Abstract
We investigated the clinical characteristics, neuroimaging findings, and final diagnosis of patients with acute isolated or prominent dysarthria who visited the emergency department (ED) between 1 January 2020 and 31 December 2021. Of 2028 patients aged ≥ 18 years with neurologic symptoms treated by a neuro-emergency expert, 75 with acute isolated or predominant dysarthria within 1 week were enrolled. Patients were categorized as having isolated dysarthria (n = 28, 37.3%) and prominent dysarthria (n = 47, 62.7%). The causes of stroke were acute ischemic stroke (AIS) (n = 37, 49.3%), transient ischemic attack (TIA) (n = 14, 18.7%), intracerebral hemorrhage (n = 1, 1.3%), and non-stroke causes (n = 23, 30.7%). The most common additional symptoms were gait disturbance or imbalance (n = 8, 15.4%) and dizziness (n = 3, 13.0%) in the stroke and non-stroke groups, respectively. The isolated dysarthria group had a higher rate of TIA (n = 7, 38.9%), single and small lesions (n = 10, 83.3%), and small-vessel occlusion in Trial of Org 101072 in acute stroke treatment (n = 8, 66.7%). Acute isolated or prominent dysarthria in the ED mostly presented as clinical symptoms of AIS, but other non-stroke and medical causes were not uncommon. In acute dysarthria with ischemic stroke, multiple territorial and small and single lesions are considered a cause.
1. Introduction
Acute dysarthria, a motor speech disorder in which speech process or production is impaired, is a neurological symptom encountered in the emergency department (ED). Acute dysarthria is common accompanied by other neurological symptoms, such as motor weakness, mainly in acute ischemic stroke at a rate of 26–56% [1,2,3]. However, it rarely occurs alone or with minor neurological symptoms in patients with acute ischemic stroke [4,5,6,7]. In the field of stroke, it has been called isolated or prominent dysarthria and is sometimes described as a special neurological syndrome, such as dysarthria-clumsy hand syndrome, dysarthria-facial paresis syndrome, and dysarthria-lingual palsy syndrome [8,9,10,11]. Pure dysarthria has been described as lacunar syndrome in internal capsule, corona radiata, small cortical, or subcortical lesions [10,12].
However, this isolated or prominent dysarthria is a challenging symptom for ED physicians because it is caused by not only acute ischemic stroke but also other conditions, such as multiple sclerosis [13]. To date, there are insufficient clinical data and comprehension of patients with isolated or prominent dysarthria in the ED environment. We investigated the clinical characteristics, neuroimaging findings, and final diagnosis of patients with acute isolated or prominent dysarthria who visited the ED. In addition, we hypothesized that there would be different clinical characteristics between isolated dysarthria and prominent dysarthria group in acute ischemic stroke or transient ischemic attack (TIA).
2. Materials and Methods
2.1. Study Design and Patient Selection
This study was conducted at the ED of an urban academic tertiary-care hospital in South Korea. This 900-bed facility has a regional emergency medical center, as designated by the government, and receives approximately 75,000 ED visits annually. In our neuro-emergency medicine project [14], a neuro-emergency expert was responsible for the emergency care of patients with neurological symptoms. Through a systematic review of electronic medical records between 1 January 2020 and 31 December 2021, patients aged ≥18 years with neurologic symptoms treated by a neuro-emergency expert in the ED were screened. Among these patients, those with acute isolated or prominent dysarthria occurring within 1 week were enrolled. Patients with aphasia, altered mentality beyond deep drowsiness, definite motor weakness (The Modified Medical Research Council [MRC] grade ≤ 4), visual field defect, or National Institutes of Health Stroke Scale (NIHSS) score ≥ 5 were excluded. Neurological examination was assessed directly by a neuro-emergency expert at admission to ED. Patients who did not recognize the symptoms themselves but confirmed mild motor weakness (modified MRC grade 4+, 5−) through neurological examination were included in this study.
Isolated dysarthria was defined as having only dysarthria and no neurological symptoms of other subjective or transient motor weakness, sensory changes, ataxia, gait disturbance, vertigo, or visual symptoms. Prominent dysarthria had other neurologic symptoms or systemic symptoms, such as fever or pain, but was defined as complaining of the main symptoms as dysarthria. NIHSS was investigated only in patients with acute ischemic stroke or TIA. All patients with TIA were received an NIHSS score of 0 with improved neurological symptoms at ED.
This study was approved by the institutional review board of Hallym University Sacred Heart Hospital, which waived the requirement for informed consent (IRB no. 2022-07-002).
2.2. Data Collection
Data on patients’ baseline characteristics (age, sex, coexisting condition, antithrombotic agent, and time interval from onset to door), additional neurological or general symptoms, neuroimaging findings, NIHSS score, final diagnosis, and Trial of Org 101072 in Acute Stroke Treatment (TOAST) [15] were obtained and analyzed. When accompanied by multiple neurological or general symptoms, the symptom evaluated to be the most severe among these was analyzed as an additional symptom.
2.3. Statistical Analyses
The normality of the data distributions was evaluated using the Kolmogorov–Smirnov test for the selection of appropriate parametric and non-parametric statistical methods. Categorical variables were analyzed using chi-square or Fisher’s exact tests and are expressed as total numbers (percentages). Continuous variables are expressed as medians (interquartile range) and were analyzed using the Mann–Whitney U test. For all comparisons, the tests were two-tailed, and between-group differences were considered significant at p < 0.05. Statistical Package for the Social Sciences for Windows ver. 18.0.0 (IBM, Armonk, NY, USA) was used for all analyses.
