Effects of Stimulus Frequency, Intensity, and Sex on the Autonomic Response to Transcutaneous Vagus Nerve Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Transcutaneous Vagus Nerve Stimulation (tVNS)
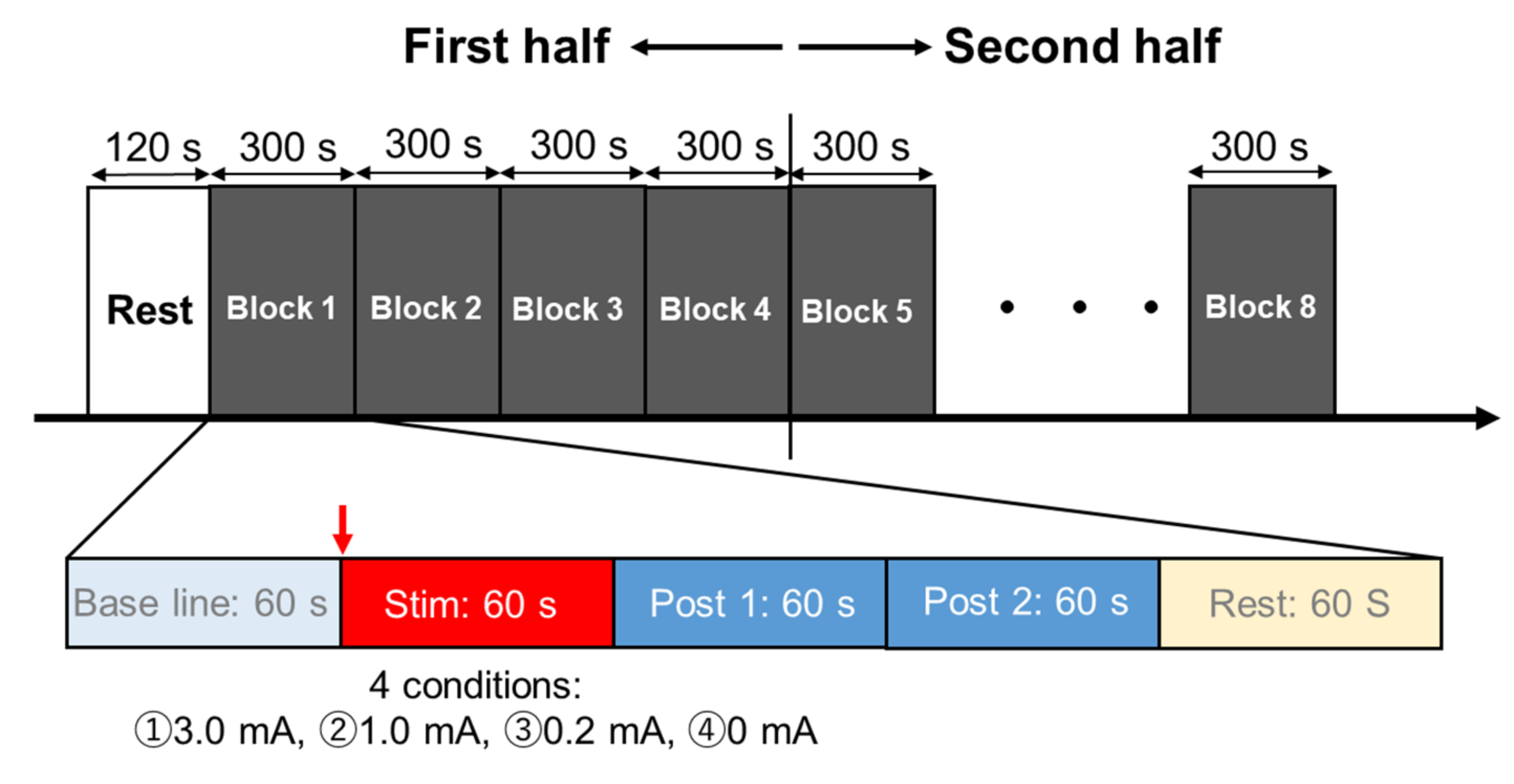
2.3. Experimental Procedures
2.4. Electrocardiogram
2.5. Data and Statistical Analyses
3. Results
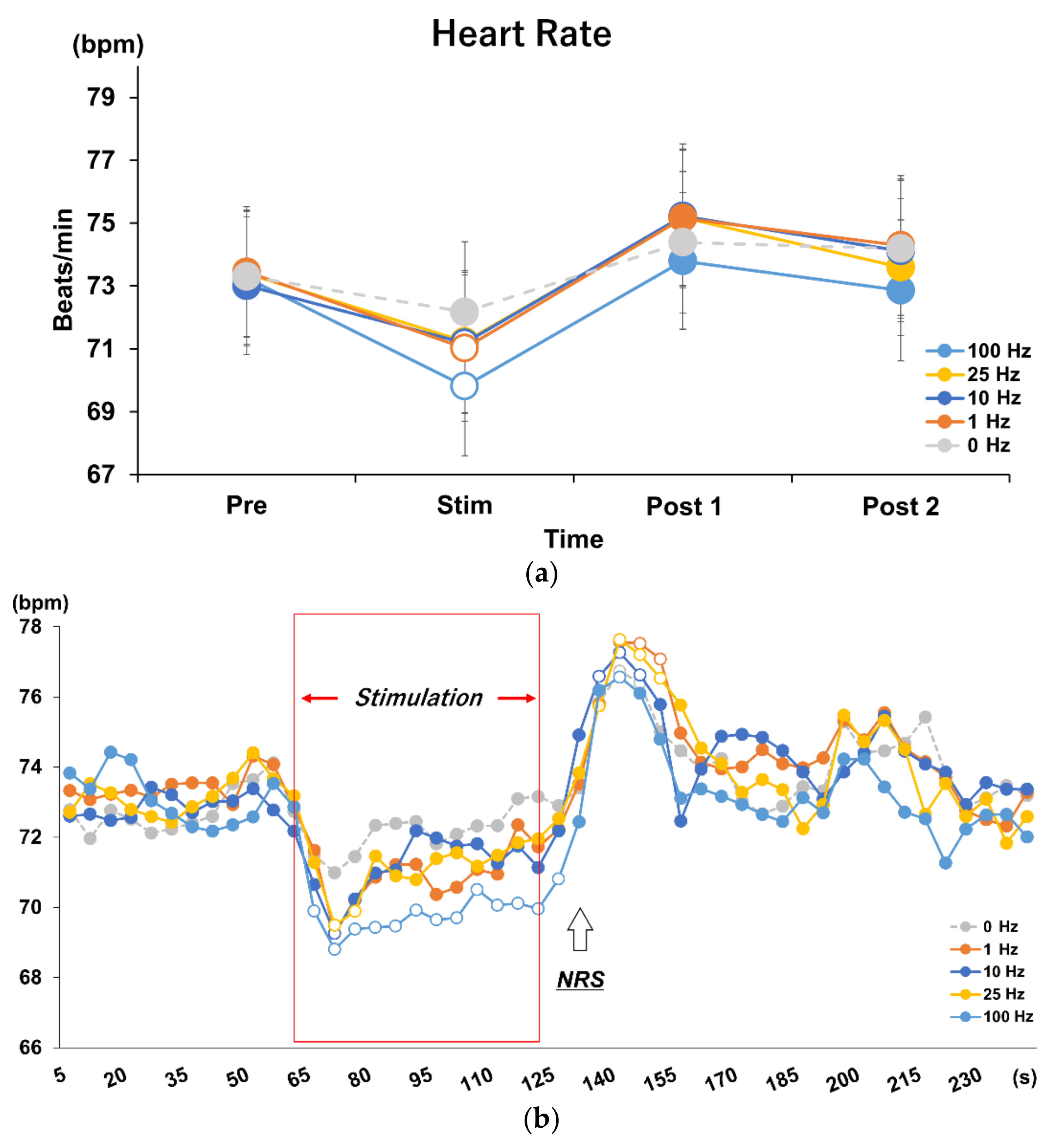
3.1. Effects of Different Stimulus Frequencies of tVNS on HR and HRV
3.2. Time Course Analysis on Every 5 s in Each Frequency Condition
3.3. Effects of Different Stimulus Intensities of tVNS on HR and HRV
3.4. Time Course Analysis on Every 5 s in Each Stimulus Intensity Condition
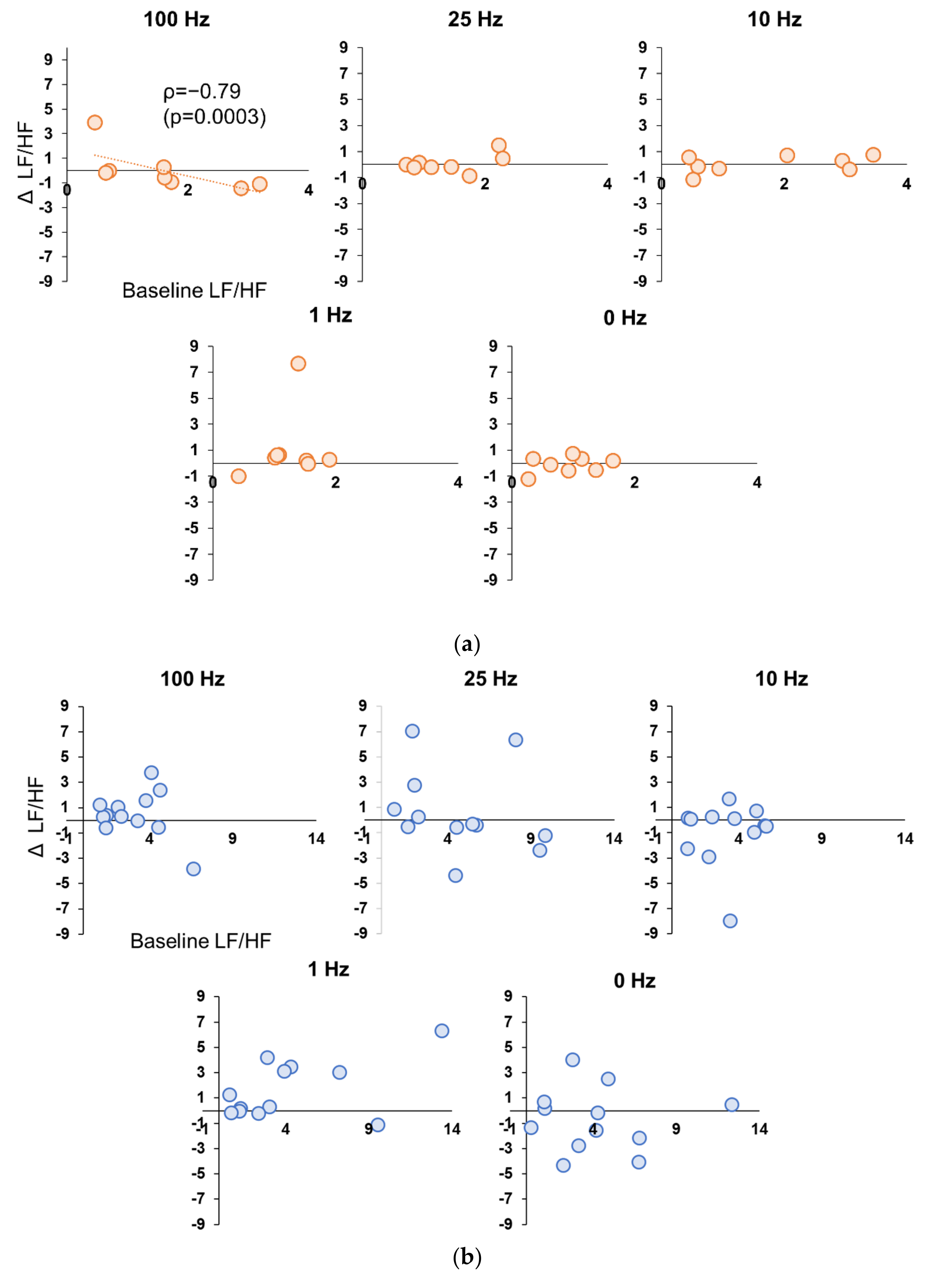
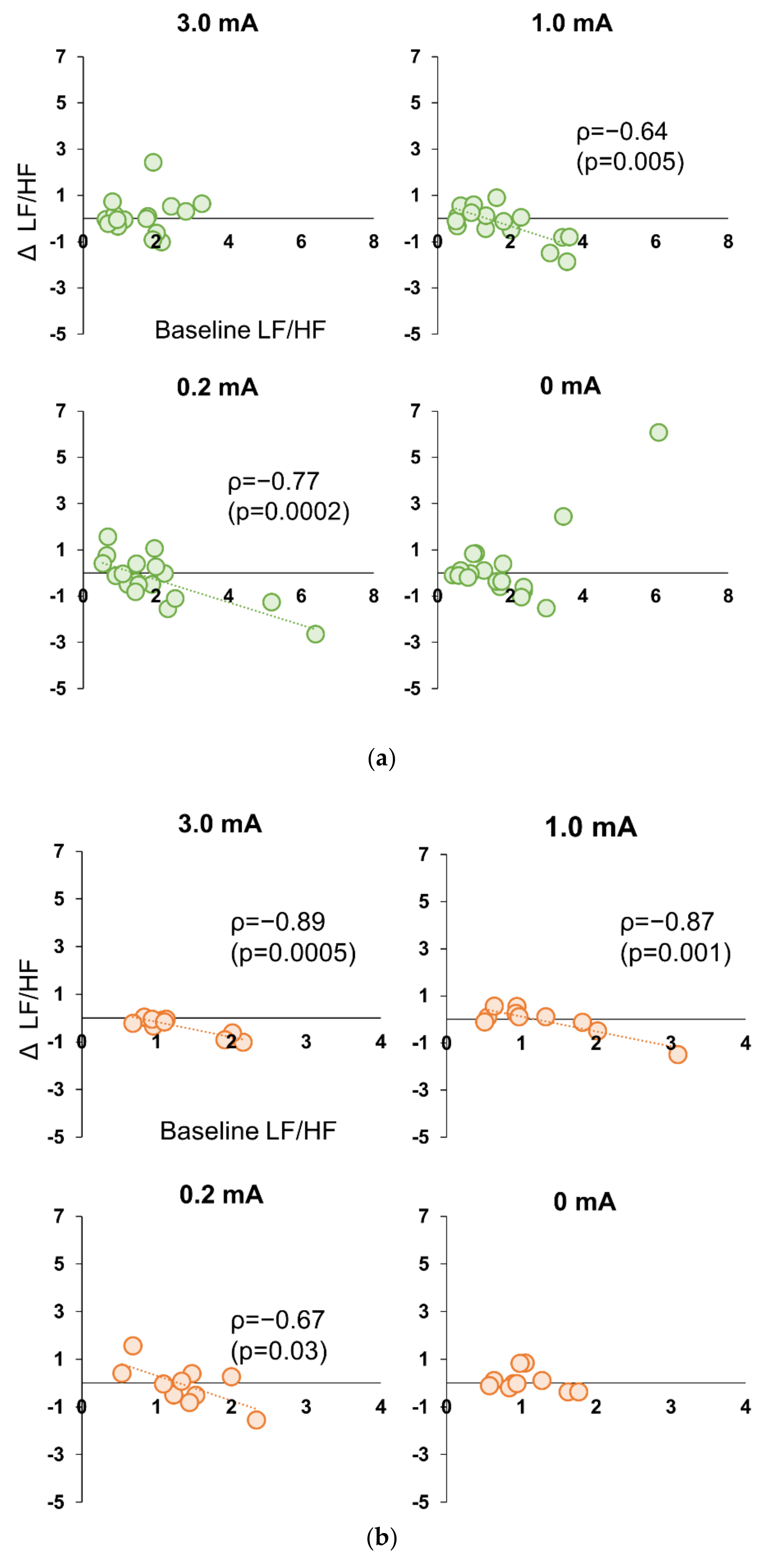
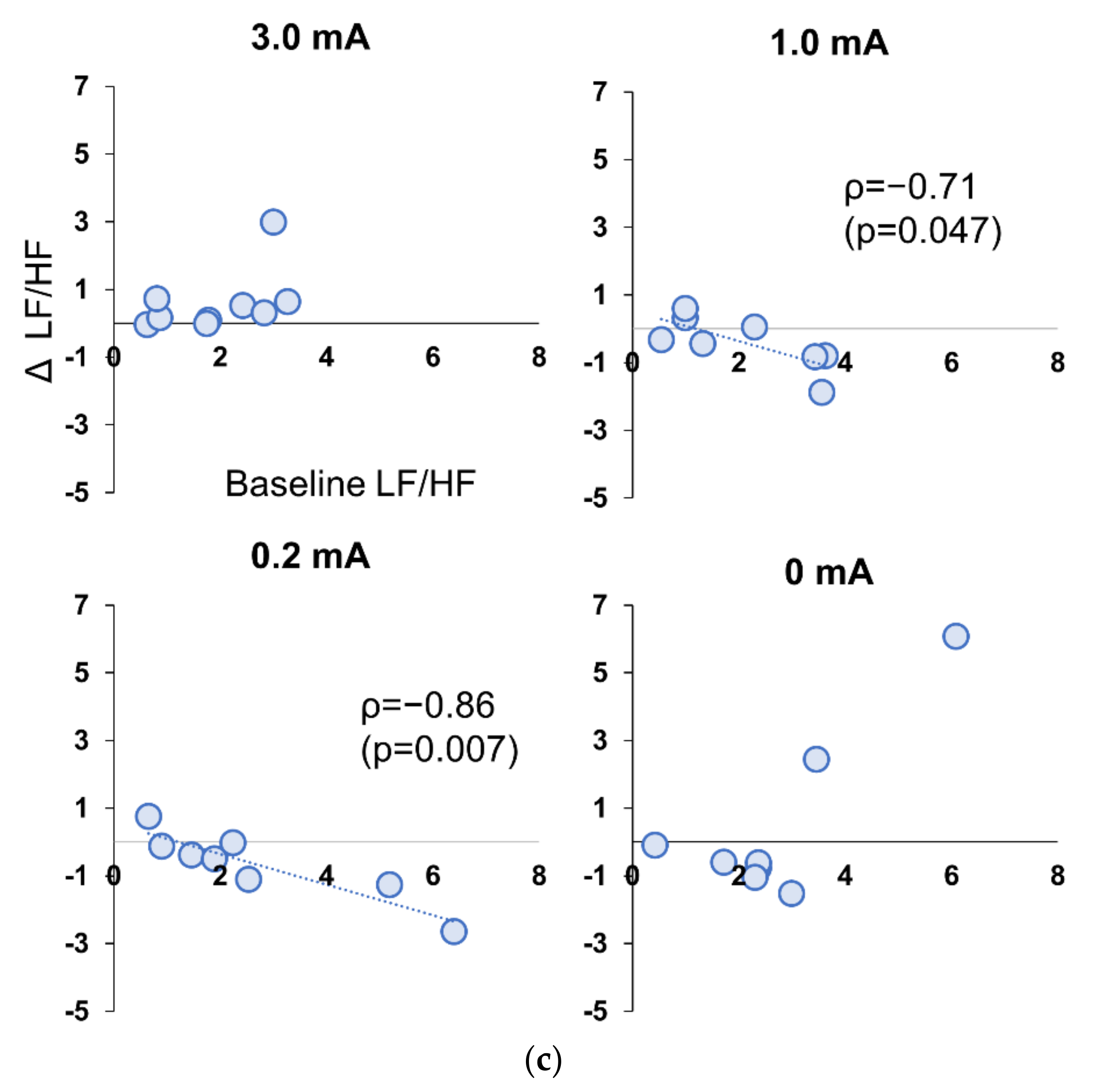
3.5. Correlation between Baseline and Stimulation Period in Experiment 1 and 2
3.6. Numerical Rating Scales: NRS in Subjective Discomfort Among Stimulus Conditions in Experiment 1
3.7. Perceptual Threshold and NRS in Subjective Discomfort among Stimulus Conditions in Experiment 2
3.8. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buschman, H.P.; Storm, C.J.; Duncker, D.J.; Verdouw, P.D.; van der Aa, H.E.; van der Kemp, P. Heart rate control via vagus nerve stimulation. Neuromodulation 2006, 9, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hachem, L.D.; Wong, S.M.; Ibrahim, G.M. The vagus afferent network: Emerging role in translational connectomics. Neurosurg. Focus 2018, 45, E2. [Google Scholar] [CrossRef] [PubMed]
