Stimulation of GABA Receptors in the Lateral Septum Rapidly Elicits Food Intake and Mediates Natural Feeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedures
2.3. Specific Experiments
2.4. Test Procedures
2.5. Drugs
2.6. Histological Procedures
2.7. Statistical Analysis
3. Results
3.1. Histology
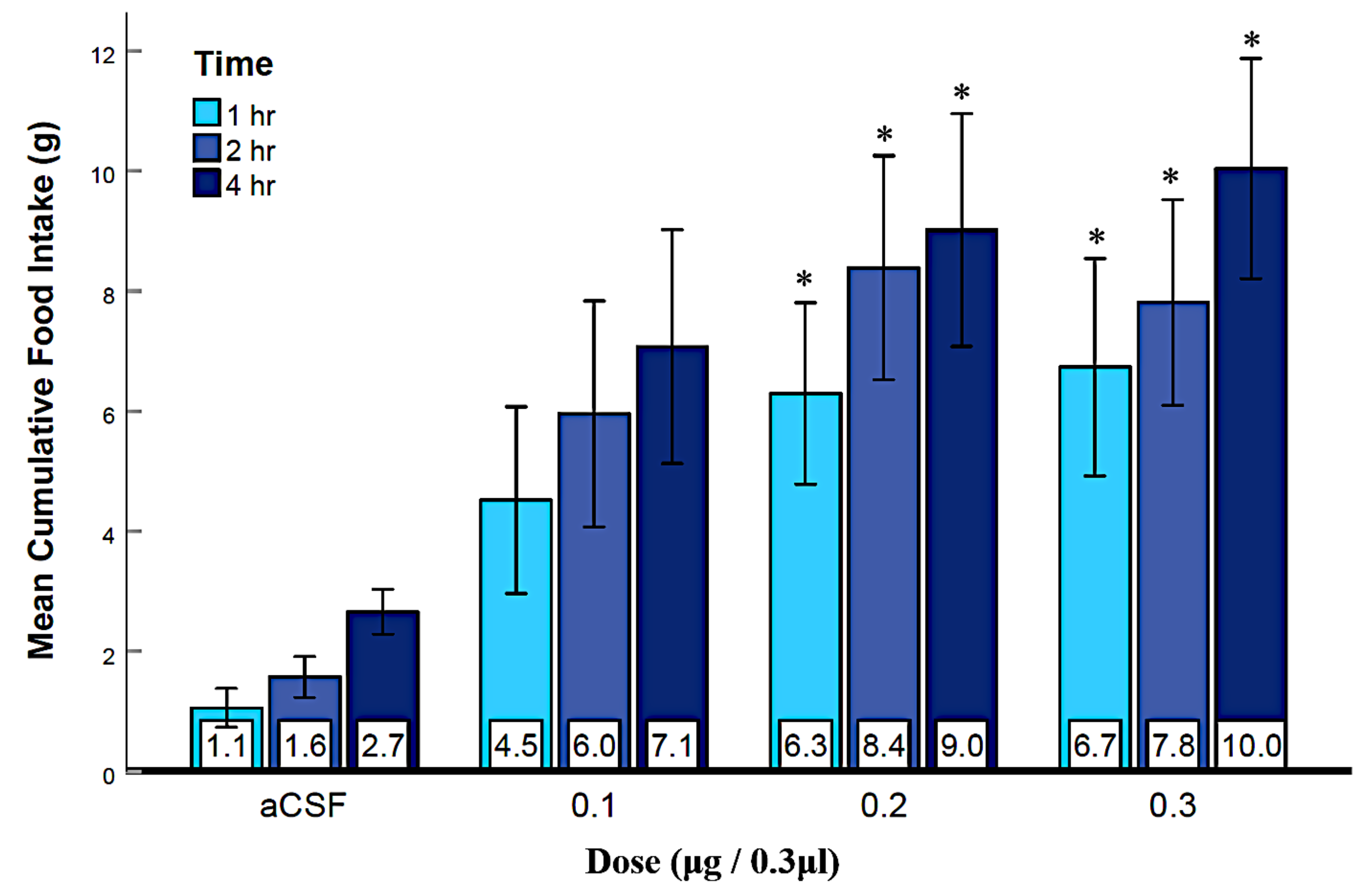
3.2. Experiment 1: Activation of LS GABAA Receptors Elicits Feeding
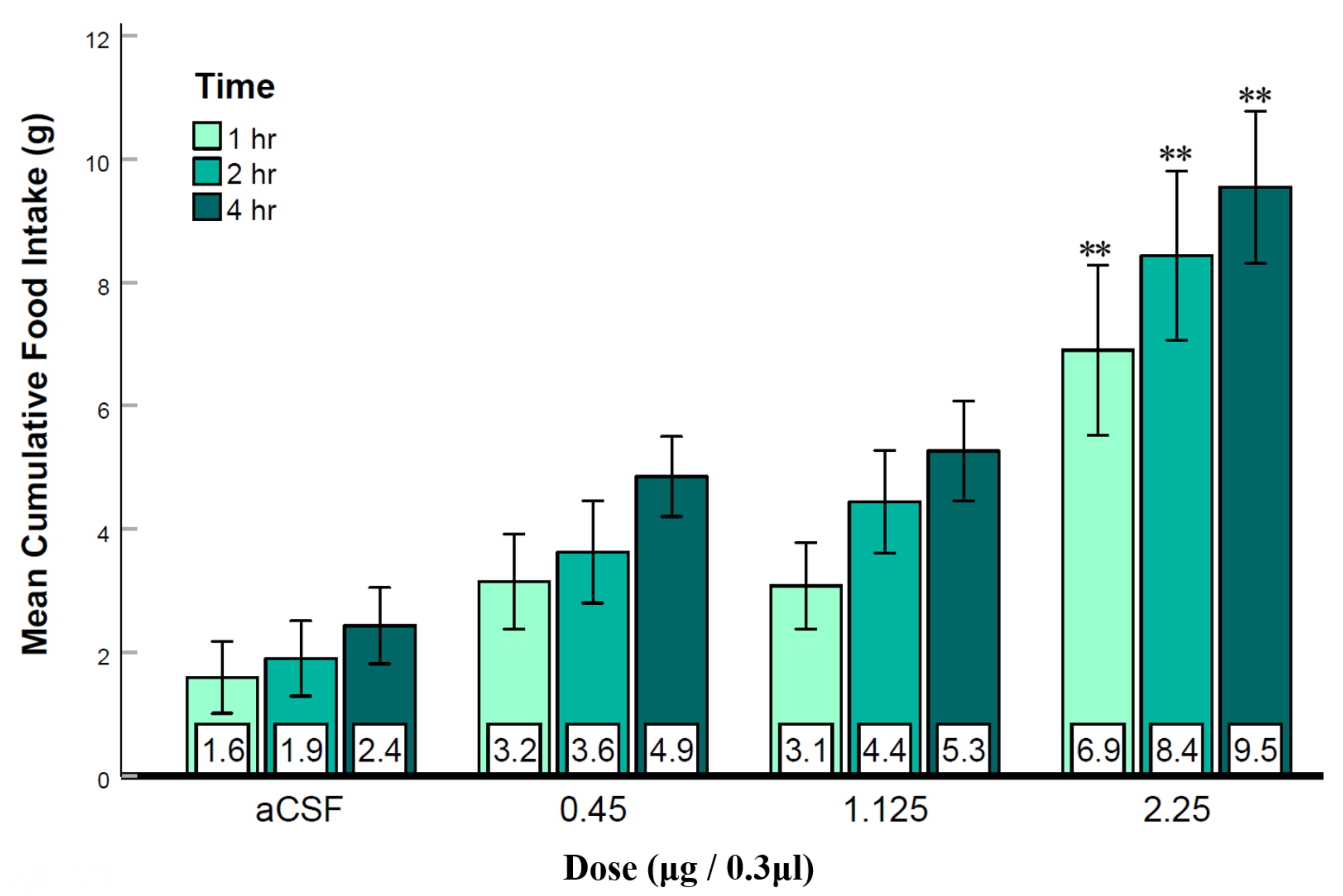
3.3. Experiment 2: Activation of LS GABAB Receptors Elicits Feeding
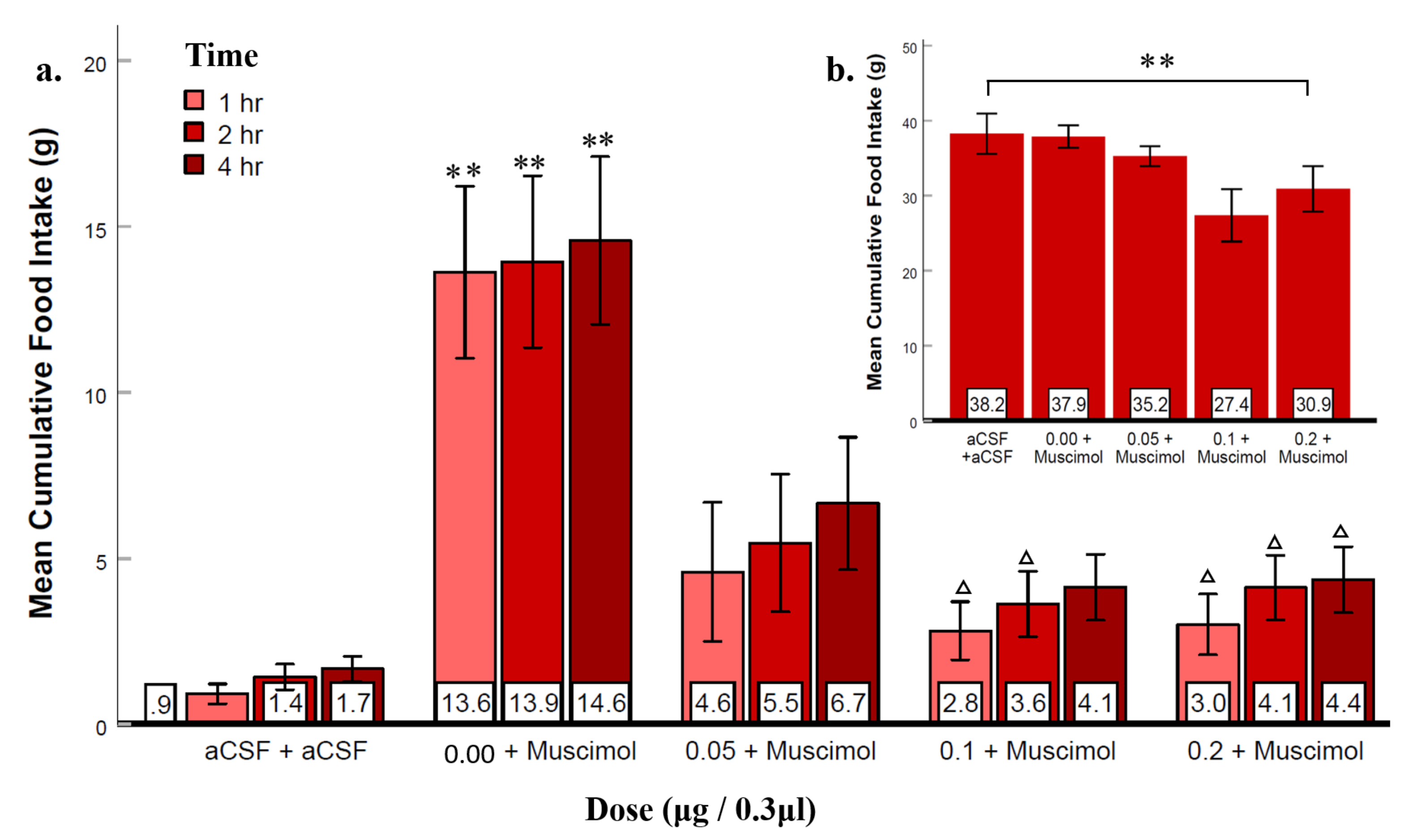
3.4. Experiment 3: Suppression of LS GABAA Receptors Reduced Agonist-Elicited Feeding
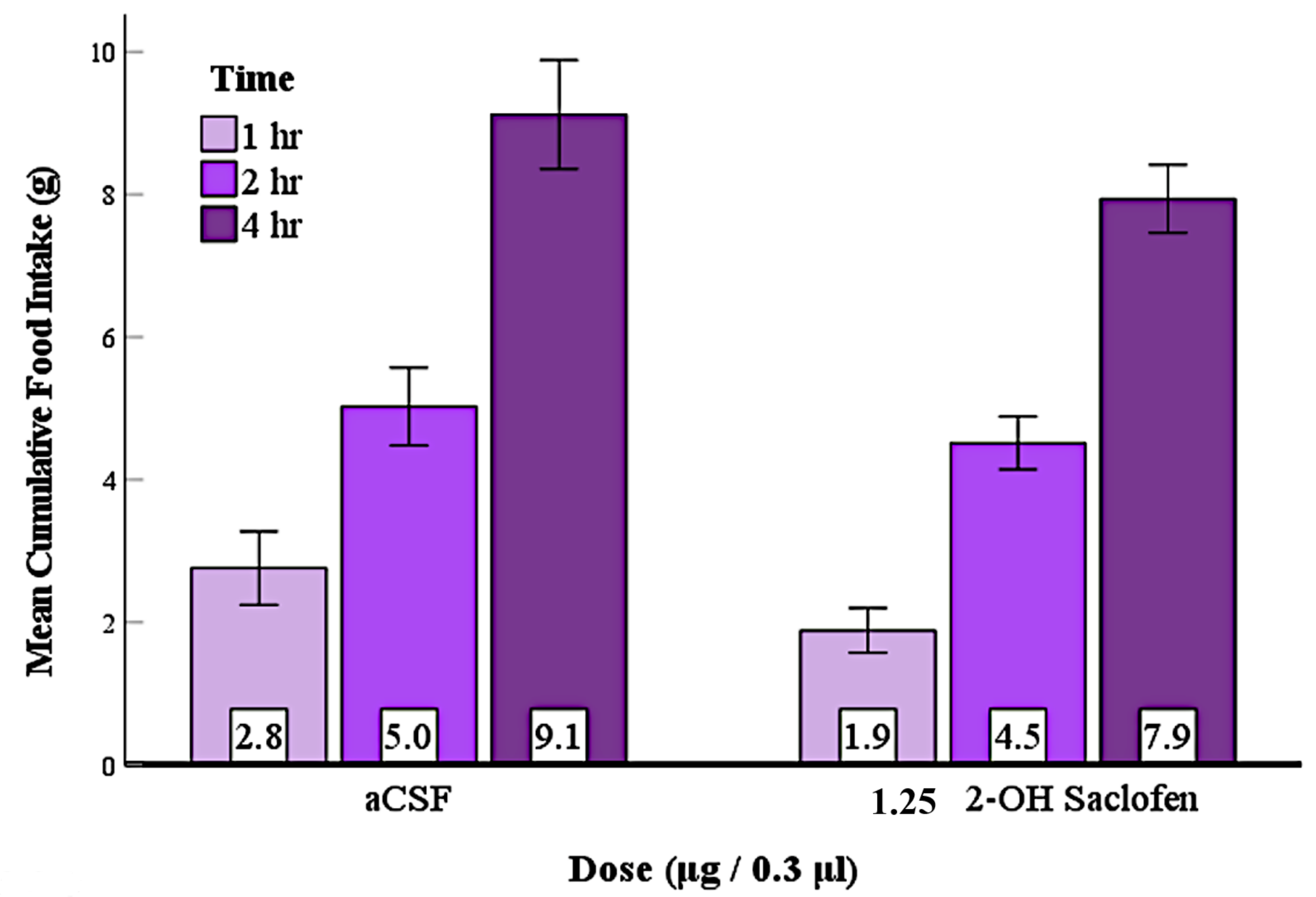
3.5. Experiment 4: Suppression of LS GABAB Receptors Reduced Agonist-Elicited Feeding
3.6. Experiment 5: Suppression of LS GABAA Receptors at the Beginning of the Dark Phase Reduced Feeding
3.7. Experiment 6: No Evidence That Suppression of LS GABAB Receptors at the Beginning of the Dark Phase Reduced Feeding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018. NCHS Data Brief, No. 360; National Center for Health Statistics: Hyattsville, MD, USA, 2020; Volume 360. [Google Scholar]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-5-TR), 15th ed.; American Psychiatric Association: Washington, DC, USA, 2022; Volume 5. [Google Scholar]