3. Results
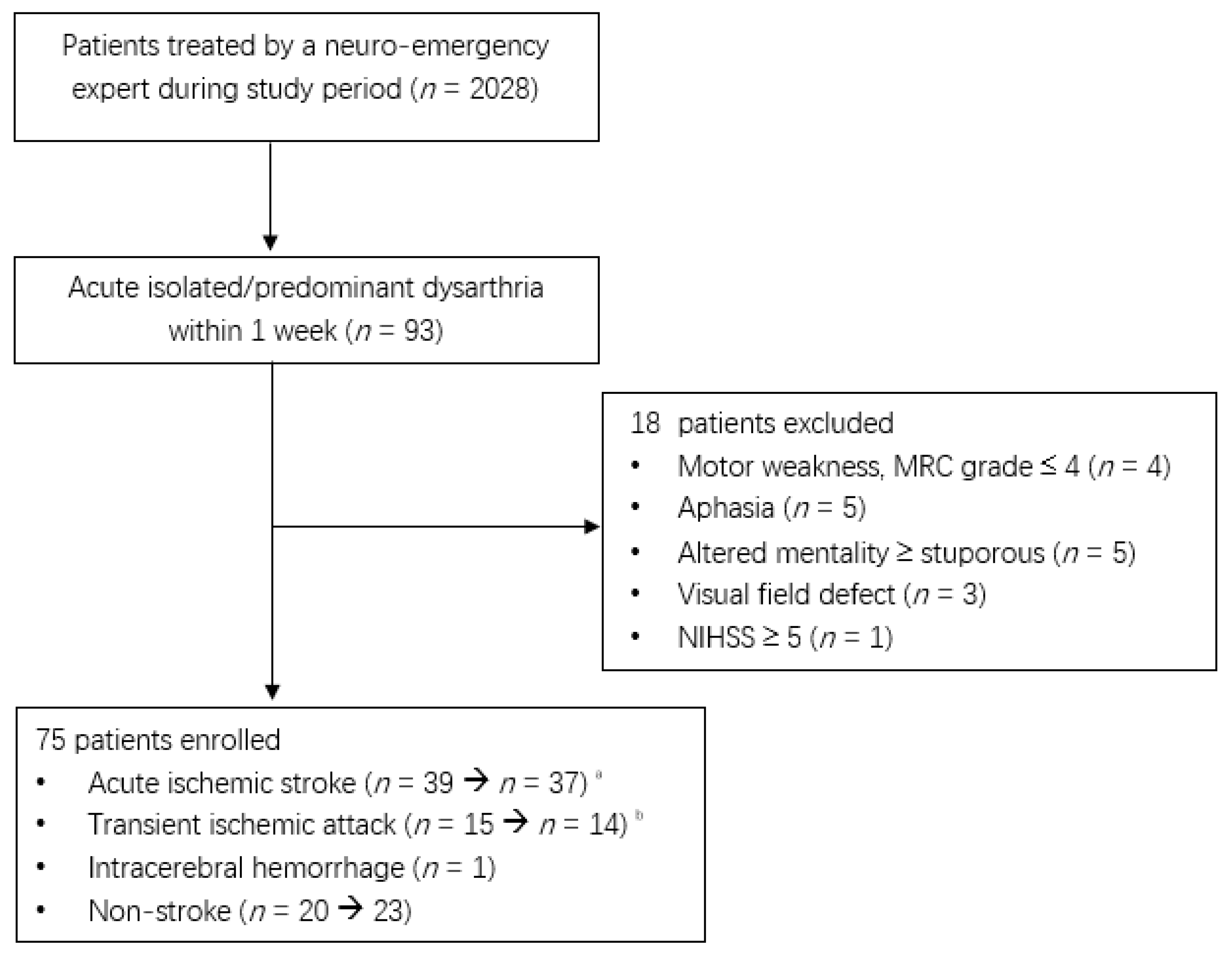
Of the 2208 patients treated by a neuro-emergency expert, 75 patients, excluding 18 patients with acute isolated or predominant dysarthria within 1 week, were enrolled in the present study (Figure 1). Two patients with an initial acute ischemic stroke were diagnosed with multiple sclerosis and multiple cranial neuropathies.
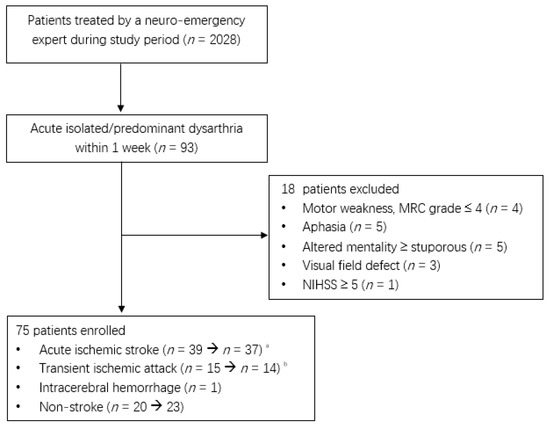
Figure 1.
Flow diagram of enrolled patients during the study period (1 January 2020 to 31 December 2021). a. Two patients were diagnosed with multiple sclerosis and multiple cranial neuropathies. b. One patient’s diagnosis was changed to focal seizures.
As shown in Table 1, the study population was divided into isolated dysarthria (n = 28, 37.3%) and prominent dysarthria (n = 47, 62.7%) groups. We noted that the median onset-to-door time of the isolated dysarthria group was shorter than that of the prominent dysarthria group (689 min vs. 1283 min, p = 0.042).

Table 1.
Characteristics of patients with isolated or prominent dysarthria.
As shown in Table 2, the causes of stroke in acute isolated or prominent dysarthria were acute ischemic stroke (n = 37, 49.3%), TIA (n = 14, 18.7%) and intracerebral hemorrhage (n = 1, 1.3%). The number of patients with non-stroke causes was 23 (30.7%), including metabolic causes with hypoglycemia and uremia (n = 7, 9.3%), toxic causes (n = 6, 8.0%), brain tumor (n = 2, 2.7%), and Parkinson’s disease (n = 2, 2.7%).