- Toffa, D.H.; Touma, L.; El Meskine, T.; Bouthillier, A.; Nguyen, D.K. Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: A critical review. Seizure 2020, 83, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Austelle, C.W.; O'Leary, G.H.; Thompson, S.; Gruber, E.; Kahn, A.; Manett, A.J.; Short, B.; Badran, B.W. A comprehensive review of vagus nerve stimulation for depression. Neuromodulation 2022, 25, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Yuan, H.; Najib, U.; Ailani, J.; Morais, A.L.; Mathew, P.G.; Liebler, E.; Tassorelli, C.; Diener, H.C. Non-invasive vagus nerve stimulation for primary headache: A clinical update. Cephalalgia 2020, 40, 1370–1384. [Google Scholar] [CrossRef]
- Hays, S.A.; Rennaker, R.L.; Kilgard, M. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013, 207, 275–299. [Google Scholar]
- Kharbanda, R.K.; van der Does, W.F.B.; van Staveren, L.N.; Taverne, Y.J.H.J.; Bogers, A.J.J.C.; de Groot, N.M.S. Vagus nerve stimulation and atrial fibrillation: Revealing the paradox. Neuromodulation 2022, 25, 356–365. [Google Scholar] [CrossRef]
- Boezaart, A.; Botha, D.A. Treatment of stage 3 COVID-19 with transcutaneous auricular vagus nerve stimulation drastically reduces interleukin-6 blood levels: A report on two cases. Neuromodulation 2021, 24, 166–167. [Google Scholar] [CrossRef]
