Small Demyelination of the Cortex May Be a Potential Marker for the Right-to-Left Shunt of the Heart
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data Collection
2.3. RLS Assessment
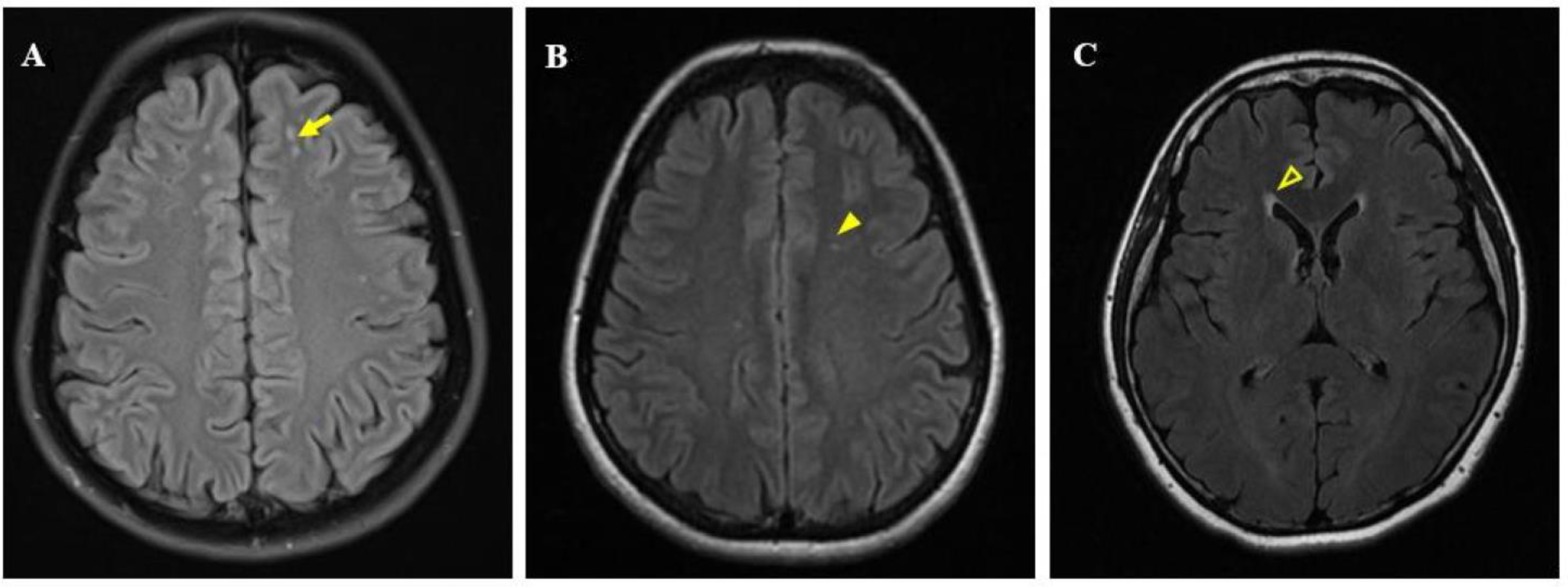
2.4. MRI Examination
2.5. Image Post-Processing Analysis and WML Evaluation
2.6. Statistical Analysis
3. Results
3.1. Comparison between the WML-Positive and WML-Negative Patients
3.2. Comparison between the Near-Cortical WML-Positive and WML-Negative Patients
3.3. Comparison of White Matter Lesions between the RLS-Positive Group and the RLS-Negative Group
3.4. Multivariate Logistic Regression Analysis of White Matter Lesions and Near-Cortical White Matter Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olesen, J. International classification of headache disorders. Lancet Neurol. 2018, 17, 396–397. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Liu, R.; Zhao, G.; Yang, X.; Qiao, X.; Feng, J. The prevalence and burden of primary headaches in china: A population-based door-to-door survey. Headache 2012, 52, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Gladstone, J.P.; Dodick, D.W. Migraine and white matter hyperintensities. Curr. Pain Headache Rep. 2005, 9, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Lip, P.Z.; Lip, G.Y. Patent foramen ovale and migraine attacks: A systematic review. Am. J. Med. 2014, 127, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Q.; Guo, Y.Z.; Gao, Y.S.; Guo, Z.N.; Niu, P.P.; Yang, Y. Effectiveness and safety of transcatheter patent foramen ovale closure for migraine (eastform) trial. Sci. Rep. 2016, 6, 39081. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.M.; Wang, B.Z.; Hao, Y.S.; Song, S.J.; Pan, M.X. The correlation between migraine and patent foramen ovale. Front. Neurol. 2020, 11, 543485. [Google Scholar] [CrossRef]
- Kruit, M.C.; Thijs, R.D.; Ferrari, M.D.; Launer, L.J.; van Buchem, M.A.; van Dijk, J.G. Syncope and orthostatic intolerance increase risk of brain lesions in migraineurs and controls. Neurology 2013, 80, 1958–1965. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.H.; Wang, S.B.; Tian, Q.; Zhong, C.; Zhang, G.L.; Li, Y.J. Right-to-left shunt and subclinical ischemic brain lesions in chinese migraineurs: A multicentre mri study. BMC Neurol. 2018, 18, 18. [Google Scholar] [CrossRef]
