Comprehensive Cortical Structural Features Predict the Efficacy of Cognitive Behavioral Therapy in Obsessive-Compulsive Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. CBT Treatment
2.3. Diagnosis and Symptom Evaluation
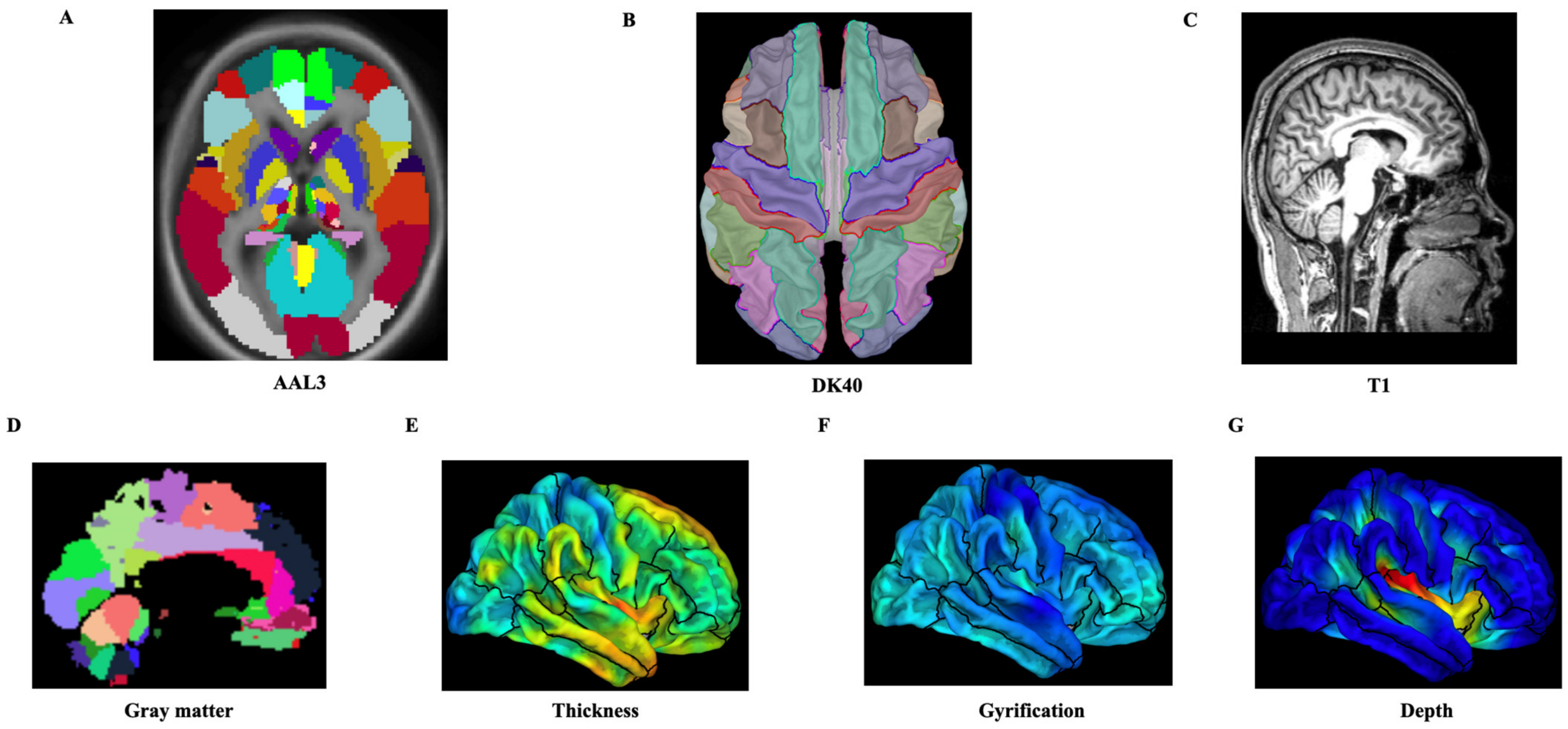
2.4. MRI Data Acquisition
2.5. MRI Data Preprocessing
2.6. Statistical Analysis
2.6.1. Clinical Data Analysis
2.6.2. Extraction of Structural Features and Analysis of the Prediction Models
3. Results
3.1. Demographic and Clinical Characteristics of OCD Patients
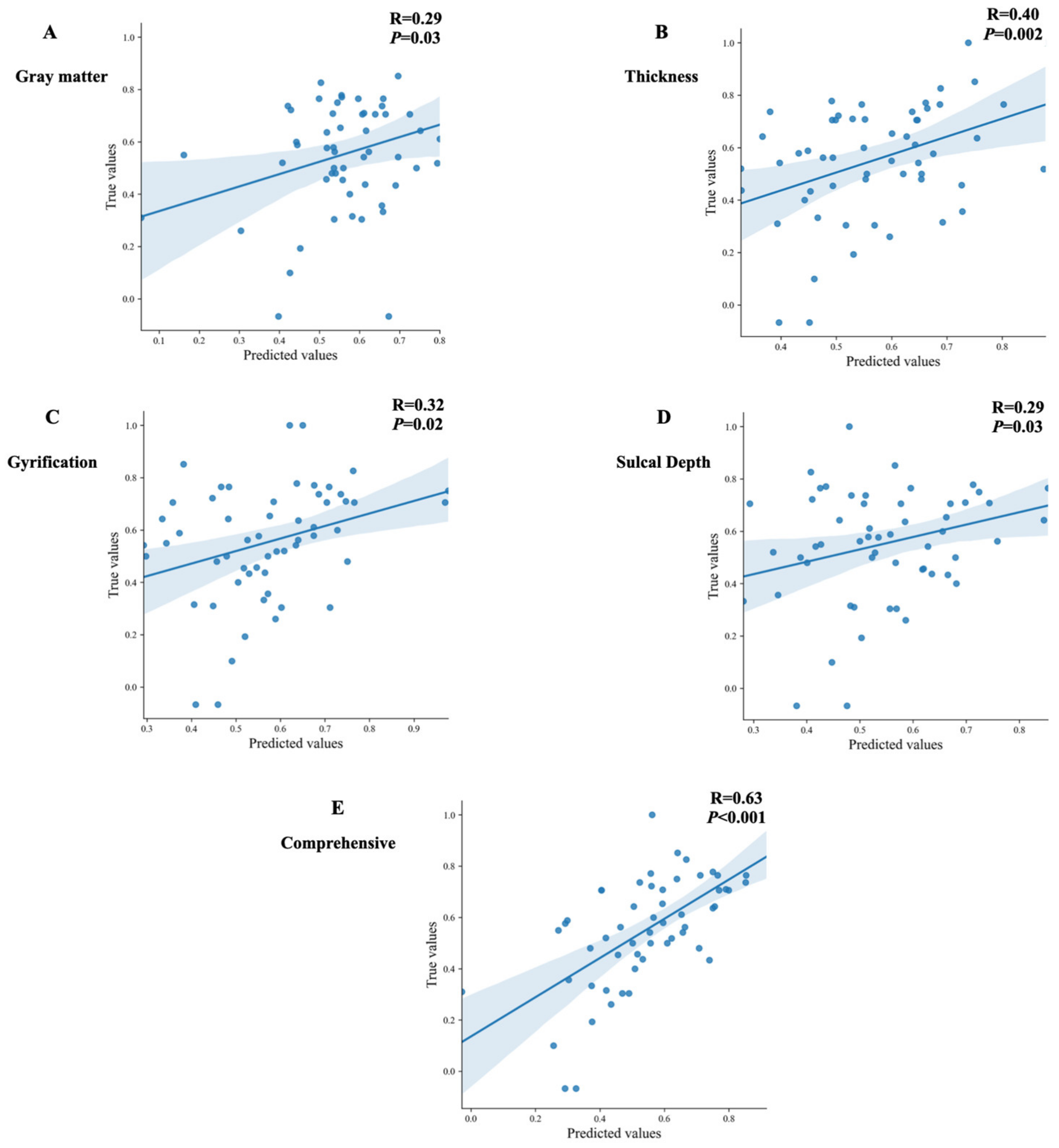
3.2. The Prediction Models Analysis Based on the Structural Features in OCD Patients
3.3. The Post Hoc Analysis of the Prediction Model with the Comprehensive Variables
4. Discussion
4.1. The Efficacy of CBT for OCD Patients
4.2. The Performance of Five Prediction Models on CBT in OCD Patients
4.3. Key Cortical Structural Feature and Regions for Predicting the Efficacy of CBT in OCD
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heyman, I.; Mataix-Cols, D.; Fineberg, N.A. Obsessive-compulsive disorder. BMJ 2006, 333, 424–429. [Google Scholar] [CrossRef]
- Stein, D.J.; Costa, D.L.C.; Lochner, C.; Miguel, E.C.; Reddy, Y.C.J.; Shavitt, R.G.; van den Heuvel, O.A.; Simpson, H.B. Obsessive-compulsive disorder. Nat. Rev. Dis. Primers 2019, 5, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Tang, Z.; Wang, X.; Ma, X.; Cheng, Y.; Wang, B.; Sun, P.; Tang, W.; Luo, J.; Wang, C.; et al. The cost of obsessive-compulsive disorder (OCD) in China: A multi-center cross-sectional survey based on hospitals. Gen. Psychiatr. 2021, 34, e100632. [Google Scholar] [CrossRef]
- Reid, J.E.; Laws, K.R.; Drummond, L.; Vismara, M.; Grancini, B.; Mpavaenda, D.; Fineberg, N.A. Cognitive behavioural therapy with exposure and response prevention in the treatment of obsessive-compulsive disorder: A systematic review and meta-analysis of randomised controlled trials. Compr. Psychiatry 2021, 106, 152223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Sun, J.; Luo, J.; Zhong, Z.X.; Li, P.; Yao, S.M.; Xiong, H.F.; Huang, F.F.; Li, Z.J. Regional homogeneity of spontaneous brain activity in adult patients with obsessive-compulsive disorder before and after cognitive behavioural therapy. J. Affect. Disord. 2015, 188, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Paszkiel, S.; Szpulak, P. Methods of Acquisition, Archiving and Biomedical Data Analysis of Brain Functioning. Adv. Intell. Syst. 2018, 720, 158–171. [Google Scholar] [CrossRef]