- Stavrakis, S.; Elkholey, K.; Morris, L.; Niewiadomska, M.; Asad, Z.U.A.; Humphrey, M.B. Neuromodulation of inflammation to treat heart failure with preserved ejection fraction: A pilot randomized clinical trial. J. Am. Heart Assoc. 2022, 11, e023582. [Google Scholar] [CrossRef]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A randomized clinical trial. JACC Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef]
- Clancy, J.A.; Mary, D.A.; Witte, K.K.; Greenwood, J.P.; Deuchars, S.A.; Deuchars, J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014, 7, 871–877. [Google Scholar] [CrossRef]
- Antonino, D.; Teixeira, A.L.; Maia-Lopes, P.M.; Souza, M.C.; Sabino-Carvalho, J.L.; Murray, A.R.; Deuchars, J.; Vianna, L.C. Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: A randomized placebo-controlled trial. Brain Stimul. 2017, 10, 875–881. [Google Scholar] [CrossRef]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef]
- Badran, B.W.; Dowdle, L.T.; Mithoefer, O.J.; LaBate, N.T.; Coatsworth, J.; Brown, J.C.; DeVries, W.H.; Austelle, C.W.; McTeague, L.M.; George, M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2018, 11, 492–500. [Google Scholar] [CrossRef]
- Badran, B.W.; Mithoefer, O.J.; Summer, C.E.; LaBate, N.T.; Glusman, C.E.; Badran, A.W.; DeVries, W.H.; Summers, P.M.; Austelle, C.W.; McTeague, L.M.; et al. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 2018, 11, 699–708. [Google Scholar] [CrossRef]
- De Couck, M.; Cserjesi, R.; Caers, R.; Zijlstra, W.P.; Widjaja, D.; Wolf, N.; Luminet, O.; Ellrich, J.; Gidron, Y. Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton. Neurosci. 2017, 203, 88–96. [Google Scholar] [CrossRef]
