Dynamic Prediction of Mechanical Thrombectomy Outcome for Acute Ischemic Stroke Patients Using Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analyses
2.3. Feature Selection
2.4. Model Development
2.5. Model Evaluation
3. Results
3.1. Study Population
3.2. Feature Selection
3.3. Model Performance
3.3.1. “Admission” Model
3.3.2. “24-Hour” Model
3.3.3. The “3-Day” Model
3.3.4. “Discharge” Model
| Testing Data | Statistical Analysis | ||||
|---|---|---|---|---|---|
| True Predicted | 0 | 1 | Total | Accuracy | 0.750 |
| 0 | 8 | 7 | 15 | Precision | 0.800 |
| 1 | 5 | 28 | 33 | Sensitivity | 0.848 |
| Total | 13 | 35 | 48 | Specificity | 0.533 |
| AUC | 0.824 | ||||
| Testing Data | Statistical Analysis | ||||
|---|---|---|---|---|---|
| True Predicted | 0 | 1 | Total | Accuracy | 0.792 |
| 0 | 12 | 3 | 15 | Precision | 0.897 |
| 1 | 7 | 26 | 33 | Sensitivity | 0.788 |
| Total | 19 | 29 | 48 | Specificity | 0.800 |
| AUC | 0.891 | ||||
| Testing Data | Statistical Analysis | ||||
|---|---|---|---|---|---|
| True Predicted | 0 | 1 | Total | Accuracy | 0.812 |
| 0 | 14 | 1 | 15 | Precision | 0.962 |
| 1 | 8 | 25 | 33 | Sensitivity | 0.758 |
| Total | 22 | 26 | 48 | Specificity | 0.933 |
| AUC | 0.931 | ||||
| Testing Data | Statistical Analysis | ||||
|---|---|---|---|---|---|
| True Predicted | 0 | 1 | Total | Accuracy | 0.875 |
| 0 | 12 | 3 | 15 | Precision | 0.909 |
| 1 | 3 | 30 | 33 | Sensitivity | 0.909 |
| Total | 17 | 31 | 48 | Specificity | 0.800 |
| AUC | 0.945 | ||||
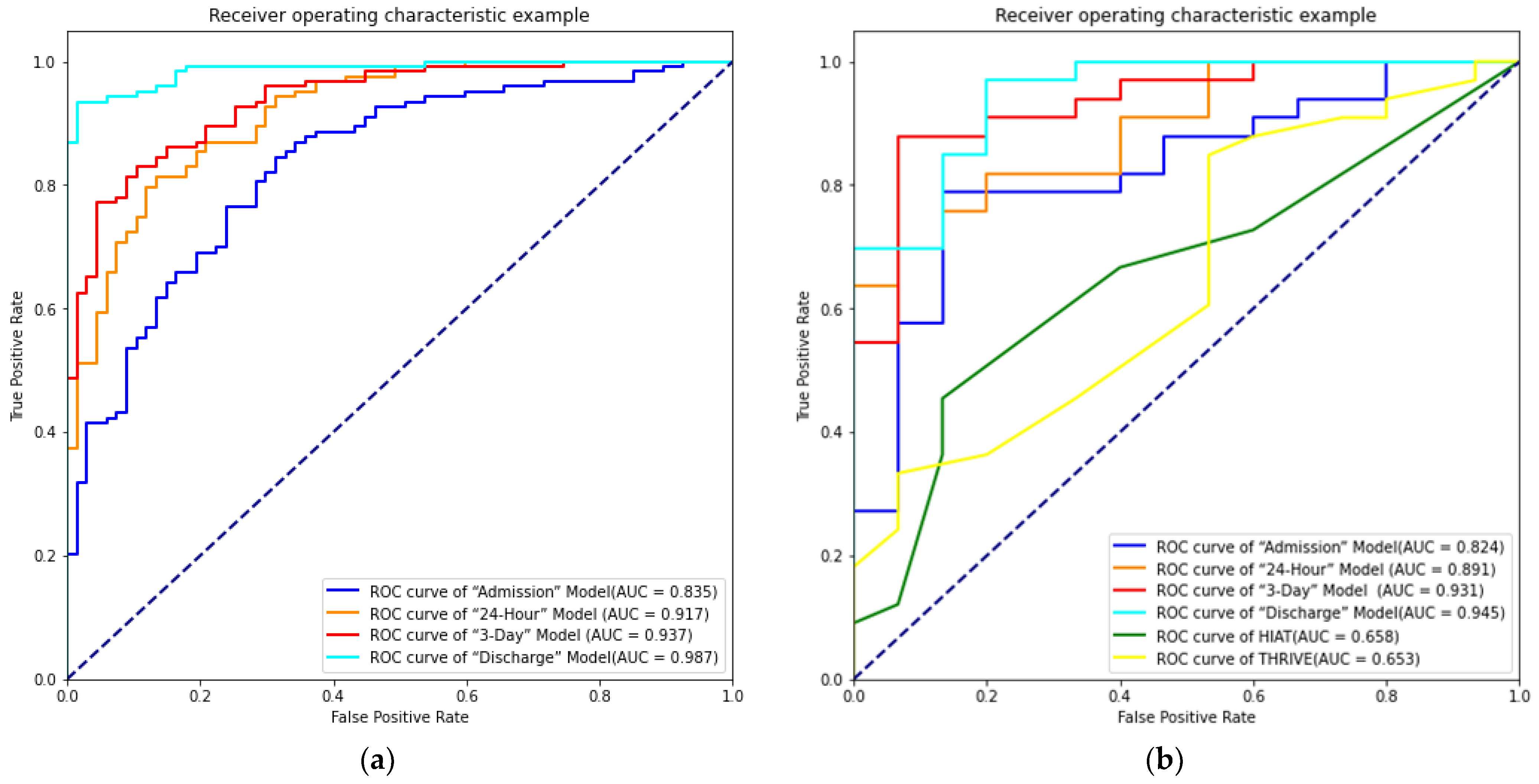
3.4. Model Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Goyal, M.; van der Lugt, A.; Menon, B.K.; Majoie, C.B.L.M.; Dippel, D.W.; Campbell, B.C.; Nogueira, R.G.; Demchuk, A.M.; Tomasello, A.; et al. Time to Treatment With Endovascular Thrombectomy and Outcomes from Ischemic Stroke: A Meta-analysis. JAMA 2016, 316, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, W.J. BEST evidence on mechanical thrombectomy for patients with vertebrobasilar occlusion. Lancet Neurol. 2020, 19, 102–103. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; De Miquel, M.A.; Molina, C.A.; Rovira, A.; Román, L.S.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [Green Version]
- Singer, O.C.; Berkefeld, J.; Nolte, C.; Bohner, G.; Haring, H.-P.; Trenkler, J.; Gröschel, K.; Müller-Forell, W.; Niederkorn, K.; Deutschmann, H.; et al. Mechanical recanalization in basilar artery occlusion: The ENDOSTROKE study. Ann. Neurol. 2015, 77, 415–424. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97 (Suppl. 2), S6–S16. [Google Scholar] [CrossRef]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef] [Green Version]