- Udo, T.; Grilo, C.M. Prevalence and correlates of DSM-5–defined eating disorders in a nationally representative sample of US adults. Biol. Psychiatry 2018, 84, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, E.P.; Ivan, V.J.; Friedman, J.M.; Stern, S.A. Higher-order inputs involved in appetite control. Biol. Psychiatry 2021, 91, 869–878. [Google Scholar] [CrossRef]
- Kosugi, K.; Yoshida, K.; Suzuki, T.; Kobayashi, K.; Yoshida, K.; Mimura, M.; Tanaka, K.F. Activation of ventral CA1 hippocampal neurons projecting to the lateral septum during feeding. Hippocampus 2021, 31, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Rizzi-Wise, C.A.; Wang, D.V. Putting together pieces of the lateral septum: Multifaceted functions and its neural pathways. eNeuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, T.P.; Chambers, R.A.; Russell, D.S. Regulation of affect by the lateral septum: Implications for neuropsychiatry. Brain Res. Rev. 2004, 46, 71–117. [Google Scholar] [CrossRef] [PubMed]
- Olds, J.; Milner, P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954, 47, 419. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, C.B.; Aston-Jones, G. Lateral septum inhibition reduces motivation for cocaine: Reversal by diazepam. Addict. Biol. 2020, 25, e12742. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.L.; Wishart, T.B. Motivated feeding behavior elicited by electrical stimulation of the septum. Physiol. Behav. 1971, 6, 105–109. [Google Scholar] [CrossRef]
- Urstadt, K.R.; Stanley, B.G. Direct hypothalamic and indirect trans-pallidal, trans-thalamic, or trans-septal control of accumbens signaling and their roles in food intake. Front. Syst. Neurosci. 2015, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Stanley, B.; Urstadt, K.; Charles, J.; Kee, T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol. Behav. 2011, 104, 40–46. [Google Scholar] [CrossRef]
- Sweeney, P.; Yang, Y. An inhibitory septum to lateral hypothalamus circuit that suppresses feeding. J. Neurosci. 2016, 36, 11185–11195. [Google Scholar] [CrossRef] [Green Version]
- Urstadt, K.R.; Kally, P.; Zaidi, S.F.; Stanley, B.G. Ipsilateral feeding-specific circuits between the nucleus accumbens shell and the lateral hypothalamus: Regulation by glutamate and GABA receptor subtypes. Neuropharmacology 2013, 67, 176–182. [Google Scholar] [CrossRef]