Table 2.
Final diagnosis of patients with isolated or prominent dysarthria.
Table 3 shows various additional symptoms of patients with prominent dysarthria. The most common additional symptoms were gait disturbance or imbalance (n = 9, 12.0%), facial palsy (n = 8, 10.7%), and mild hemiplegia (n = 5, 6.7%) in order of frequency. This trend was similar to the stroke group, but dizziness was the most common additional symptom in the non-stroke group (n = 3, 13.0%).

Table 3.
Various additional symptoms in patients with prominent dysarthria.
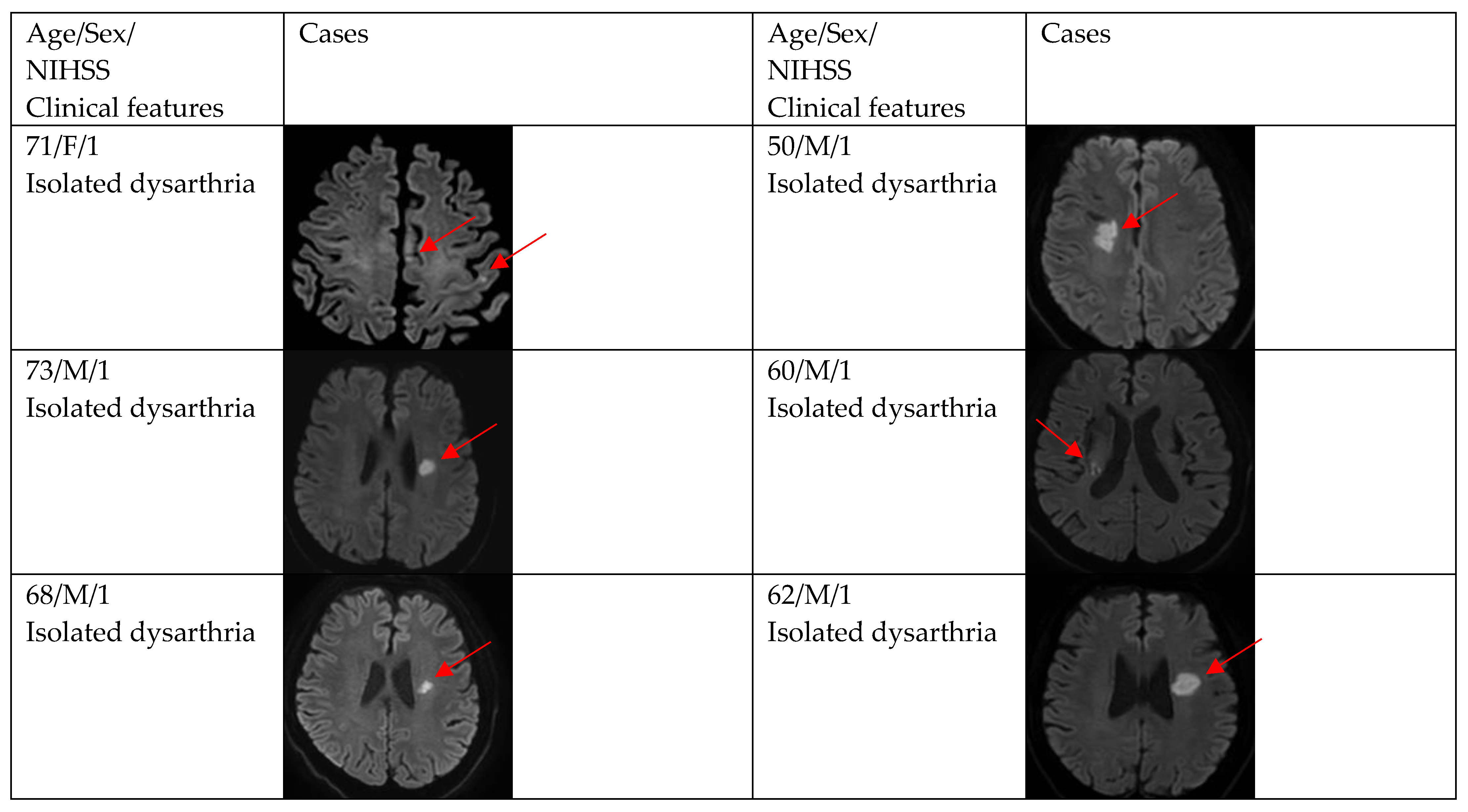