- Flint, A.C.; Cullen, S.P.; Faigeles, B.S.; Rao, V.A. Predicting long-term outcome after endovascular stroke treatment: The totaled health risks in vascular events score. AJNR Am. J. Neuroradiol. 2010, 31, 1192–1196. [Google Scholar] [CrossRef] [Green Version]
- . Almekhlafi, M.; Davalos, A.; Bonafe, A.; Chapot, R.; Gralla, J.; Pereira, V.; Goyal, M. Impact of age and baseline NIHSS scores on clinical outcomes in the mechanical thrombectomy using solitaire FR in acute ischemic stroke study. AJNR Am. J. Neuroradiol. 2014, 35, 1337–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hametner, C.; Kellert, L.; Ringleb, P.A. Impact of sex in stroke thrombolysis: A coarsened exact matching study. BMC Neurol. 2015, 15, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linfante, I.; Starosciak, A.K.; Walker, G.R.; Dabus, G.; Castonguay, A.C.; Gupta, R.; Sun, C.-H.J.; Martin, C.; Holloway, W.E.; Mueller-Kronast, N.; et al. Predictors of poor outcome despite recanalization: A multiple regression analysis of the NASA registry. J. Neurointerv. Surg. 2016, 8, 224–229. [Google Scholar] [CrossRef]
- Hallevi, H.; Barreto, A.D.; Liebeskind, D.S.; Morales, M.M.; Martin-Schild, S.B.; Abraham, A.T.; Gadia, J.; Saver, J.L.; Grotta, J.C.; Savitz, S.I.; et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke 2009, 40, 1780–1785. [Google Scholar] [CrossRef] [Green Version]
- Liggins, J.T.P.; Yoo, A.J.; Mishra, N.; Wheeler, H.M.; Straka, M.; Leslie-Mazwi, T.M.; Chaudhry, Z.A.; Kemp, S.; Mlynash, M.; Bammer, R.; et al. A score based on age and DWI volume predicts poor outcome following endovascular treatment for acute ischemic stroke. Int. J. Stroke 2015, 10, 705–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansberg, M.G.; Christensen, S.; Kemp, S.; Mlynash, M.; Mishra, N.; Federau, C.; Tsai, J.P.; Kim, S.; Nogueria, R.G.; Jovin, T.; et al. Computed tomographic perfusion to Predict Response to Recanalization in ischemic stroke. Ann. Neurol. 2017, 81, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Elijovich, L.; Goyal, N.; Mainali, S.; Hoit, D.; Arthur, A.S.; Whitehead, M.; Choudhri, A.F. CTA collateral score predicts infarct volume and clinical outcome after endovascular therapy for acute ischemic stroke: A retrospective chart review. J. Neurointerv. Surg. 2016, 8, 559–562. [Google Scholar] [CrossRef]
- Rangaraju, S.; Liggins, J.T.; Aghaebrahim, A.; Streib, C.; Sun, C.; Gupta, R.; Nogueira, R.; Frankel, M.; Mlynash, M.; Lansberg, M.; et al. Pittsburgh outcomes after stroke thrombectomy score predicts outcomes after endovascular therapy for anterior circulation large vessel occlusions. Stroke 2014, 45, 2298–2304. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, K.P.; Hathidara, M.Y.; Danala, G.; Xu, C.; McCoy, T.M.; Sidorov, E.V.; Zheng, B.; Bohnstedt, B.N.; Ray, B. Corrigendum to: Predicting Clinical Outcome After Mechanical Thrombectomy: The GADIS (Gender, Age, Diabetes Mellitus History, Infarct Volume, and Current Smoker) Score [World Neurosurgery 134 (2020) e1130-e1142]. World Neurosurg. 2020, 138, 606. [Google Scholar] [CrossRef]