- Machetanz, K.; Berelidze, L.; Guggenberger, R.; Gharabaghi, A. Transcutaneous auricular vagus nerve stimulation and heart rate variability: Analysis of parameters and targets. Auton. Neurosci. 2021, 236, 102894. [Google Scholar] [CrossRef]
- Borges, U.; Laborde, S.; Raab, M. Influence of transcutaneous vagus nerve stimulation on cardiac vagal activity: Not different from sham stimulation and no effect of stimulation intensity. PLoS ONE 2019, 14, e0223848. [Google Scholar] [CrossRef]
- Ghani, S.; Vilensky, J.; Turner, B.; Tubbs, R.S.; Loukas, M. Meta-analysis of vagus nerve stimulation treatment for epilepsy: Correlation between device setting parameters and acute response. Childs Nerv. Syst. 2015, 31, 2291–2304. [Google Scholar] [CrossRef]
- Sclocco, R.; Garcia, R.G.; Kettner, N.W.; Fisher, H.P.; Isenburg, K.; Makarovsky, M.; Stowell, J.A.; Goldstein, J.; Barbieri, R.; Napadow, V. Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimul. 2020, 13, 970–978. [Google Scholar] [CrossRef]
- Jiao, J.; Harreby, K.R.; Sevcencu, C.; Jensen, W. Optimal vagus nerve stimulation frequency for suppression of spike-and-wave seizures in rats. Artif. Organs 2016, 40, E120–E127. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Singh, J.P.; Parks, K.A.; Katritsis, D.G.; Stavrakis, S.; Armoundas, A.A. Low-level tragus stimulation modulates atrial alternans and fibrillation burden in patients with paroxysmal atrial fibrillation. J. Am. Heart Assoc. 