- Atmaca, M.; Yildirim, H.; Yilmaz, S.; Caglar, N.; Mermi, O.; Korkmaz, S.; Akaslan, U.; Gurok, M.G.; Kekilli, Y.; Turkcapar, H. Orbito-frontal cortex and thalamus volumes in the patients with obsessive-compulsive disorder before and after cognitive behavioral therapy. Int. J. Psychiatry Med. 2018, 53, 243–255. [Google Scholar] [CrossRef]
- Huyser, C.; van den Heuvel, O.A.; Wolters, L.H.; de Haan, E.; Boer, F.; Veltman, D.J. Increased orbital frontal gray matter volume after cognitive behavioural therapy in paediatric obsessive compulsive disorder. World J. Biol. Psychiatry 2013, 14, 319–331. [Google Scholar] [CrossRef]
- Lazaro, L.; Bargallo, N.; Castro-Fornieles, J.; Falcon, C.; Andres, S.; Calvo, R.; Junque, C. Brain changes in children and adolescents with obsessive-compulsive disorder before and after treatment: A voxel-based morphometric MRI study. Psychiatry Res. 2009, 172, 140–146. [Google Scholar] [CrossRef]
- Tsuchiyagaito, A.; Hirano, Y.; Asano, K.; Oshima, F.; Nagaoka, S.; Takebayashi, Y.; Matsumoto, K.; Masuda, Y.; Iyo, M.; Shimizu, E.; et al. Cognitive-Behavioral Therapy for Obsessive-Compulsive Disorder with and without Autism Spectrum Disorder: Gray Matter Differences Associated with Poor Outcome. Front. Psychiatry 2017, 8, 143. [Google Scholar] [CrossRef]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 2014, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, M.J.; Cardoso, M.J.; Ridgway, G.R.; Modat, M.; Leung, K.K.; Rohrer, J.D.; Fox, N.C.; Ourselin, S. A comparison of voxel and surface based cortical thickness estimation methods. NeuroImage 2011, 57, 856–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, P.; Anstey, K.J.; Wen, W.; Sachdev, P.S.; Cherbuin, N. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav. Brain Res. 2015, 287, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Jockwitz, C.; Caspers, S.; Lux, S.; Jutten, K.; Schleicher, A.; Eickhoff, S.B.; Amunts, K.; Zilles, K. Age- and function-related regional changes in cortical folding of the default mode network in older adults. Brain Struct. Funct. 2017, 222, 83–99. [Google Scholar] [CrossRef]