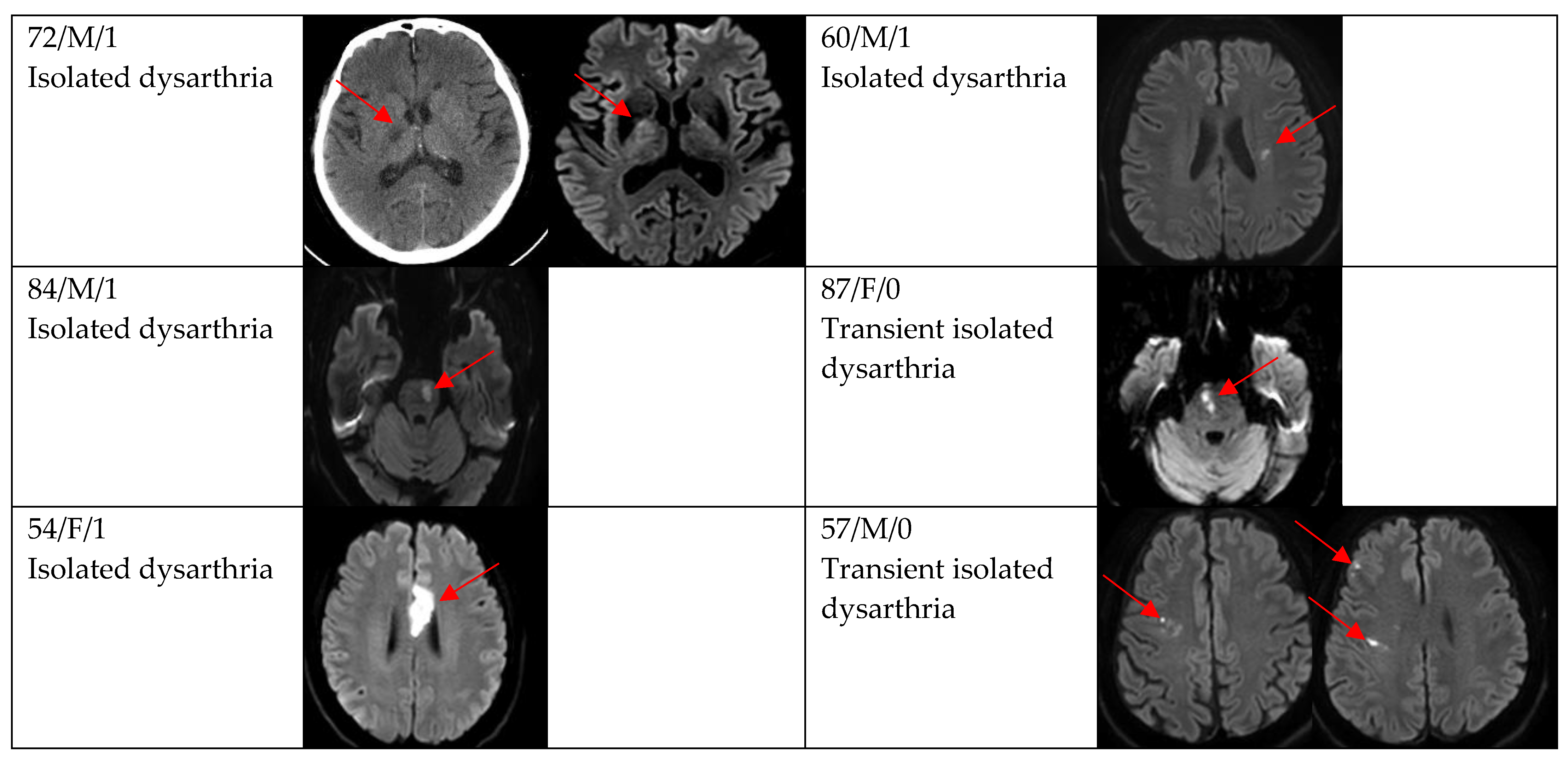
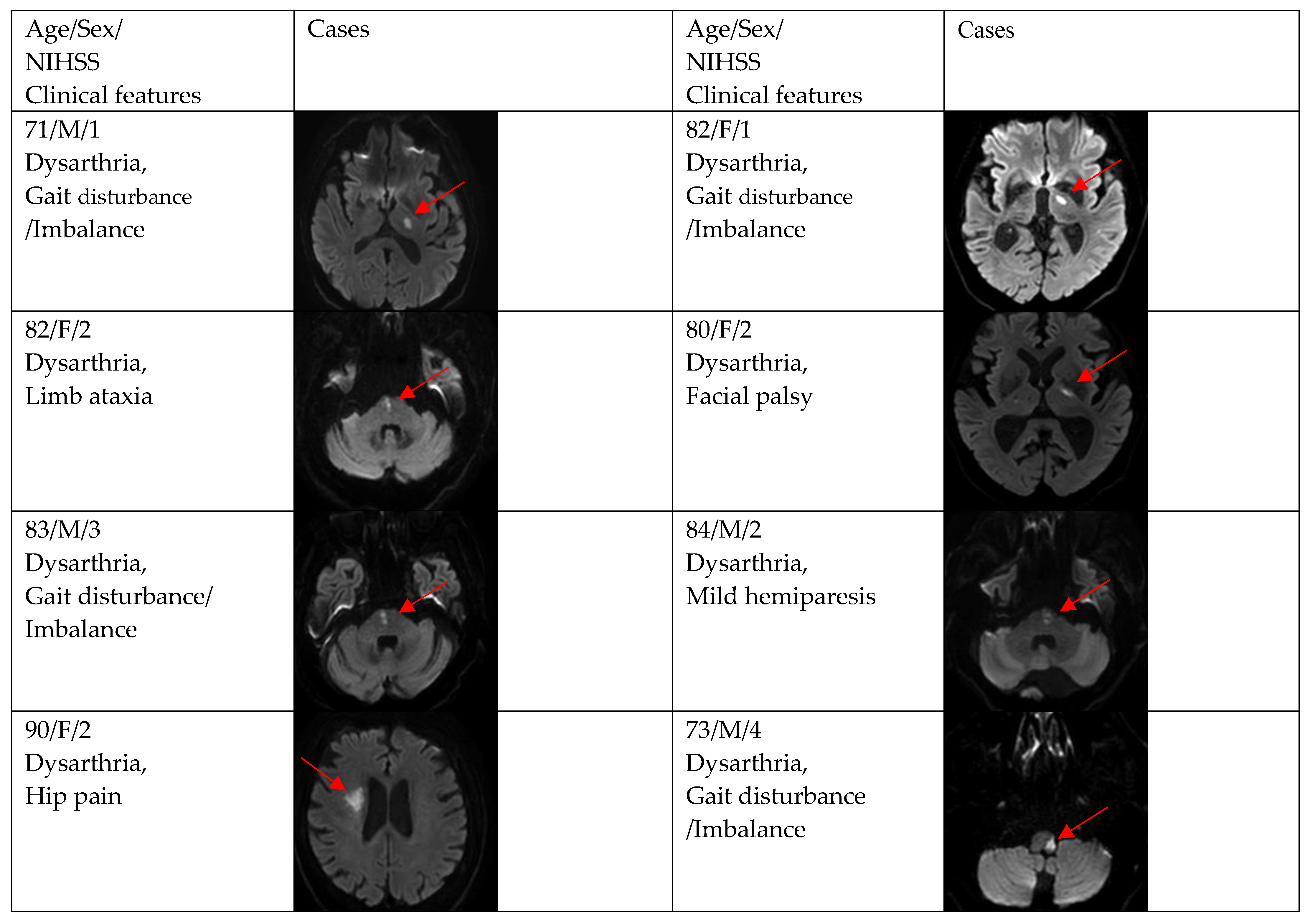
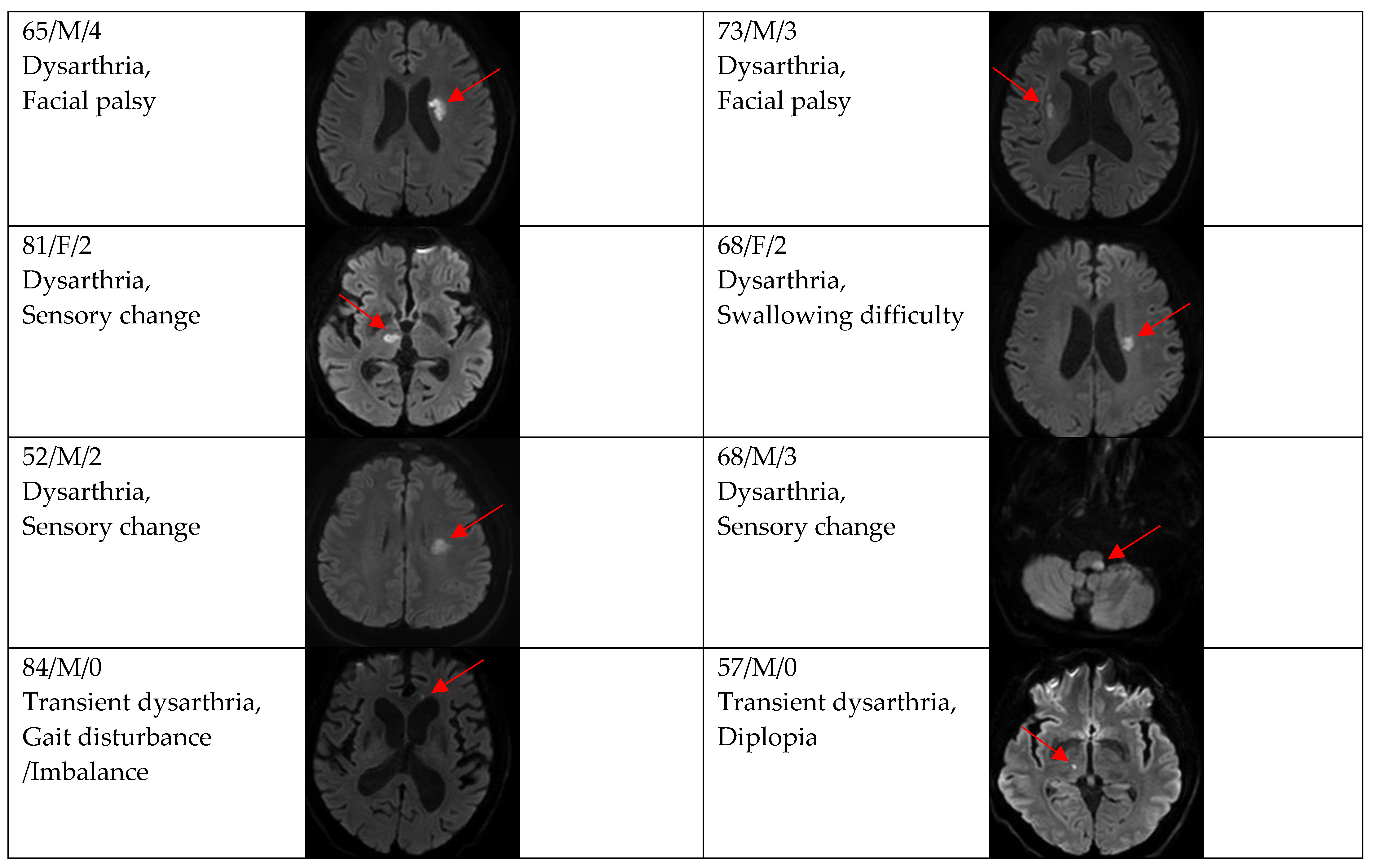
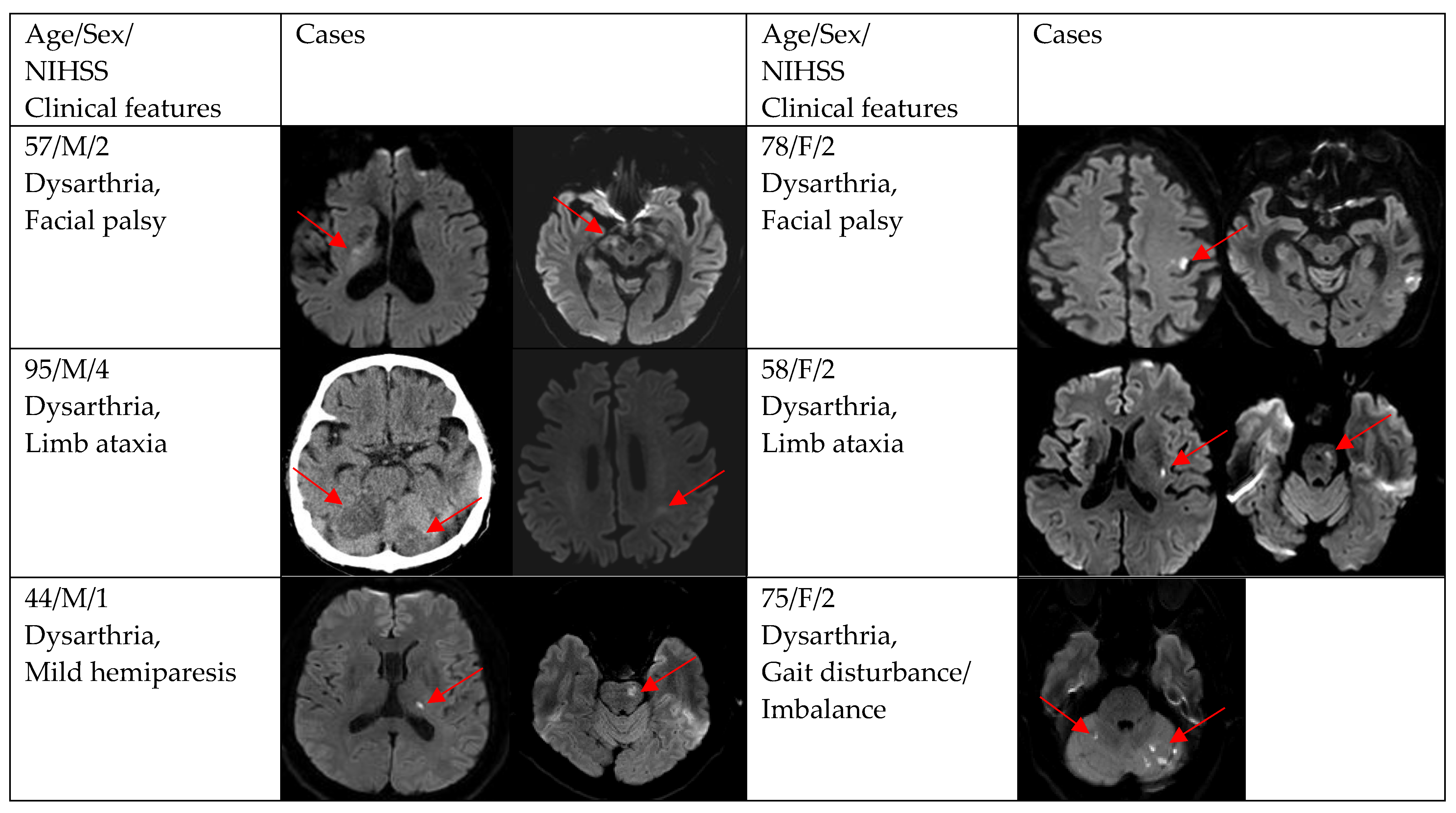
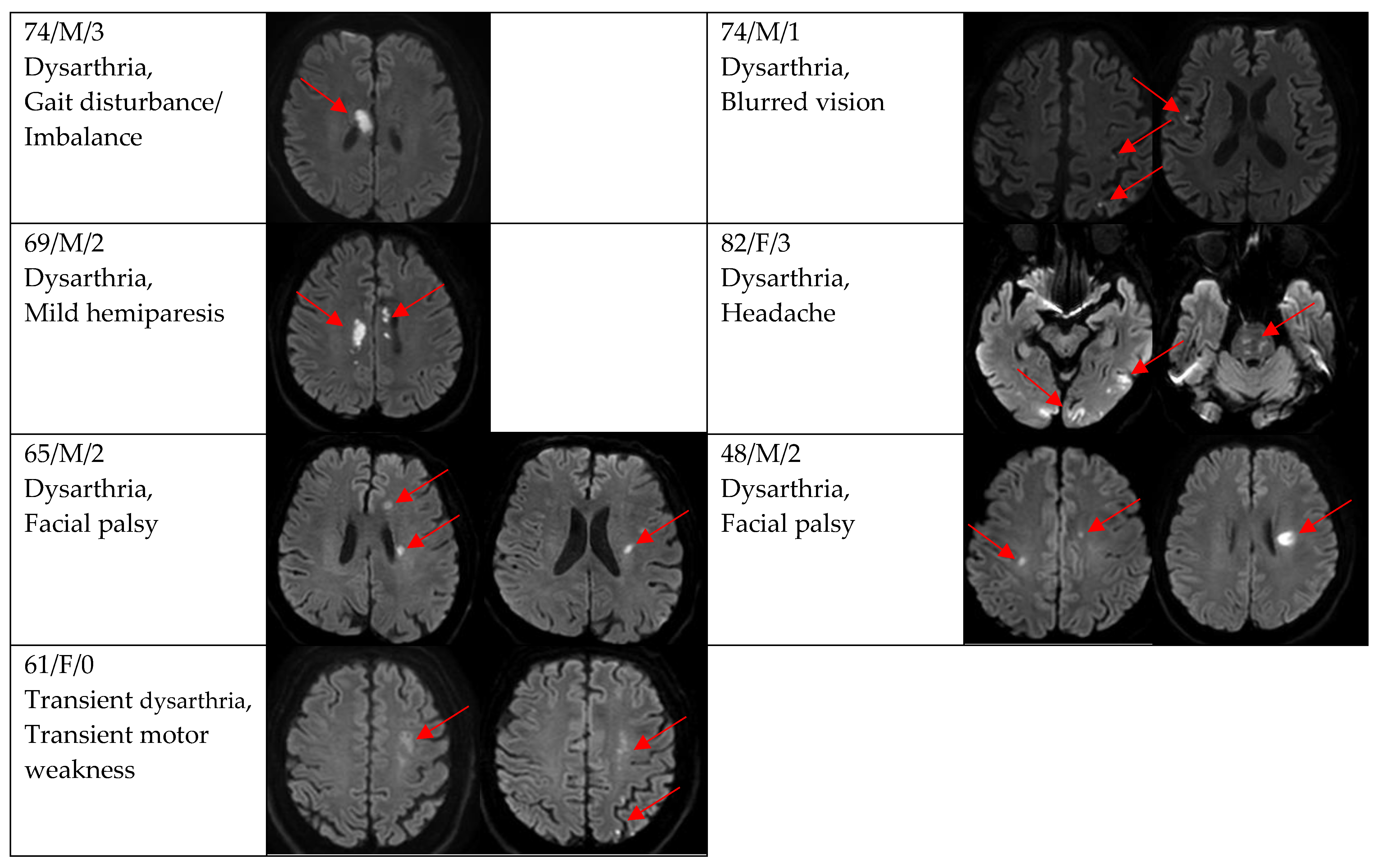
Figure 2, Figure 3 and Figure 4 show acute stroke lesions on diffusion-weighted imaging (DWI). In the isolated dysarthria group, 10 patients had small and single lesions, and 2 patients had multiple or territorial lesions on DWI. In the prominent dysarthria group, 16 patients had small and single lesions, and 13 patients had multiple or territorial lesions on DWI.