- Li, X.; Zou, Y.; Hu, J.; Li, X.M.; Huang, C.P.; Shan, Y.J.; Nyame, L.; Zhao, Z.; Sun, C.; Ibrahim, M.; et al. A NAC nomogram to predict the probability of three-month unfavorable outcome in Chinese acute ischemic stroke patients treated with mechanical thrombectomy. Int. J. Neurosci. 2021, 131, 163–169. [Google Scholar] [CrossRef]
- Cappellari, M.; Mangiafico, S.; Saia, V.; Pracucci, G.; Nappini, S.; Nencini, P.; Konda, D.; Sallustio, F.; Vallone, S.; Zini, A.; et al. IER-START nomogram for prediction of three-month unfavorable outcome after thrombectomy for stroke. Int. J. Stroke 2020, 15, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Zeng, W.H.; Cheng, C.Y.; Chen, S.H.; Lin, C.R. Using a Multiclass Machine Learning Model to Predict the Outcome of Acute Ischemic Stroke Requiring Reperfusion Therapy. Diagnostics 2021, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Oishi, N.; Ishii, A.; Ono, I.; Ogura, T.; Sunohara, T.; Chihara, H.; Fukumitsu, R.; Okawa, M.; Yamana, N.; et al. Predicting Clinical Outcomes of Large Vessel Occlusion before Mechanical Thrombectomy Using Machine Learning. Stroke 2019, 50, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Van Os, H.J.A.; Ramos, L.A.; Hilbert, A.; van Leeuwen, M.; Van Walderveen, M.A.A.; Kruyt, N.D.; Dippel, D.W.J.; Steyerberg, E.W.; Van Der Schaaf, I.C.; Lingsma, H.F.; et al. Predicting Outcome of Endovascular Treatment for Acute Ischemic Stroke: Potential Value of Machine Learning Algorithms. Front. Neurol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Asadi, H.; Dowling, R.; Yan, B.; Mitchell, P. Machine learning for outcome prediction of acute ischemic stroke post intra-arterial therapy. PLoS ONE 2014, 9, e88225. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.M. A multi-scale fusion CNN model based on adaptive transfer learning for multi-class MI-classification in BCI system. BioRxiv 2022. [Google Scholar] [CrossRef]
- Sharma, R.; Kim, M.; Gupta, A. Motor imagery classification in brain-machine interface with machine learning algorithms: Classical approach to multi-layer perceptron model. Biomed. Signal. Process. 2022, 71, 103101. [Google Scholar] [CrossRef]
- Kapgate, D. Efficient Quadcopter Flight Control Using Hybrid SSVEP + P300 Visual Brain Computer Interface. Int. J. Hum-Comput Int. 2022, 38, 42–52. [Google Scholar] [CrossRef]