- Zahm, D.S.; Parsley, K.P.; Schwartz, Z.M.; Cheng, A.Y. On lateral septum-like characteristics of outputs from the accumbal hedonic “hotspot” of Peciña and Berridge with commentary on the transitional nature of basal forebrain “boundaries”. J. Comp. Neurol. 2013, 521, 50–68. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, E.P.; Tan, B.; Pomeranz, L.E.; Ivan, V.; Fetcho, R.; Schneeberger, M.; Doerig, K.R.; Liston, C.; Friedman, J.M.; Stern, S.A. A limbic circuit selectively links active escape to food suppression. eLife 2020, 9, e58894. [Google Scholar] [CrossRef]
- Terrill, S.J.; Jackson, C.M.; Greene, H.E.; Lilly, N.; Maske, C.B.; Vallejo, S.; Williams, D.L. Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R124–R132. [Google Scholar] [CrossRef] [Green Version]
- Calderwood, M.T.; Tseng, A.; Stanley, B.G. Lateral septum mu opioid receptors in stimulation of feeding. Brain Res. 2020, 1734, 146648. [Google Scholar] [CrossRef]
- Stanley, B.G.; Lanthier, D.; Leibowitz, S.F. Multiple brain sites sensitive to feeding stimulation by opioid agonists: A cannula-mapping study. Pharmacol. Biochem. Behav. 1988, 31, 825–832. [Google Scholar] [CrossRef]