- Rus, O.G.; Reess, T.J.; Wagner, G.; Zaudig, M.; Zimmer, C.; Koch, K. Hypogyrification in obsessive-compulsive disorder. Psychol. Med. 2017, 47, 1053–1061. [Google Scholar] [CrossRef]
- Wobrock, T.; Gruber, O.; McIntosh, A.M.; Kraft, S.; Klinghardt, A.; Scherk, H.; Reith, W.; Schneider-Axmann, T.; Lawrie, S.M.; Falkai, P.; et al. Reduced prefrontal gyrification in obsessive-compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Venkatasubramanian, G.; Zutshi, A.; Jindal, S.; Srikanth, S.G.; Kovoor, J.M.; Kumar, J.K.; Janardhan Reddy, Y.C. Comprehensive evaluation of cortical structure abnormalities in drug-naive, adult patients with obsessive-compulsive disorder: A surface-based morphometry study. J. Psychiatr. Res. 2012, 46, 1161–1168. [Google Scholar] [CrossRef]
- Fan, Q.; Palaniyappan, L.; Tan, L.; Wang, J.; Wang, X.; Li, C.; Zhang, T.; Jiang, K.; Xiao, Z.; Liddle, P.F. Surface anatomical profile of the cerebral cortex in obsessive-compulsive disorder: A study of cortical thickness, folding and surface area. Psychol. Med. 2013, 43, 1081–1091. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.C.; Lin, C.P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Winkler, A.M.; Kochunov, P.; Blangero, J.; Almasy, L.; Zilles, K.; Fox, P.T.; Duggirala, R.; Glahn, D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 2010, 53, 1135–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, R.; Yang, X.; Luo, J.; Wang, P.; Meng, F.; Xia, M.; He, Y.; Zhao, T.; Li, Z. The effects of cognitive behavioral therapy on the whole brain structural connectome in unmedicated patients with obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110037. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, X.; Greenshaw, A.J.; Li, S.; Luo, J.; Han, H.; Liu, J.; Zhong, Z.; Guo, Z.; Xiong, H.; et al. The effects of cognitive behavioral therapy on resting-state functional brain network in drug-naive patients with obsessive-compulsive disorder. Brain Behav. 2018, 8, e00963. [Google Scholar] [CrossRef] [PubMed]