- Watts, J.; Khojandi, A.; Shylo, O.; Ramdhani, R.A. Machine Learning’s Application in Deep Brain Stimulation for Parkinson’s Disease: A Review. Brain Sci. 2020, 10, 809. [Google Scholar] [CrossRef]
- Thorsen-Meyer, H.C.; Nielsen, A.B.; Nielsen, A.P.; Kaas-Hansen, B.S.; Toft, P.; Schierbeck, J.; Strøm, T.; Chmura, P.J.; Heimann, M.; Dybdahl, L.; et al. Dynamic and explainable machine learning prediction of mortality in patients in the intensive care unit: A retrospective study of high-frequency data in electronic patient records. Lancet Digit Health 2020, 2, e179–e191. [Google Scholar] [CrossRef]
- Khera, R.; Haimovich, J.; Hurley, N.C.; McNamara, R.; Spertus, J.A.; Desai, N.; Rumsfeld, J.S.; Masoudi, F.A.; Huang, C.; Normand, S.-L.; et al. Use of Machine Learning Models to Predict Death after Acute Myocardial Infarction. JAMA Cardiol. 2021, 6, 633–641. [Google Scholar] [CrossRef] [PubMed]
- von Kummer, R.; Broderick, J.P.; Campbell, B.C.V.; Demchuk, A.; Goyal, M.; Hill, M.D.; Treurniet, K.M.; Majoie, C.B.L.M.; Marquering, H.A.; Mazya, M.V.; et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events after Ischemic Stroke and Reperfusion Therapy. Stroke 2015, 46, 2981–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Han, D.; Huang, T.; Zhang, X.; Lu, H.; Shen, S.; Lyu, J.; Wang, H. Predicting ICU Mort.tality in Rheumatic Heart Disease: Comparison of XGBoost and Logistic Regression. Front. Cardiovasc. Med. 2022, 9, 847206. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Peterson, T.A.; Seo, K.Y.; Han, H.W.; Kang, J.I. Discovery of Depression-Associated Factors from a Nationwide Population-Based Survey: Epidemiological Study Using Machine Learning and Network Analysis. J. Med. Internet Res. 2021, 23, e27344. [Google Scholar] [CrossRef]
- Hou, N.; Li, M.; He, L.; Xie, B.; Wang, L.; Zhang, R.; Yu, Y.; Sun, X.; Pan, Z.; Wang, K. Predicting 30-days mortality for MIMIC-III patients with sepsis-3: A machine learning approach using XGboost. J. Transl. Med. 2020, 18, 462. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Selvarajah, S.; Kaur, G.; Haniff, J.; Cheong, K.C.; Hiong, T.G.; van der Graaf, Y.; Bots, M.L. Comparison of the Framingham Risk Score, SCORE and WHO/ISH cardiovascular risk prediction models in an Asian population. Int. J. Cardiol. 2014, 176, 211–218. [Google Scholar] [CrossRef] [Green Version]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Maldaner, N.; Zeitlberger, A.M.; Sosnova, M.; Goldberg, J.; Fung, C.; Bervini, D.; May, A.; Bijlenga, P.; Schaller, K.; Roethlis-berger, M.; et al. Development of a Complication- and Treatment-Aware Prediction Model for Favorable Functional Outcome in Aneurysmal Subarachnoid Hemorrhage Based on Machine Learning. Neurosurgery 2021, 88, E150–E157. [Google Scholar] [CrossRef]