- Pierobon, P. An interesting molecule:γ-aminobutyric acid. What can we learn from Hydra Polyps? Brain Sci. 2021, 11, 437. [Google Scholar] [CrossRef]
- Köhler, C.; Chan-Palay, V. Distribution of gamma aminobutyric acid containing neurons and terminals in the septal area. Anat. Embryol. 1983, 167, 53–65. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Cassidy, R.M.; Mangieri, L.R.; Zhu, C.; Huang, X.; Jiang, Z.; Justice, N.J.; Xu, Y.; Arenkiel, B.R.; et al. Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, P.; Yang, Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; Lenglos, C.; Timofeeva, E. Activation of GABAA and GABAB receptors in the lateral septum increases sucrose intake by differential stimulation of sucrose licking activity. Behav. Brain Res. 2014, 273, 82–88. [Google Scholar] [CrossRef]
- Calderwood, M.T.; Tseng, A.; Gabriella, I.; Stanley, B.G. Feeding behavior elicited by mu opioid and GABA receptor activation in the lateral septum. Pharmacol. Biochem. Behav. 2022, 217, 173395. [Google Scholar] [CrossRef]
- Calderwood, M.T. The Role of Lateral Septal Opioid and GABAA Receptors in Feeding Behavior. Doctoral Dissertation, University of California, Riverside, CA, USA, 2020. Available online: https://escholarship.org/uc/item/8ns6r2v8 (accessed on 14 June 2022).