2021, 10, e020865. [Google Scholar] [CrossRef] [PubMed]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus nerve stimulation and the cardiovascular system. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.J.; Hu, Y.; Jackman, W.M.; Nakagawa, H.; Lockwood, D.; Lazzara, R.; Po, S.S. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 2015, 65, 867–875. [Google Scholar] [CrossRef]
- Nearing, B.D.; Libbus, I.; Amurthur, B.; Kenknight, B.H.; Verrier, R.L. Acute autonomic engagement assessed by heart rate dynamics during vagus nerve stimulation in patients with heart failure in the ANTHEM-HF Trial. J. Cardiovasc. Electrophysiol. 2016, 27, 1072–1077. [Google Scholar] [CrossRef]
- Geng, D.; Liu, X.; Wang, Y.; Wang, J. The effect of transcutaneous auricular vagus nerve stimulation on HRV in healthy young people. PLoS ONE 2022, 17, e0263833. [Google Scholar] [CrossRef]
- Peuker, E.T.; Filler, T.J. The nerve supply of the human auricle. Clin. Anat. 2002, 15, 35–37. [Google Scholar] [CrossRef]
- Henssen, D.J.H.A.; Derks, B.; van Doorn, M.; Verhoogt, N.C.; Staats, P.; Vissers, K.; Van Cappellen van Walsum, A.M. Visualizing the trigeminovagal complex in the human medulla by combining ex-vivo ultra-high resolution structural MRI and polarized light imaging microscopy. Sci. Rep. 2019, 9, 11305. [Google Scholar] [CrossRef]
- Alexander, G.M.; McNamara, J.O. Vagus nerve stimulation elevates seizure threshold in the kindling model. Epilepsia 2012, 53, 2043–2052. [Google Scholar] [CrossRef]