- Reznik, M.E.; Yaghi, S.; Jayaraman, M.V.; McTaggart, R.A.; Hemendinger, M.; Mac Grory, B.C.; Burton, T.M.; Cutting, S.M.; Siket, M.S.; Madsen, T.E.; et al. Baseline NIH Stroke Scale is an inferior predictor of functional outcome in the era of acute stroke intervention. Int. J. Stroke 2018, 13, 806–810. [Google Scholar] [CrossRef]
- Jeong, H.-G.; Kim, B.J.; Choi, J.C.; Hong, K.-S.; Yang, M.H.; Jung, C.; Han, M.-K.; Bae, H.-J. Posttreatment National Institutes of Health Stroke Scale Is Superior to the Initial Score or Thrombolysis in Cerebral Ischemia for 3-Month Outcome. Stroke 2018, 49, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Fonseca, A.C.; Freitas, A.T.; e Melo, T.P.; Francisco, A.P.; Ferro, J.M.; Oliveira, A.L. Using Machine Learning to Improve the Prediction of Functional Outcome in Ischemic Stroke Patients. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 15, 1953–1959. [Google Scholar] [CrossRef] [PubMed]

| Variables | Favorable Outcome (mRS 0–2) | Unfavorable Outcome (mRS 3–6) | p-Value |
|---|---|---|---|
| Patients, n | 82 | 156 | |
| Demographics | |||
| Age, years, median (IQR) | 65 (58–74) | 72 (60–81) | 0.001 * |
| Sex, n (%) | 0.592 | ||
| Male | 59 (72) | 107 (68.6) | |
| Female | 23 (28) | 49 (31.4) | |
| Smoking, n (%) | 28 (34.1) | 49 (31.4) | 0.668 |
| Medical history, n (%) | |||
| Transient ischemic attack | 0 (0) | 3 (1.9) | 0.110 * |
| Previous cerebral infarction | 12 (14.6) | 29 (18.6) | 0.443 |
| Previous cerebral hemorrhage | 1 (1.2) | 6 (3.8) | 0.462 |
| Diabetes mellitus | 18 (22) | 31 (19.9) | 0.706 |
| Hypertension | 57 (69.5) | 108 (69.5) | 0.964 |
| Hyperlipidemia | 7 (8.5) | 12 (7.7) | 0.819 |
| Coronary artery disease | 15 (18.3) | 44 (28.2) | 0.092 * |
| Atrial fibrillation | 25 (30.5) | 58 (37.2) | 0.303 |
| Baseline data | |||
| NIHSS score on admission, median (IQR) | 11 (7–16) | 17 (13–22) | <0.0001 * |
| Systolic pressure, mmHg, median (IQR) | 138 (124–155) | 142 (129–160) | 0.299 |
| Diastolic pressure, mmHg, median (IQR) | 83 (74–93) | 86 (76–99) | 0.154 * |
| INR, median (IQR) | 0.98 (0.93–1.09) | 1.02 (0.935–1.12) | 0.092 * |
| HbA1c, mmol/L, median (IQR) | 5.80 (5.50–6.53) | 5.90 (5.50–6.50) | 0.856 |
| TC, mmol/L, median (IQR) | 4.32 (3.43–4.98) | 4.08 (3.43–4.83) | 0.300 |
| TG, mmol/L, median (IQR) | 1.12 (0.82–1.68) | 1.05 (0.76–1.47) | 0.205 |
| LDL, mmol/L, median (IQR) | 2.66 (1.98–3.24) | 2.23 (1.80–2.83) | 0.019 * |
| FBG, mmol/L, median (IQR) | 6.04 (5.08–7.35) | 6.48 (5.60–7.99) | 0.028 * |
| PLT, μmol/L, median (IQR) | 193.00 (150.75–235.50) | 172.5 (143.00–212.50) | 0.017 * |