- Hojjati, S.H.; Ebrahimzadeh, A.; Khazaee, A.; Babajani-Feremi, A. Predicting conversion from MCI to AD by integrating rs-fMRI and structural MRI. Comput. Biol. Med. 2018, 102, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Cheng, Y.; Ping, L.; Xu, J.; Shen, Z.; Jiang, L.; Shi, L.; Yang, S.; Lu, Y.; Xu, X. Support Vector Machine Classification of Obsessive-Compulsive Disorder Based on Whole-Brain Volumetry and Diffusion Tensor Imaging. Front. Psychiatry 2018, 9, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reess, T.J.; Rus, O.G.; Gursel, D.A.; Schmitz-Koep, B.; Wagner, G.; Berberich, G.; Koch, K. Network-based decoupling of local gyrification in obsessive-compulsive disorder. Hum. Brain Mapp. 2018, 39, 3216–3226. [Google Scholar] [CrossRef] [Green Version]
- Nakao, T.; Okada, K.; Kanba, S. Neurobiological model of obsessive-compulsive disorder: Evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin. Neurosci. 2014, 68, 587–605. [Google Scholar] [CrossRef]
- Gunturkun, O.; Strockens, F.; Ocklenburg, S. Brain Lateralization: A Comparative Perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef]
- Maril, S.; Hermesh, H.; Gross-Isseroff, R.; Tomer, R. Spatial attention and neural asymmetry in obsessive-compulsive disorder. Psychiatry Res. 2007, 153, 189–193. [Google Scholar] [CrossRef]
- Rao, N.P.; Arasappa, R.; Reddy, N.N.; Venkatasubramanian, G.; Reddy, Y.C.J. Lateralisation abnormalities in obsessive-compulsive disorder: A line bisection study. Acta Neuropsychiatr. 2015, 27, 242–247. [Google Scholar] [CrossRef]
- Ischebeck, M.; Endrass, T.; Simon, D.; Kathmann, N. Altered frontal EEG asymmetry in obsessive-compulsive disorder. Psychophysiology 2014, 51, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Simpson, H.B.; Fallon, B.A.; Rossi, S.; Lisanby, S.H. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2010, 13, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Ahmari, S.E. A Framework for Understanding the Emerging Role of Corticolimbic-Ventral Striatal Networks in OCD-Associated Repetitive Behaviors. Front. Syst. Neurosci. 2015, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Sesack, S.R. GABA-Containing Neurons in the Rat Ventral Tegmental Area Project to the Prefrontal Cortex. Synapse 2000, 38, 114–123. [Google Scholar]
- Salamone, J.D.; Correa, M.; Farrar, A.; Mingote, S.M. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 2007, 191, 461–482. [Google Scholar] [CrossRef]
- Boedhoe, P.S.W.; van Rooij, D.; Hoogman, M.; Twisk, J.W.R.; Schmaal, L.; Abe, Y.; Alonso, P.; Ameis, S.H.; Anikin, A.; Anticevic, A.; et al. Subcortical Brain Volume, Regional Cortical Thickness, and Cortical Surface Area Across Disorders: Findings From the ENIGMA ADHD, ASD, and OCD Working Groups. Am. J. Psychiatry 2020, 177, 834–843. [Google Scholar] [CrossRef]