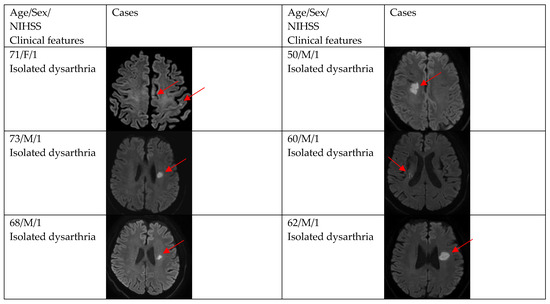
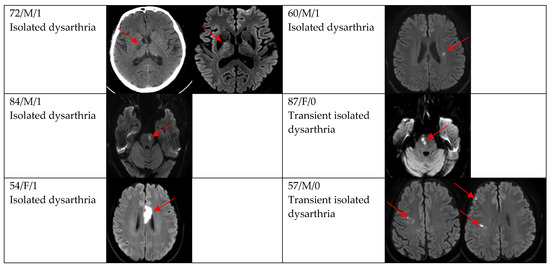
Figure 2.
Clinical characteristics and neuroimaging findings of patients with ischemic stroke or TIA presenting with dysarthria only. Except for the bottom two cases, all showed a single lesion on DWI.
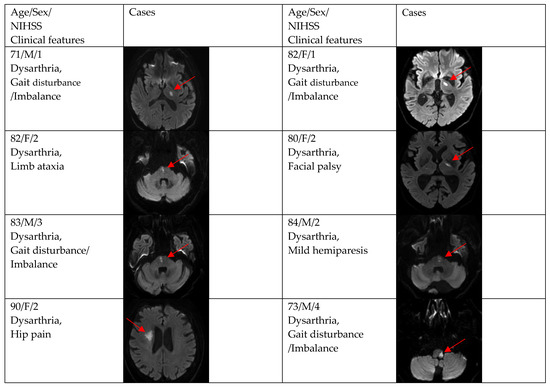
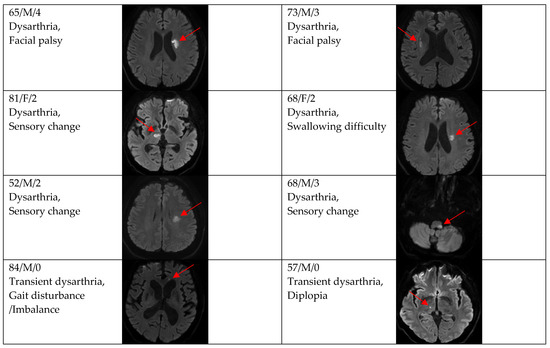
Figure 3.
Clinical characteristics and neuroimaging findings of patients with ischemic stroke or TIA presenting with prominent dysarthria and small and single lesions.
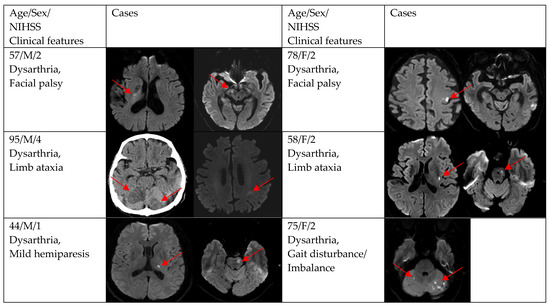
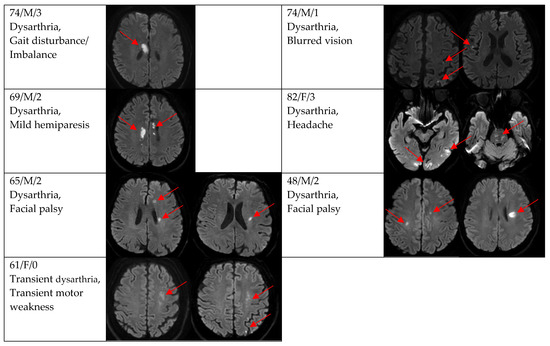
Figure 4.
Clinical characteristics and neuroimaging findings of patients with ischemic stroke or TIA presenting with prominent dysarthria and multiple territorial lesions.