| UA, μmol/L, median (IQR) | 284.90 (233.00–357.00) | 313.50 (232.32–396.75) | 0.125 * |
| HCY, μmol/L, median (IQR) | 12.46 (10.70–16.76) | 13.18 (10.97–16.64) | 0.433 |
| Creatinine, μmol/L, median (IQR) | 67.00 (58.37–77.00) | 78.00 (61.63–94.00) | 0.003 * |
| Anterior circulation stroke, n (%) | 60 (73.2) | 126 (80.8) | 0.178 * |
| Posterior circulation stroke, n (%) | 22 (26.8) | 30 (19.2) | 0.178 * |
| TOAST classification, n (%) | 0.119 * | ||
| Large artery atherosclerosis | 47 (57.3) | 69 (44.2) | |
| Cardioembolism | 32 (39.0) | 75 (48.1) | |
| Others | 3 (3.7) | 12 (7.7) | |
| Interventional characteristics | |||
| Interval from groin puncture to recanalization, min, median (IQR) | 60 (50–85) | 81 (59–130) | 0.004 * |
| Interval from onset to treatment, min, median (IQR) | 290 (230–411) | 280 (206–413) | 0.240 |
| Endovascular therapy, n (%) | 0.144 * | ||
| Tirofiban | 29 (35.4) | 41 (26.3) | |
| No tirofiban | 53 (64.6) | 115 (73.7) | |
| IV thrombolysis, n (%) | 0.806 | ||
| No thrombolysis | 45 (54.9) | 83 (53.2) | |
| Thrombolysis | 37 (45.1) | 73 (46.8) | |
| Post interventional characteristics | |||
| sICH, n (%) | 0 (0) | 18 (11.5) | 0.001 * |
| NIHSS score after 24-hour, median (IQR) | 5 (2–10) | 17 (12–31) | <0.0001 * |
| NIHSS score after 3-day, median (IQR) | 3 (2–7) | 18 (10–34) | <0.0001 * |
| NIHSS score on discharge, median (IQR) | 2 (1–3) | 16 (8–34) | <0.0001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Yang, T.; Zhang, J.; Wang, X.; Cui, X.; Chen, N.; Zhou, J.; Jiang, F.; Zhu, J.; Zou, J. Dynamic Prediction of Mechanical Thrombectomy Outcome for Acute Ischemic Stroke Patients Using Machine Learning. Brain Sci. 2022, 12, 938. https://doi.org/10.3390/brainsci12070938
Hu Y, Yang T, Zhang J, Wang X, Cui X, Chen N, Zhou J, Jiang F, Zhu J, Zou J. Dynamic Prediction of Mechanical Thrombectomy Outcome for Acute Ischemic Stroke Patients Using Machine Learning. Brain Sciences. 2022; 12(7):938. https://doi.org/10.3390/brainsci12070938
Chicago/Turabian StyleHu, Yixing, Tongtong Yang, Juan Zhang, Xixi Wang, Xiaoli Cui, Nihong Chen, Junshan Zhou, Fuping Jiang, Junrong Zhu, and Jianjun Zou. 2022. "Dynamic Prediction of Mechanical Thrombectomy Outcome for Acute Ischemic Stroke Patients Using Machine Learning" Brain Sciences 12, no. 7: 938. https://doi.org/10.3390/brainsci12070938
APA StyleHu, Y., Yang, T., Zhang, J., Wang, X., Cui, X., Chen, N., Zhou, J., Jiang, F., Zhu, J., & Zou, J. (2022). Dynamic Prediction of Mechanical Thrombectomy Outcome for Acute Ischemic Stroke Patients Using Machine Learning. Brain Sciences, 12(7), 938. https://doi.org/10.3390/brainsci12070938





