Topologic Efficiency Abnormalities of the Connectome in Asymptomatic Patients with Leukoaraiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Acquisition
2.3. DKI Analysis
2.4. Topological Parameters
2.5. TBSS
2.6. Statistical Analysis
2.7. Reproducibility Analysis
3. Results
3.1. Demographic Data
3.2. Overall Topology of WM Networks
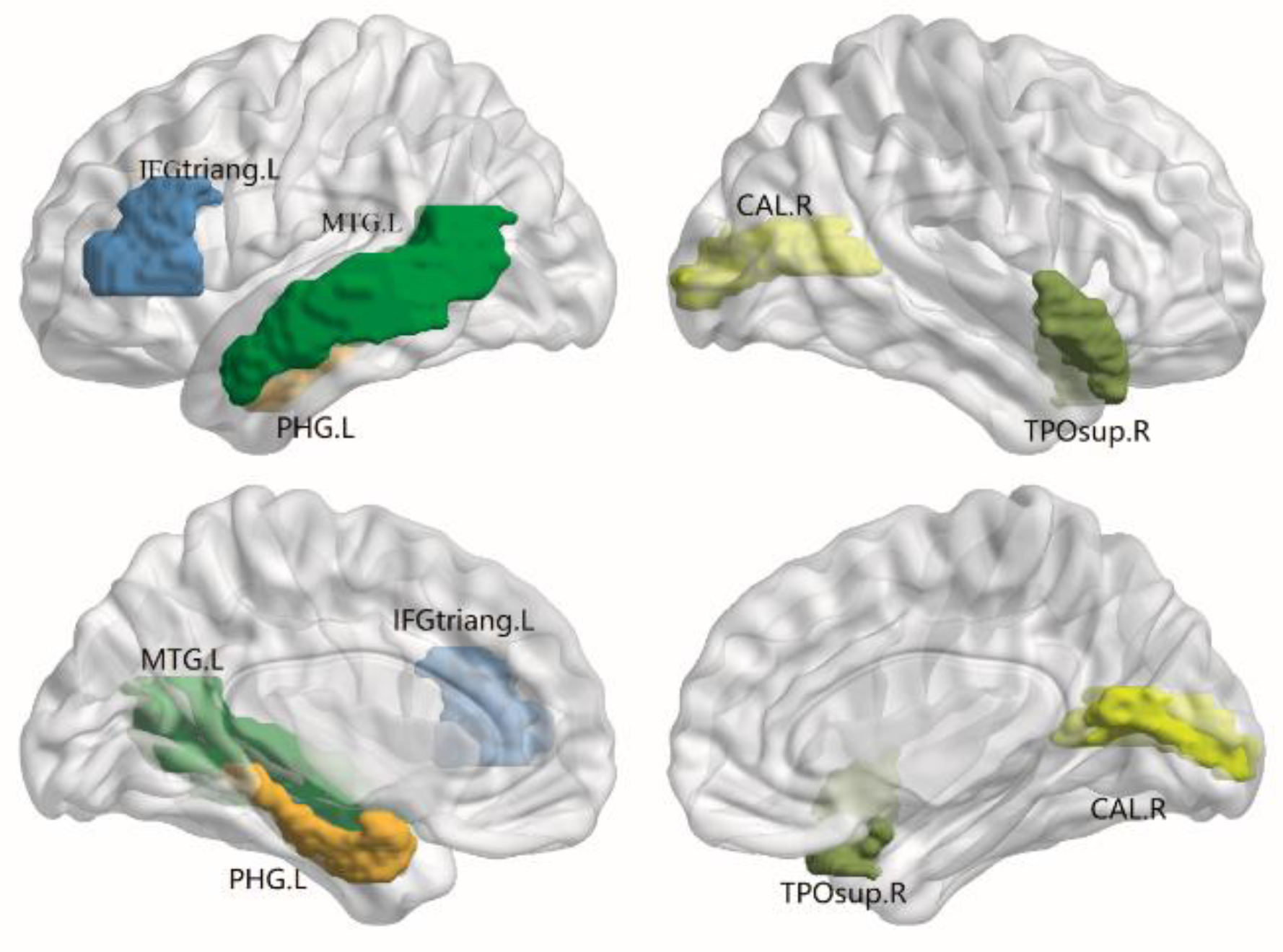
3.3. Alterations in Nodal Efficiency
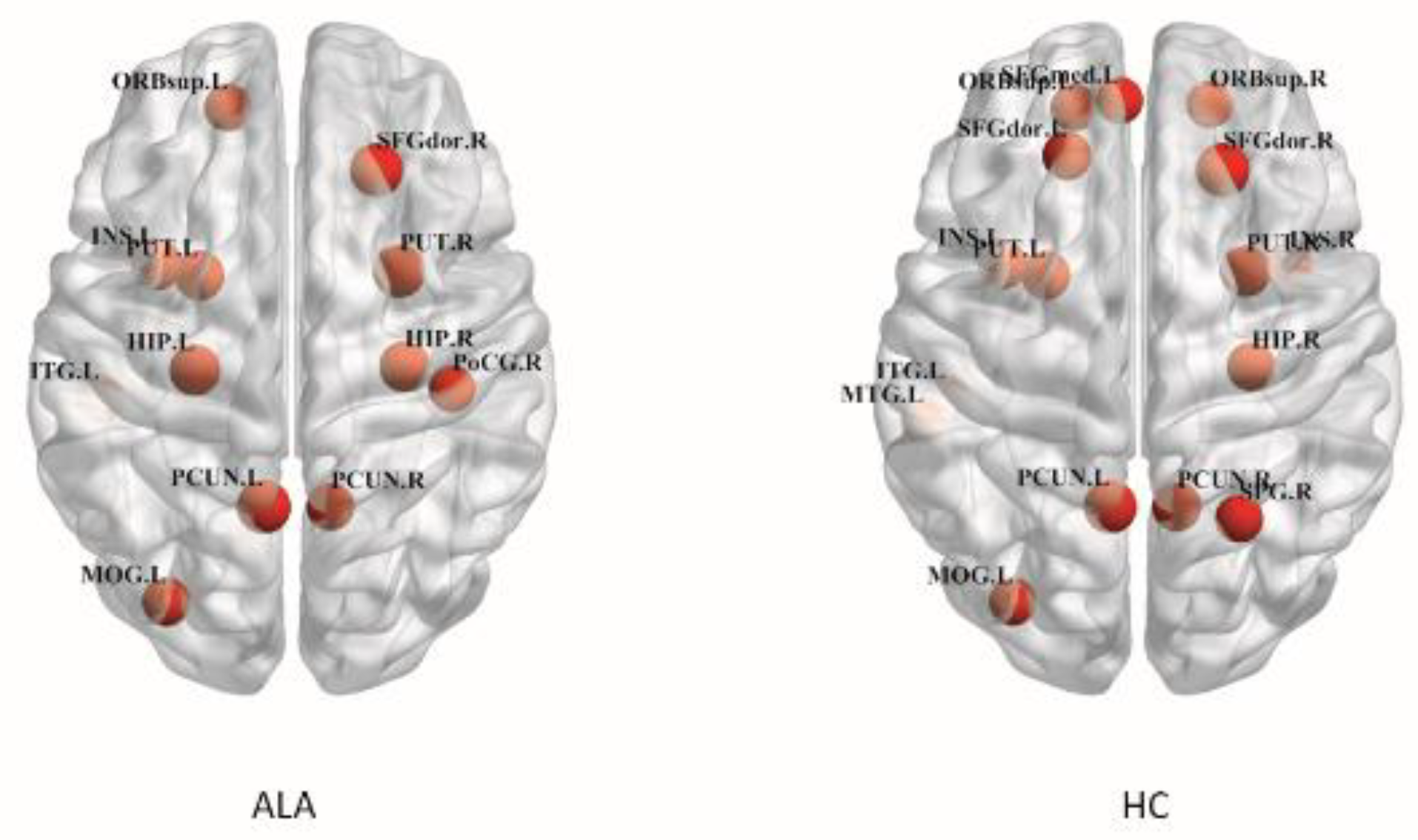
3.4. Hub
3.5. WM Abnormalities Identified by TBSS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Sato, J.; Vandewouw, M.M.; Bando, N.; Branson, H.M.; O’Connor, D.L.; Unger, S.L.; Taylor, M.J. White matter alterations and cognitive outcomes in children born very low birth weight. Neuroimage Clin. 2021, 32, 102843. [Google Scholar] [CrossRef] [PubMed]
- Llorente, I.L.; Xie, Y.; Mazzitelli, J.A.; Hatanaka, E.A.; Cinkornpumin, J.; Miller, D.R.; Lin, Y.; Lowry, W.E.; Carmichael, S.T. Patient-derived glial enriched progenitors repair functional deficits due to white matter stroke and vascular dementia in rodents. Sci. Transl. Med. 2021, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Savarraj, J.P.J.; Kitagawa, R.; Kim, D.H.; Choi, H.A. White matter connectivity for early prediction of Alzheimer’s disease. Technol. Health Care 2022, 30, 17–28. [Google Scholar] [CrossRef]
- Guan, S.; Kong, X.; Duan, S.; Ren, Q.; Huang, Z.; Li, Y.; Wang, W.; Gong, G.; Meng, X.; Ma, X. Neuroimaging Anomalies in Community-Dwelling Asymptomatic Adults with Very Early-Stage White Matter Hyperintensity. Front. Aging Neurosci. 2021, 13, 715434. [Google Scholar] [CrossRef]
- Kerkovsky, M.; Stulik, J.; Dostal, M.; Kuhn, M.; Losak, J.; Praksova, P.; Hulova, M.; Bednarik, J.; Sprlakova-Pukova, A.; Mechl, M. Structural and functional MRI correlates of T2 hyperintensities of brain white matter in young neurologically asymptomatic adults. Eur. Radiol. 2019, 29, 7027–7036. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Chen, H.; Wang, W.; Wang, Y.; Liang, Y.; Zhang, Y. Structural changes in white matter lesion patients and their correlation with cognitive impairment. Neuropsychiatr. Dis. Treat. 