- Syversen, I.F.; Witter, M.P.; Kobro-Flatmoen, A.; Goa, P.E.; Navarro Schroder, T.; Doeller, C.F. Structural connectivity-based segmentation of the human entorhinal cortex. Neuroimage 2021, 245, 118723. [Google Scholar] [CrossRef]
- Nebli, A.; Rekik, I. Gender differences in cortical morphological networks. Brain Imaging Behav. 2020, 14, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.; Calhoun, V.D.; van Erp, T.G.M.; Bustillo, J.; Damaraju, E.; Turner, J.A.; Du, Y.; Yang, J.; Chen, J.; Yu, Q.; et al. Multimodal Fusion With Reference: Searching for Joint Neuromarkers of Working Memory Deficits in Schizophrenia. IEEE Trans. Med. Imaging 2018, 37, 93–105. [Google Scholar] [CrossRef] [Green Version]


| Session | Main Technique | Week |
|---|---|---|
| The 1st–2nd session | Therapeutic alliance establishment, information collection and assessment, psychoeducation, and normalization. | The 1st week |
| The 3rd–6th session | Case conceptualization, identification of cognitive distortion, plan and implementation of behavioral experiments, and challenging and correction of the distorted cognitions, including on-site and homework exercises. | The 2nd–4th week |
| The 7th–12th session | Introduction of exposure, creation of an anxiety hierarchy and planning of exposure, and the conduct of ERP practice, including therapist-assisted and self-administered practice. | The 5th–10th week |
| The 13th–14th session | Treatment review to consolidate treatment effects and prevent relapse. | The 11th–12th week |
| Characteristics | Baseline (n = 56) | 12 Weeks (n = 56) | p Value (Paired−t Test) |
|---|---|---|---|
| Age | 28.02 ± 6.7 | -- | |
| Gender (male/female) | 37/19 | -- | |
| Education level (years) | 15.5 ± 2.3 | -- | |
| Illness duration | 10.35 ± 7.5 | -- | |
| Y−BOCS score | |||
| Total | 23.43 ± 5.84 | 10.68± 6.86 | <0.001 |
| Obsession | 12.8 ± 4.6 | 5.3 ± 3.9 | <0.001 |
| Compulsion | 11.3 ± 4.1 | 5.4 ± 3.2 | <0.001 |
| HAMD−17 score | 6.29 ± 4.06 | 2.32 ± 2.83 | <0.001 |
| HAMA score | 8.09 ± 5.89 | 2.61 ± 3.33 | <0.001 |
| Selected Features | Brain Regions | MAE | MSE | R2 |
|---|---|---|---|---|