- Carandina, A.; Rodrigues, G.D.; Di Francesco, P.; Filtz, A.; Bellocchi, C.; Furlan, L.; Carugo, S.; Montano, N.; Tobaldini, E. Effects of transcutaneous auricular vagus nerve stimulation on cardiovascular autonomic control in health and disease. Auton. Neurosci. 2021, 236, 102893. [Google Scholar] [CrossRef]
- Frangos, E.; Komisaruk, B.R. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul. 2017, 10, 19–27. [Google Scholar] [CrossRef]
- Poppa, T.; Benschop, L.; Horczak, P.; Vanderhasselt, M.A.; Carrette, E.; Bechara, A.; Baeken, C.; Vonck, K. Auricular transcutaneous vagus nerve stimulation modulates the heart-evoked potential. Brain Stimul. 2022, 15, 260–269. [Google Scholar] [CrossRef]
- Jiang, Y.; Po, S.S.; Amil, F.; Dasari, T.W. Non-invasive low-level tragus stimulation in cardiovascular diseases. Arrhythmia Electrophysiol. Rev. 2020, 9, 40–46. [Google Scholar] [CrossRef]
- Wang, Y.; Po, S.S.; Scherlag, B.J.; Yu, L.; Jiang, H. The role of low-level vagus nerve stimulation in cardiac therapy. Expert Rev. Med. Devices 2019, 16, 675–682. [Google Scholar] [CrossRef]
- Tran, N.; Asad, Z.; Elkholey, K.; Scherlag, B.J.; Po, S.S.; Stavrakis, S. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J. Cardiovasc. Transl. Res. 2019, 12, 221–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Ilsar, I.; Sabbah, H.N.; Ben David, T.; Mazgalev, T.N. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: Therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm 2009, 6, 244–250. [Google Scholar] [CrossRef]