2019, 15, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zou, L.; Tang, X.; Zhu, W.; Zhang, G.; Qin, Y.; Zhu, W. Changes of white matter integrity and structural network connectivity in nondemented cerebral small-vessel disease. J. Magn. Reson Imaging 2020, 51, 1162–1169. [Google Scholar] [CrossRef]
- Le Bihan, D. Diffusion, confusion and functional MRI. Neuroimage 2012, 62, 1131–1136. [Google Scholar] [CrossRef]
- Glenn, G.R.; Helpern, J.A.; Tabesh, A.; Jensen, J.H. Optimization of white matter fiber tractography with diffusional kurtosis imaging. NMR Biomed. 2015, 28, 1245–1256. [Google Scholar] [CrossRef]
- Grinberg, F.; Maximov, I.I.; Farrher, E.; Neuner, I.; Amort, L.; Thonnessen, H.; Oberwelland, E.; Konrad, K.; Shah, N.J. Diffusion kurtosis metrics as biomarkers of microstructural development: A comparative study of a group of children and a group of adults. Neuroimage 2017, 144, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Andica, C.; Kamagata, K.; Hatano, T.; Saito, Y.; Ogaki, K.; Hattori, N.; Aoki, S. MR Biomarkers of Degenerative Brain Disorders Derived From Diffusion Imaging. J. Magn. Reson. Imaging 2020, 52, 1620–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, A.; Jensen, J.H.; Ardekani, B.A.; Helpern, J.A. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn. Reson. Med. 2011, 65, 823–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Collins, D.L.; Neelin, P.; Peters, T.M.; Evans, A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994, 18, 192–205. [Google Scholar] [CrossRef]
- Cui, Z.; Zhong, S.; Xu, P.; He, Y.; Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. Corrigendum: GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9, 458. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer. a network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [Green Version]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Y.; He, Y.; Wang, J.; Xia, M.; Yu, C.; Dong, H.; Ye, J.; Butzkueven, H.; Li, K.; et al. Altered topological organization of white matter structural networks in patients with neuromyelitis optica. PLoS ONE 2012, 7, e48846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, L.C. Centrality in social networks conceptual clarification. Soc. Netw. 1978, 1, 215–239. