mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Perez, A.; Barbaro, R.P.; Johns, J.M.; Magnuson, T.R.; Piven, J.; Crawley, J.N. Sociability and Preference for Social Novelty in Five Inbred Strains: An Approach to Assess Autistic-like Behavior in Mice. Genes Brain Behav. 2004, 3, 287–302. [Google Scholar] [CrossRef]
- Illumina 16s Metagenomic Sequencing Library Preparation Guide. Available online: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 13 April 2022).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017, 1–15. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Mu, C.; Choudhary, A.; Mayengbam, S.; Barrett, K.T.; Rho, J.M.; Shearer, J.; Scantlebury, M.H. Seizure Modulation by the Gut Microbiota and Tryptophan-Kynurenine Metabolism in an Animal Model of Infantile Spasms. eBioMedicine 2022, 76, 103833. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Lee, L.C.; Liong, C.Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Halouska, S.; Powers, R. Utilities for Quantifying Separation in PCA/PLS-DA Scores Plots. Anal. Biochem. 2013, 433, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for Significance Testing of PLS and OPLS® Models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Herbst, E.A.F.; Holloway, G.P. Permeabilization of Brain Tissue in Situ Enables Multiregion Analysis of Mitochondrial Function in a Single Mouse Brain. J. Physiol. 2015, 593, 787–801. [Google Scholar] [CrossRef] [Green Version]
- Mu, C.; Tompkins, T.A.; Rho, J.M.; Scantlebury, M.H.; Shearer, J. Gut-Based Manipulations Spur Hippocampal Mitochondrial Bioenergetics in a Model of Pediatric Epilepsy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2022, 1868, 166446. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting Outliers When Fitting Data with Nonlinear Regression—A New Method Based on Robust Nonlinear Regression and the False Discovery Rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Chao, O.Y.; Yunger, R.; Yang, Y.M. Behavioral Assessments of BTBR T+Itpr3tf/J Mice by Tests of Object Attention and Elevated Open Platform: Implications for an Animal Model of Psychiatric Comorbidity in Autism. Behav. Brain Res. 2018, 347, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated Three-Chambered Social Approach Task for Mice. Curr. Protoc. Neurosci. 2011, 56, 8.26.1–8.26.16. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, J.; McMurdie, P.J.; Dethlefsen, L.; Relman, D.A.; Holmes, S. Comparisons of Distance Methods for Combining Covariates and Abundances in Microbiome Studies. Pac. Symp. Biocomput. 2012, 2012, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The Good, the Bad, and the Ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, B.; McVeigh, C.; Bendriss, G.; Chaari, A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef]
- Hashemi, A.; Villa, C.R.; Comelli, E.M. Probiotics in Early Life: A Preventative and Treatment Approach. Food Funct. 2016, 7, 1752–1768. [Google Scholar] [CrossRef]
- McTighe, S.M.; Neal, S.J.; Lin, Q.; Hughes, Z.A.; Smith, D.G. The BTBR Mouse Model of Autism Spectrum Disorders Has Learning and Attentional Impairments and Alterations in Acetylcholine and Kynurenic Acid in Prefrontal Cortex. PLoS ONE 2013, 8, e62189. [Google Scholar] [CrossRef]
- Filippello, P.; Flavia, M.; Sorrenti, L.; Marino, F.; Chilà, P. Attachment and Social Behavior in Children’s Autistic Disorders. Life Span. Disabil. 2015, 1, 101–118. [Google Scholar]
- Dissanayake, C.; Crossley, S.A. Proximity and Sociable Behaviours in Autism: Evidence for Attachment. J. Child Psychol. Psychiatry 1996, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.A.M.V.; Kay, S.; Bienenstock, J. Mouse Strain Affects Behavioral and Neuroendocrine Stress Responses Following Administration of Probiotic Lactobacillus Rhamnosus JB-1 or Traditional Antidepressant Fluoxetine. Front. Neurosci. 2018, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral Treatment with Lactobacillus Rhamnosus Attenuates Behavioural Deficits and Immune Changes in Chronic Social Stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Hemarajata, P.; Versalovic, J. Effects of Probiotics on Gut Microbiota: Mechanisms of Intestinal Immunomodulation and Neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing Study of Fecal Microflora of Autistic and Control Children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Joyce, S.A.; Moloney, G.; Burokas, A.; Sherwin, E.; Arboleya, S.; Flynn, I.; Khochanskiy, D.; Moya-Pérez, A.; Peterson, V.; et al. Microbiota-Related Changes in Bile Acid & Tryptophan Metabolism Are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine 2017, 24, 166. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Liang, J.; Dai, M.; Wang, J.; Luo, J.; Zhang, Z.; Jing, J. Altered Gut Microbiota in Chinese Children with Autism Spectrum Disorders. Front. Cell Infect. Microbiol. 2019, 9, 40. [Google Scholar] [CrossRef]
- Kang, D.W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in Fecal Microbial Metabolites and Microbiota of Children with Autism Spectrum Disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Bhattarai, Y. Microbiota-Gut-Brain Axis: Interaction of Gut Microbes and Their Metabolites with Host Epithelial Barriers. J. Neurogastroenterol. Motil. 2018, 30, e13366. [Google Scholar] [CrossRef]