- Koppen, H.; Palm-Meinders, I.H.; Mess, W.H.; Keunen, R.W.; Terwindt, G.M.; Launer, L.J. Systemic right-to-left shunts, ischemic brain lesions, and persistent migraine activity. Neurology 2016, 86, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Yoon, G.J.; Kim, J.T.; Chang, J.; Kim, D.E.; Cho, B.H.; Lee, J.H. Right-to-left shunts as a cause of juxtacortical spots in patients with migraine. Eur. J. Neurol. 2012, 19, 1086–1092. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.E.; Diamond, M.; Freitag, F.; Reed, M.L.; Stewart, W.F. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007, 68, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacGregor, E.A. Migraine. Ann. Intern. Med. 2017, 166, ITC49–ITC64. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the sustainable development goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- Teshome, M.K.; Najib, K.; Nwagbara, C.C.; Akinseye, O.A.; Ibebuogu, U.N. Patent foramen ovale: A comprehensive review. Curr. Probl. Cardiol. 2020, 45, 100392. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, D.; Lan, L.; Fan, Y. Multiple factors involved in the pathogenesis of white matter lesions. Biomed Res. Int. 2017, 2017, 9372050. [Google Scholar] [CrossRef]
- Kumar, P.; Kijima, Y.; West, B.H.; Tobis, J.M. The connection between patent foramen ovale and migraine. Neuroimaging Clin. N. Am. 2019, 29, 261–270. [Google Scholar] [CrossRef]
- Mok, V.; Ding, D.; Fu, J.; Xiong, Y.; Chu, W.W.; Wang, D. Transcranial doppler ultrasound for screening cerebral small vessel disease: A community study. Stroke 2012, 43, 2791–2793. [Google Scholar] [CrossRef]
- Poels, M.M.; Zaccai, K.; Verwoert, G.C.; Vernooij, M.W.; Hofman, A.; van der Lugt, A. Arterial stiffness and cerebral small vessel disease: The rotterdam scan study. Stroke 2012, 43, 2637–2642. [Google Scholar] [CrossRef] [Green Version]
- Tobis, J.M.; Azarbal, B. Does patent foramen ovale promote cryptogenic stroke and migraine headache? Tex. Heart Inst. J. 2005, 32, 362–365. [Google Scholar]
- Park, H.K.; Lee, S.Y.; Kim, S.E.; Yun, C.H.; Kim, S.H. Small deep white matter lesions are associated with right-to-left shunts in migraineurs. J. Neurol. 2011, 258, 427–433. [Google Scholar] [CrossRef]
- Iwasaki, A.; Suzuki, K.; Takekawa, H.; Takashima, R.; Suzuki, A.; Suzuki, S. The relationship between right-to-left shunt and brain white matter lesions in japanese patients with migraine: A single center study. J. Headache Pain 2017, 18, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altamura, C.; Paolucci, M.; Costa, C.M.; Brunelli, N.; Cascio Rizzo, A.; Cecchi, G. Right-to-left shunt and the clinical features of migraine with aura: Earlier but not more. Cerebrovasc. Dis. 2019, 47, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Migraine. Lancet 2004, 363, 381–391. [Google Scholar] [CrossRef]