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-X.; Zhang, H.-Y.; Peng, Z.-K.; Xu, Y.; Tang, H.; Wu, J.-T.; Xu, J. Divergent topological networks in Alzheimer’s disease: A diffusion kurtosis imaging analysis. Transl. Neurodegener. 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lu, T.; Xie, C.; Wang, Q.; Wang, Y.; Cao, X.; Su, Y.; Wang, Z.; Zhang, Z. Functional Disorganization of Small-World Brain Networks in Patients with Ischemic Leukoaraiosis. Front. Aging Neurosci. 2020, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Petrovic, K.; Ropele, S.; Enzinger, C.; Fazekas, F. Progression of leukoaraiosis and cognition. Stroke 2007, 38, 2619–2625. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hu, J.; Zhang, S.; Fan, W.; Wen, L.; Wang, G.; Zhang, D. Changes in Resting-State Cerebral Activity in Women with Polycystic Ovary Syndrome: A Functional MR Imaging Study. Front. Endocrinol. 2020, 11, 603279. [Google Scholar] [CrossRef]
- Zheng, W.; Su, Z.; Liu, X.; Zhang, H.; Han, Y.; Song, H.; Lu, J.; Li, K.; Wang, Z. Modulation of functional activity and connectivity by acupuncture in patients with Alzheimer disease as measured by resting-state fMRI. PLoS ONE 2018, 13, e0196933. [Google Scholar] [CrossRef] [Green Version]
- Celle, S.; Boutet, C.; Annweiler, C.; Ceresetti, R.; Pichot, V.; Barthelemy, J.C.; Roche, F. Leukoaraiosis and Gray Matter Volume Alteration in Older Adults: The PROOF Study. Front. Neurosci. 2021, 15, 747569. [Google Scholar] [CrossRef]
- Yang, Y.L.; Deng, H.X.; Xing, G.Y.; Xia, X.L.; Li, H.F. Brain functional network connectivity based on a visual task. visual information processing-related brain regions are significantly activated in the task state. Neural Regen. Res. 2015, 10, 298–307. [Google Scholar] [CrossRef]