- Hulsey, D.R.; Riley, J.R.; Loerwald, K.W.; Rennaker, R.L., 2nd; Kilgard, M.P.; Hays, S.A. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol. 2017, 289, 21–30. [Google Scholar] [CrossRef]
- Morrison, R.A.; Hulsey, D.R.; Adcock, K.S.; Rennaker, R.L., 2nd; Kilgard, M.P.; Hays, S.A. Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul. 2019, 12, 256–262. [Google Scholar] [CrossRef]
- Clancy, J.A.; Deuchars, S.A.; Deuchars, J. The wonders of the Wanderer. Exp. Physiol. 2013, 98, 38–45. [Google Scholar] [CrossRef]
- Licursi de Alcântara, A.C.; Salgado, H.C.; Fazan, V.S. Morphology and morphometry of the vagus nerve in male and female spontaneously hypertensive rats. Brain Res. 2008, 1197, 170–180. [Google Scholar] [CrossRef]
- Collins, J.J.; Lin, C.E.; Berthoud, H.R.; Papka, R.E. Vagal afferents from the uterus and cervix provide direct connections to the brainstem. Cell Tissue Res. 1999, 295, 43–54. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ushiroyama, T.; Kimura, T.; Hayashi, T.; Moritani, T. Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. Biopsychosoc. Med. 2007, 1, 24. [Google Scholar] [CrossRef]
- Oladosu, F.A.; Hellman, K.M.; Ham, P.J.; Kochlefl, L.E.; Datta, A.; Garrison, E.F.; Steiner, N.D.; Roth, G.E.; Tu, F.F. Persistent autonomic dysfunction and bladder sensitivity in primary dysmenorrhea. Sci. Rep. 2019, 9, 2194. [Google Scholar] [CrossRef]
- Foy, M.R.; Baudry, M.; Akopian, G.K.; Thompson, R.F. Regulation of hippocampal synaptic plasticity by estrogen and progesterone. Vitam Horm 2010, 82, 219–239. [Google Scholar]