- Candee, M.S.; McCandless, R.T.; Moore, K.R.; Arrington, C.B.; Minich, L.L.; Bale, J.F., Jr. White matter lesions in children and adolescents with migraine. Pediatr. Neurol. 2013, 49, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Mas, J.L.; Derumeaux, G.; Guillon, B.; Massardier, E.; Hosseini, H.; Mechtouff, L. Patent foramen ovale closure or anticoagulation vs. Antiplatelets after stroke. N. Engl. J. Med. 2017, 377, 1011–1021. [Google Scholar] [CrossRef]
- Sondergaard, L.; Kasner, S.E.; Rhodes, J.F.; Andersen, G.; Iversen, H.K.; Nielsen-Kudsk, J.E. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N. Engl. J. Med. 2017, 377, 1033–1042. [Google Scholar] [CrossRef]
- Saver, J.L.; Carroll, J.D.; Thaler, D.E.; Smalling, R.W.; MacDonald, L.A.; Marks, D.S. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N. Engl. J. Med. 2017, 377, 1022–1032. [Google Scholar] [CrossRef]

| Clinical Data | WMLs(+) (n = 71 ) | WMLs(−) (n = 130 ) | p |
|---|---|---|---|
| Age (y) | 33.56 ± 7.56 | 32.17 ± 7.46 | 0.197 |
| Female, n (%) | 40 (56.3) | 77 (59.2) | 0.691 |
| Diabetes, n (%) | 1 (1.4) | 2 (1.5) | 0.715 a |
| Hypertension, n (%) | 6 (8.5) | 5 (3.8) | 0.148 |
| Duration of migraine, n (year) | 5 (2, 11) | 6.5 (3, 11.25) | 0.243 |
| Severity | 4.94 ± 1.88 | 4.69 ± 1.92 | 0.776 |
| Migraine with aura, n (%) | 11 (15.5) | 34 (26.2) | 0.691 |
| Family history of migraine, n (%) | 13 (18.3) | 30 (23.1) | 0.431 |
| RLS | 39 (54.9) | 56 (43.1) | 0.168 |
| RLS Grades | |||
| 0 | 32 (45.1) | 74 (56.9) | 0.210 b |
| I | 17 (23.9) | 32 (24.6) | |
| II | 8 (11.3) | 10 (7.7) | |
| III | 14 (19.7) | 14 (10.8) |
| Clinical Data | Near-Cortical WMLs(+) (n = 56) | Near-Cortical WMLs(−) (n = 145) | p |
|---|---|---|---|
| Age (y) | 34.11 ± 7.96 | 32.10 ± 7.28 | 0.068 |
| Female, n (%) | 33 (58.9) | 84 (57.9) | 0.898 |
| Diabetes, n (%) | 1 (1.78) | 2 (1.37) | 0.627 a |
| Hypertension, n (%) | 6 (10.7) | 5 (3.4) | 0.076 a |
| Duration of migraine, n(year) | 6 (2, 11) | 6 (3, 11) | 0.636 |
| Severity | 5.13 ± 1.91 | 4.65 ± 1.89 | 0.415 |
| Migraine with aura, n (%) | 10 (17.9) | 24 (16.6) | 0.825 |
| Family history of migraine, n (%) | 11 (19.6) | 32 (22.1) | 0.707 |
| RLS, n (%) | 35 (62.5) | 60 (41.4) | 0.007 |
| RLS Grades | |||
| 0 | 21 (37.5) | 85 (58.6) | 0.001 b |
| I | 14 (25.0) | 35 (24.1) | |
| II | 8 (14.3) | 10 (6.9) | |
| III | 13 (23.2) | 15 (10.4) |
| Number of WMLs | RLS Grade | |||
|---|---|---|---|---|
| 0 | I | II | III | |
| 0–5 | 97 | 46 | 14 | 25 |
| 6–10 | 5 | 2 | 3 | 3 |
| 11–15 | 3 | 0 | 1 | 0 |
| 16–20 | 1 | 1 | 0 | 0 |
| Univariate Logistic Regression | Multivariate Logistic Regression a | |||||
|---|---|---|---|---|---|---|
| Lesion Location | RLS (+) | RLS (−) | OR (95%CI) | p-Value | OR (95%CI) | p-Value |