| GMV | Right superior frontal gyrus−medial Left Heschl’s gyrus Left temporal pole: superior temporal gyrus Left lobule X of the cerebellar hemisphere Left lateral geniculate Right anterior cingulate cortex−subgenual Left ventral tegmental area Right ventral tegmental area Right lobule III of vermis Right raphe nucleus–median | 0.18 | 0.05 | −0.02 |
| Cortical thickness | Left caudal middle frontal Right entorhinal Left fusiform Right fusiform Left isthmus cingulate Left lingual Right lingual Left parahippocampal Left transverse temporal Right transverse temporal | 0.17 | 0.04 | 0.12 |
| Gyrification value | Right fusiform Right isthmus cingulate Right lateral occipital Left lateral orbitofrontal Right paracentral Right pericalcarine Right posterior cingulate Left superior frontal Left temporal pole Left transverse temporal | 0.18 | 0.05 | −0.03 |
| Sulcal depth | Left cuneus Left entorhinal Left fusiform Left lateral occipital Left lingual Left parahippocampal Right parsopercularis Left pericalcarine Left rostral middle frontal | 0.18 | 0.05 | −0.02 |
| Comprehensive variables | 0.14 | 0.03 | 0.36 | |
| GMV | Left CER10 Left VTA Right VTA | |||
| Cortical thickness | Right entorhinal | |||
| Gyrification value | Right isthmus cingulate | |||
| Sulcal depth | Left superior frontal Left fusiform Left lateral occipital Left parahippocampal Left rostral middle frontal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-Y.; Liu, R.; Luo, J.; Huang, F.-F.; Wang, P.-C.; Yang, X.-J.; Wu, H.; Zhou, Y.; Li, Z.-J. Comprehensive Cortical Structural Features Predict the Efficacy of Cognitive Behavioral Therapy in Obsessive-Compulsive Disorder. Brain Sci. 2022, 12, 921. https://doi.org/10.3390/brainsci12070921
Yang X-Y, Liu R, Luo J, Huang F-F, Wang P-C, Yang X-J, Wu H, Zhou Y, Li Z-J. Comprehensive Cortical Structural Features Predict the Efficacy of Cognitive Behavioral Therapy in Obsessive-Compulsive Disorder. Brain Sciences. 2022; 12(7):921. https://doi.org/10.3390/brainsci12070921
Chicago/Turabian StyleYang, Xiang-Yun, Rui Liu, Jia Luo, Fang-Fang Huang, Peng-Chong Wang, Xiao-Jie Yang, Hang Wu, Yuan Zhou, and Zhan-Jiang Li. 2022. "Comprehensive Cortical Structural Features Predict the Efficacy of Cognitive Behavioral Therapy in Obsessive-Compulsive Disorder" Brain Sciences 12, no. 7: 921. https://doi.org/10.3390/brainsci12070921