- Kersten, A.; Strubbe, J.H.; Spiteri, N.J. Meal patterning of rats with changes in day length and food availability. Physiol. Behav. 1980, 25, 953–958. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Page, A.J.; Christie, S.; Symonds, E.; Li, H. Circadian regulation of appetite and time restricted feeding. Physiol. Behav. 2020, 220, 112873. [Google Scholar] [CrossRef]
- Sieghart, W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006, 54, 231–263. [Google Scholar]
- Johnston, G.A. Muscimol as an ionotropic GABA receptor agonist. Neurochem. Res. 2014, 39, 1942–1947. [Google Scholar] [CrossRef]
- Bitran, D.; Dugan, M.; Renda, P.; Ellis, R.; Foley, M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999, 850, 217–224. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; Contreras, C.M.; Saavedra, M.; Luna-Baltazar, I.; López-Rubalcava, C. Participation of the lateral septal nuclei (LSN) in the antidepressant-like actions of progesterone in the forced swimming test (FST). Behav. Brain Res. 2002, 134, 175–183. [Google Scholar] [CrossRef]
- Izquierdo, I.; da Cunha, C.; Rosat, R.; Jerusalinsky, D.; Ferreira, M.B.C.; Medina, J.H. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav. Neural Biol. 1992, 58, 16–26. [Google Scholar] [CrossRef]
- Richter, J.A.; Gormley, J.M. Inhibition of high-affinity choline uptake in the rat hippocampus by in vivo injection of phenobarbital in the medial septum. J. Pharmacol. Exp. Ther. 1986, 237, 563–568. [Google Scholar] [PubMed]
- Froestl, W. Chemistry and pharmacology of GABAB receptor ligands. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 58, pp. 19–62. [Google Scholar]
- Urwyler, S.; Gjoni, T.; Koljatić, J.; Dupuis, D.S. Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: Effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology 2005, 48, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mauchly, J.W. Significance test for sphericity of a normal n-variate distribution. Ann. Math. Stat. 1940, 11, 204–209. [Google Scholar] [CrossRef]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Bonferroni, C. Teoria statistica delle classi e calcolo delle probabilita. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commericiali di Firenze 1936, 8, 3–62. [Google Scholar]
- Gabriella, I. Dataset of lateral septum—GABA receptors feeding study. Open Sci. Framew. 2022. [Google Scholar] [CrossRef]
- Wang, C.; Kotz, C.M. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R358–R367. [Google Scholar] [CrossRef] [Green Version]
- Sindelar, D.K.; Palmiter, R.D.; Woods, S.C.; Schwartz, M.W. Attenuated feeding responses to circadian and palatability cues in mice lacking neuropeptide Y. Peptides 2005, 26, 2597–2602. [Google Scholar] [CrossRef]
- Strubbe, J.H.; van Dijk, G. The temporal organization of ingestive behaviour and its interaction with regulation of energy balance. Neurosci. Biobehav. Rev. 2002, 26, 485–498. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriella, I.; Tseng, A.; Sanchez, K.O.; Shah, H.; Stanley, B.G. Stimulation of GABA Receptors in the Lateral Septum Rapidly Elicits Food Intake and Mediates Natural Feeding. Brain Sci. 2022, 12, 848. https://doi.org/10.3390/brainsci12070848
Gabriella I, Tseng A, Sanchez KO, Shah H, Stanley BG. Stimulation of GABA Receptors in the Lateral Septum Rapidly Elicits Food Intake and Mediates Natural Feeding. Brain Sciences. 2022; 12(7):848. https://doi.org/10.3390/brainsci12070848
Chicago/Turabian StyleGabriella, Ivett, Andy Tseng, Kevin O. Sanchez, Himani Shah, and Billy Glenn Stanley. 2022. "Stimulation of GABA Receptors in the Lateral Septum Rapidly Elicits Food Intake and Mediates Natural Feeding" Brain Sciences 12, no. 7: 848. https://doi.org/10.3390/brainsci12070848
APA StyleGabriella, I., Tseng, A., Sanchez, K. O., Shah, H., & Stanley, B. G. (2022). Stimulation of GABA Receptors in the Lateral Septum Rapidly Elicits Food Intake and Mediates Natural Feeding. Brain Sciences, 12(7), 848. https://doi.org/10.3390/brainsci12070848