| Near-cortical, n (%) | 35 (36.8) | 21 (19.8) | 2.36 (1.253, 4.451) | 0.008 | 2.54 (1.326, 4.863) | 0.005 |
| Paraventricular, n (%) | 13 (13.7) | 22 (20.8) | 0.61 (0.286, 1.282) | 0.190 | 0.67 (0.310, 1.444) | 0.307 |
| Deep brain, n (%) | 5 (5.3) | 12 (11.3) | 0.65 (0.257, 1.658) | 0.370 | 0.68 (0.264, 1.732) | 0.415 |
| ACA blood supply area, n (%) | 27 (28.4) | 15 (14.2) | 2.41 (1.190, 4.875) | 0.015 | 2.53 (1.238, 5.168) | 0.011 |
| MCA blood supply area, n (%) | 22 (23.2) | 24 (22.6) | 1.03 (0.533, 1.990) | 0.931 | 1.07 (0.545, 2.100) | 0.845 |
| PCA blood supply area, n (%) | 3 (3.2) | 8 (7.5) | 0.40 (0.103, 1.552) | 0.185 | 0.45 (0.114, 1.795) | 0.259 |
| WMLs | Near-Cortical WMLs | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95%CI) | p-Value | Adjusted OR (95%) | p-Value | Crude OR (95%CI) | p-Value | Adjusted OR (95%) | p-Value | |
| Age | 1.03 (0.986, 1.066) | 0.209 | 1.03 (0.985, 1.070) | 0.211 | 1.04 (0.994, 1.080) | 0.091 | 1.04 (0.996, 1.092) | 0.072 |
| Sex | 1.13 (0.627, 2.021) | 0.691 | 1.19 (0.641, 2.207) | 0.583 | 1.04 (0.557, 1.949) | 0.163 | 1.07 (0.548, 2.107) | 0.835 |
| Duration of migraine | 0.98 (0.934, 1.031) | 0.464 | 0.97 (0.919, 1.027) | 0.309 | 1.00 (0.954, 1.057) | 0.870 | 0.99 (0.929, 1.045) | 0.619 |
| Severity | 1.07 (0.921, 1.247) | 0.370 | 1.14 (0.956, 1.363) | 0.144 | 1.14 (0.970, 1.338) | 0.113 | 1.18 (0.971, 1.422) | 0.097 |
| Migraine with aura | 1.17 (0.535, 2.570) | 0.691 | 1.21 (0.498, 2.920) | 0.678 | 0.91 (0.405, 2.055) | 0.825 | 0.95 (0.373, 2.429) | 0.918 |
| Family history | 1.34 (0.647, 2.768) | 0.432 | 1.40 (0.625, 3.117) | 0.415 | 1.16 (0.538, 2.495) | 0.707 | 1.43 (0.608, 3.371) | 0.411 |
| RLS | 1.61 (0.900, 2.883) | 0.109 | 1.80 (0.979, 3.277) | 0.058 | 2.36 (1.253, 4.451) | 0.008 | 2.69 (1.386, 5.219) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, J.; Wan, M.; Li, N.; Wang, J.; Cai, X.; Fan, D.; Fu, Y. Small Demyelination of the Cortex May Be a Potential Marker for the Right-to-Left Shunt of the Heart. Brain Sci. 2022, 12, 884. https://doi.org/10.3390/brainsci12070884
Huo J, Wan M, Li N, Wang J, Cai X, Fan D, Fu Y. Small Demyelination of the Cortex May Be a Potential Marker for the Right-to-Left Shunt of the Heart. Brain Sciences. 2022; 12(7):884. https://doi.org/10.3390/brainsci12070884
Chicago/Turabian StyleHuo, Junyan, Mengxia Wan, Nan Li, Juan Wang, Xiao Cai, Dongsheng Fan, and Yu Fu. 2022. "Small Demyelination of the Cortex May Be a Potential Marker for the Right-to-Left Shunt of the Heart" Brain Sciences 12, no. 7: 884. https://doi.org/10.3390/brainsci12070884