- Goyal, A.; Wang, T.; Dubinkina, V.; Maslov, S. Ecology-Guided Prediction of Cross-Feeding Interactions in the Human Gut Microbiome. Nat. Commun. 2021, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Beller, A.; Kruglov, A.; Durek, P.; von Goetze, V.; Werner, K.; Heinz, G.A.; Ninnemann, J.; Lehmann, K.; Maier, R.; Hoffmann, U.; et al. Specific Microbiota Enhances Intestinal IgA Levels by Inducing TGF-β in T Follicular Helper Cells of Peyer’s Patches in Mice. Eur. J. Immunol. 2020, 50, 783–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, J.L.; Ley, R.E. The Human Gut Bacteria Christensenellaceae Are Widespread, Heritable, and Associated with Health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Schultz, S.; Brigida, A.L.; Antonucci, N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, H.E.; Guo, Y.; Coleman, K.; Ousley, O.; Miller, A.H. Association of IL-12p70 and IL-6:IL-10 Ratio with Autism-Related Behaviors in 22q11.2 Deletion Syndrome: A Preliminary Report. Brain Behav. Immun. 2013, 31, 76. [Google Scholar] [CrossRef] [Green Version]
- Moradi, K.; Ashraf-Ganjouei, A.; Tavolinejad, H.; Bagheri, S.; Akhondzadeh, S. The Interplay between Gut Microbiota and Autism Spectrum Disorders: A Focus on Immunological Pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110091. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the Immune Response in the Brain: IL-10 and Its Regulation. J. Neuroinflamm. 2016, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Saghazadeh, A.; Ataeinia, B.; Keynejad, K.; Abdolalizadeh, A.; Hirbod-Mobarakeh, A.; Rezaei, N. Anti-Inflammatory Cytokines in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Cytokine 2019, 123, 154740. [Google Scholar] [CrossRef]
- Ye, J.; Wu, W.; Li, Y.; Li, L. Influences of the Gut Microbiota on DNA Methylation and Histone Modification. Dig. Dis. Sci. 2017, 62, 5. [Google Scholar] [CrossRef]
- Alvarez-Curto, E.; Milligan, G. Metabolism Meets Immunity: The Role of Free Fatty Acid Receptors in the Immune System. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F.; et al. Butyrate Enhances Mitochondrial Function during Oxidative Stress in Cell Lines from Boys with Autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Bennuri, S.C.; Murray, K.F.; Buie, T.; Winter, H.; Frye, R.E. Mitochondrial Dysfunction in the Gastrointestinal Mucosa of Children with Autism: A Blinded Case-Control Study. PLoS ONE 2017, 12, e0186377. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic Perturbance in Autism Spectrum Disorders: A Metabolomics Study. J. Proteome Res. 2012, 11, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.L.; Aman, M.G.; Arnold, L.E. Neurochemical Correlates of Autistic Disorder: A Review of the Literature. Res. Dev. Disabil. 2006, 27, 254–289. [Google Scholar] [CrossRef] [Green Version]
- Langley, E.A.; Krykbaeva, M.; Blusztajn, J.K.; Mellott, T.J. High Maternal Choline Consumption during Pregnancy and Nursing Alleviates Deficits in Social Interaction and Improves Anxiety-like Behaviors in the BTBR T + Itpr3tf/J Mouse Model of Autism. Behav. Brain Res. 2015, 278, 210–220. [Google Scholar] [CrossRef]
- Eissa, N.; Jayaprakash, P.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ Tf/J Mouse Model of Autism. Biomolecules 2020, 10, 1251. [Google Scholar] [CrossRef]
- Rae, C.D. A Guide to the Metabolic Pathways and Function of Metabolites Observed in Human Brain 1H Magnetic Resonance Spectra. Neuroch. Res. 2014, 39, 1–36. [Google Scholar] [CrossRef]
- Semba, R.D. Perspective: The Potential Role of Circulating Lysophosphatidylcholine in Neuroprotection against Alzheimer Disease. Adv. Nutr. 2020, 11, 760–772. [Google Scholar] [CrossRef]
- Doliba, N.M.; Babsky, A.M.; Doliba, N.M.; Wehrli, S.L.; Osbakken, M.D. AMP Promotes Oxygen Consumption and ATP Synthesis in Heart Mitochondria through the Adenylate Kinase Reaction: An NMR Spectroscopy and Polarography Study. Cell Biochem. Funct. 2015, 33, 67–72. [Google Scholar] [CrossRef]
- Farinella, Z.; Fiumara, A.; Bianciardi, P.; Guarcello, V.; Rizza, V. Effects of Carnitine and Acetylcarnitine Isomers on Mitochondrial Respiration. Int. J. Tissue React. 1984, 6, 141–143. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pochakom, A.; Mu, C.; Rho, J.M.; Tompkins, T.A.; Mayengbam, S.; Shearer, J. Selective Probiotic Treatment Positively Modulates the Microbiota–Gut–Brain Axis in the BTBR Mouse Model of Autism. Brain Sci. 2022, 12, 781. https://doi.org/10.3390/brainsci12060781
Pochakom A, Mu C, Rho JM, Tompkins TA, Mayengbam S, Shearer J. Selective Probiotic Treatment Positively Modulates the Microbiota–Gut–Brain Axis in the BTBR Mouse Model of Autism. Brain Sciences. 2022; 12(6):781. https://doi.org/10.3390/brainsci12060781
Chicago/Turabian StylePochakom, Angela, Chunlong Mu, Jong M. Rho, Thomas A. Tompkins, Shyamchand Mayengbam, and Jane Shearer. 2022. "Selective Probiotic Treatment Positively Modulates the Microbiota–Gut–Brain Axis in the BTBR Mouse Model of Autism" Brain Sciences 12, no. 6: 781. https://doi.org/10.3390/brainsci12060781
APA StylePochakom, A., Mu, C., Rho, J. M., Tompkins, T. A., Mayengbam, S., & Shearer, J. (2022). Selective Probiotic Treatment Positively Modulates the Microbiota–Gut–Brain Axis in the BTBR Mouse Model of Autism. Brain Sciences, 12(6), 781. https://doi.org/10.3390/brainsci12060781