- Thiebaut de Schotten, M.; Urbanski, M.; Valabregue, R.; Bayle, D.J.; Volle, E. Subdivision of the occipital lobes: An anatomical and functional MRI connectivity study. Cortex 2014, 56, 121–137. [Google Scholar] [CrossRef]
- Garcia-Alberca, J.M.; Mendoza, S.; Gris, E.; Royo, J.L.; Cruz-Gamero, J.M.; Garcia-Casares, N. White matter lesions and temporal atrophy are associated with cognitive and neuropsychiatric symptoms in patients with hypertension and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2020, 35, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Ramos Bernardes da Silva Filho, S.; Oliveira Barbosa, J.H.; Rondinoni, C.; Dos Santos, A.C.; Garrido Salmon, C.E.; da Costa Lima, N.K.; Ferriolli, E.; Moriguti, J.C. Neuro-degeneration profile of Alzheimer’s patients: A brain morphometry study. Neuroimage Clin. 2017, 15, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zimmerman, M.E.; Narkhede, A.; Nasrabady, S.E.; Tosto, G.; Meier, I.B.; Benzinger, T.L.S.; Marcus, D.S.; Fagan, A.M.; Fox, N.C.; et al. White matter hyperintensities and the mediating role of cerebral amyloid angiopathy in dominantly-inherited Alzheimer’s disease. PLoS ONE 2018, 13, e0195838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, F.A.; Muraskin, J.; Tosto, G.; Narkhede, A.; Wasserman, B.T.; Griffith, E.Y.; Guzman, V.A.; Meier, I.B.; Zimmerman, M.E.; Brickman, A.M.; et al. White matter hyperintensities and cerebral amyloidosis: Necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013, 70, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beason-Held, L.L.; Hohman, T.J.; Venkatraman, V.; An, Y.; Resnick, S.M. Brain network changes and memory decline in aging. Brain Imaging Behav. 2017, 11, 859–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ALA (n = 35) | HC (n = 20) | p-Value | |
|---|---|---|---|
| Age | 63 (8.86) | 59 (9.22) | 0.353 |
| Education years | 10.11 (2.44) | 9.55 (1.97) | 0.308 |
| Sex | 21 (60%) | 10 (56%) | 0.777 |
| Hypertension | 10 (38.5%) | 6 (33.3%) | 0.911 |
| MMSE | 28.45 (0.70) | 28.79 (0.79) | 0.117 |
| MoCA | 28.28 (0.85) | 28.73 (0.80) | 0.145 |