- Mukai, H.; Kimoto, T.; Hojo, Y.; Kawato, S.; Murakami, G.; Higo, S.; Hatanaka, Y.; Ogiue-Ikeda, M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim. Biophys. Acta 2010, 1800, 1030–1044. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokota, H.; Edama, M.; Hirabayashi, R.; Sekine, C.; Otsuru, N.; Saito, K.; Kojima, S.; Miyaguchi, S.; Onishi, H. Effects of Stimulus Frequency, Intensity, and Sex on the Autonomic Response to Transcutaneous Vagus Nerve Stimulation. Brain Sci. 2022, 12, 1038. https://doi.org/10.3390/brainsci12081038
Yokota H, Edama M, Hirabayashi R, Sekine C, Otsuru N, Saito K, Kojima S, Miyaguchi S, Onishi H. Effects of Stimulus Frequency, Intensity, and Sex on the Autonomic Response to Transcutaneous Vagus Nerve Stimulation. Brain Sciences. 2022; 12(8):1038. https://doi.org/10.3390/brainsci12081038
Chicago/Turabian StyleYokota, Hirotake, Mutsuaki Edama, Ryo Hirabayashi, Chie Sekine, Naofumi Otsuru, Kei Saito, Sho Kojima, Shota Miyaguchi, and Hideaki Onishi. 2022. "Effects of Stimulus Frequency, Intensity, and Sex on the Autonomic Response to Transcutaneous Vagus Nerve Stimulation" Brain Sciences 12, no. 8: 1038. https://doi.org/10.3390/brainsci12081038
APA StyleYokota, H., Edama, M., Hirabayashi, R., Sekine, C., Otsuru, N., Saito, K., Kojima, S., Miyaguchi, S., & Onishi, H. (2022). Effects of Stimulus Frequency, Intensity, and Sex on the Autonomic Response to Transcutaneous Vagus Nerve Stimulation. Brain Sciences, 12(8), 1038. https://doi.org/10.3390/brainsci12081038