| KT | DT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MK | KFA | AK | RK | MD | FA | AD | RD | ||
| Lp | ALA | 4.11 (0.5) | 8.99 (1.31) | 6.71 (1.1) | 4.95 (0.74) | 3.81 (0.51) | 11.98 (3.11) | 3.77 (0.42) | 4.61 (0.64) |
| HC | 4.26 (0.62) | 9.03 (1.12) | 6.86 (1.03) | 4.79 (0.92) | 3.96 (0.62) | 12.04 (3.52) | 3.86 (0.52) | 4.52 (0.71) | |
| Cp | ALA | 0.23 (0.01) * | 0.17 (0.02) | 0.18 (0.10) * | 0.13 (0.03) * | 0.12 (0.01) | 0.17 (0.01) | 0.13 (0.13) | 0.13 (0.01) |
| HC | 0.28 (0.01) | 0.18 (0.02) | 0.22 (0.13) | 0.18 (0.02) | 0.13 (0.01) | 0.18 (0.01) | 0.13 (0.12) | 0.13 (0.01) | |
| Eg | ALA | 0.2 (0.12) | 0.1 (0.07) | 0.14 (0.02) | 0.21 (0.13) | 0.24 (0.02) | 0.13 (0.02) | 0.31 (0.18) | 0.21 (0.06) |
| HC | 0.22 (0.01) | 0.1 (0.02) | 0.15 (0.01) | 0.21 (0.02) | 0.25 (0.00) | 0.13 (0.01) | 0.32 (0.01) | 0.23 (0.35) | |
| Eloc | ALA | 0.41 (0.32) * | 0.13 (0.02) * | 0.31 (0.21) * | 0.48 (0.03) * | 0.50 (0.02) | 0.17 (0.02) | 0.56 (0.02) | 0.44 (0.26) |
| HC | 0.44 (0.01) | 0.17 (0.01) | 0.34 (0.15) | 0.53 (0.02) | 0.51 (0.01) | 0.17 (0.01) | 0.57 (0.01) | 0.44 (0.13) | |
| σ | ALA | 1.50 (0.11) | 1.44 (0.13) | 1.78 (0.01) | 1.45 (0.31) | 1.49 (0.21) | 1.31 (0.11) | 1.29 (0.14) | 1.64 (0.34) |
| HC | 1.47 (0.13) | 1.43 (0.13) | 1.76 (0.04) | 1.33 (0.15) | 1.57 (0.17) | 1.30 (0.11) | 1.31 (0.13) | 1.72 (0.27) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Zhang, H.-Y.; Wang, R.; Cheng, D.-S.; Ye, J. Topologic Efficiency Abnormalities of the Connectome in Asymptomatic Patients with Leukoaraiosis. Brain Sci. 2022, 12, 784. https://doi.org/10.3390/brainsci12060784
Yao S, Zhang H-Y, Wang R, Cheng D-S, Ye J. Topologic Efficiency Abnormalities of the Connectome in Asymptomatic Patients with Leukoaraiosis. Brain Sciences. 2022; 12(6):784. https://doi.org/10.3390/brainsci12060784
Chicago/Turabian StyleYao, Shun, Hong-Ying Zhang, Ren Wang, Ding-Sheng Cheng, and Jing Ye. 2022. "Topologic Efficiency Abnormalities of the Connectome in Asymptomatic Patients with Leukoaraiosis" Brain Sciences 12, no. 6: 784. https://doi.org/10.3390/brainsci12060784