In the sub-analysis of acute ischemic stroke or TIA, Table 4 shows the comparison between the isolated dysarthria group (n = 18, 35.3%) and the prominent dysarthria group (n = 33, 64.7%). The isolated dysarthria group had a higher proportion of TIA and lower proportion of DWI positivity than the prominent dysarthria group (n = 7, 38.9% vs. 7, 21.2%, p = 0.204, and n = 12, 66.7% vs. 29, 87.9%, p = 0.129). In terms of NIHSS, except for one patient with a score of 0, all patients had a score of 1 in the isolated dysarthria group. In the prominent group, 14 (48.3%) patients had a score of 2, five patients (17.2%) had a score of 3, and four patients (13.8%) had a score of 1. As for the TOAST classification in DWI positivity, the percentage of small vessel disease was the highest in both groups (n = 8, 66.7% vs. n = 15, 51.7%).

Table 4.
Comparison between isolated and prominent dysarthria in patients with acute ischemic stroke or TIA.
4. Discussion
In the present study, we investigated the clinical characteristics, neuroimaging findings, and diagnoses of patients with acute isolated or prominent dysarthria visiting the ED.
Acute dysarthria is a common neurological symptom encountered in the ED and occurs in various neurological and medical conditions. Interestingly, acute dysarthria rarely occurs alone or is a major symptom. In acute ischemic stroke, acute dysarthria is often accompanied by limb weakness and rarely occurs alone or with minor neurological symptoms without motor weakness [7,8,10,13]. Therefore, there has been little clinical interest in acute dysarthria, and previous studies have shown limited data on acute stroke. Moreover, the definition of acutely isolated dysarthria and the criteria of enrolled patients were described heterogeneously in each study.
In clinical practice, it is natural for emergency physicians to first consider ischemic stroke, which has a golden time for treating patients with acute dysarthria. In the present study, acute ischemic stroke accounted for most causes at 69.3%, supporting the evidence that clinicians should pay attention to the causes of stroke. However, hemorrhagic stroke was the cause in only one patient, presumably because symptoms tended to be more severe and complicated in patients with hemorrhagic stroke visiting the ED. Even though we reviewed the data thoroughly, only one case of isolated dysarthria in hemorrhagic stroke was identified [10]. However, since 30.7% of dysarthria cases in the present study were caused by other non-stroke causes, clinicians should consider that acute dysarthria in the ED may also be caused by neurological and medical causes other than stroke. Neurological diseases, such as brain tumor, Parkinson’s disease, multiple sclerosis, seizure, and multiple cranial neuropathies, as well as metabolic causes, toxic causes, aortic dissection, infectious causes, and psychiatric disorders, can cause acute dysarthria; therefore, a comprehensive approach is needed.
In terms of additional symptoms, the common symptoms in the stroke group were facial palsy (13.5%) and gait disturbance or imbalance (15.4%). Similar to our results, Urban [7] and Kim [9] reported that facial palsy was commonly accompanied by 58.8% and 69.2%. In our DWI findings, we confirmed that the lesion involved the corticobulbar fiber (corona radiata, internal capsule, and subcortex) in all cases of facial palsy. Although the authors cannot clearly explain why dysarthria is often accompanied by gait disturbance, we were able to confirm that patients with gait disturbance or imbalance had lesions in the thalamus, brainstem, cerebellum, and frontal lobe on DWI findings [16].
Generally, it is reasonable to assume that patients with mild neurological symptoms may have small lesions on magnetic resonance imaging. The isolated dysarthria group had a higher rate of TIA, single and small lesions, and small-vessel occlusion in TOAST than the prominent dysarthria group, albeit without reaching statistical significance, owing to the small sample size. However, we need to pay attention clinically to the finding that even mild isolated symptoms can be caused by multiple or territorial lesions or large vessel occlusion [7].
The strength of our study is that the patients’ neurological evaluation was performed continuously and consistently by a single neuro-emergency expert in the ED, and both NIHSS and TOAST were evaluated, recorded, and analyzed. On the other hand, this study has limitations in that it had a retrospective design, the sample size was small, and it did not include all emergency center patients.
5. Conclusions
Acute isolated or prominent dysarthria in the ED mostly presented as clinical symptoms of AIS, but other non-stroke and medical causes were not uncommon. In acute dysarthria with ischemic stroke, multiple territorial lesions are considered a cause, in addition to small and single lesions.
Author Contributions
S.-H.L., S.-O.H. and J.-H.K. designed the study and collected the data. W.-S.Y., Y.-S.P. and T.-J.P. analyzed the data and results. S.-H.L., S.-O.H. and J.-H.K. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of our institution (approval No. 2022-07-002).
Informed Consent Statement
Patient consent was waived due to the purely observational, retrospective, and non-interventional nature of the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stipancic, K.L.; Borders, J.C.; Brates, D.; Thibeault, S.L. Prospective Investigation of Incidence and Co-Occurrence of Dysphagia, Dysarthria, and Aphasia Following Ischemic Stroke. Am. J. Speech Lang. Pathol. 2019, 28, 188–194. [Google Scholar] [CrossRef]
- Flowers, H.L.; Silver, F.L.; Fang, J.; Rochon, E.; Martino, R. The incidence, co-occurrence, and predictors of dysphagia, dysarthria, and aphasia after first-ever acute ischemic stroke. J. Commun. Disord. 2013, 46, 238–248. [Google Scholar] [CrossRef]
- Vidovic, M.; Sinanovic, O.; Sabaskic, L.; Haticic, A.; Brkic, E. Incidence and types of speech disorders in stroke patients. Acta Clin. Croat. 2011, 50, 491–494. [Google Scholar]
- Arboix, A.; Bell, Y.; García-Eroles, L.; Massons, J.; Comes, E.; Balcells, M.; Targa, C. Clinical Study of 35 Patients With Dysarthria-Clumsy Hand Syndrome. J. Neurol. Neurosurg. Psychiatry 2004, 75, 231–234. [Google Scholar] [PubMed]
- De Cock, E.; Batens, K.; Hemelsoet, D.; Boon, P.; Oostra, K.; De Herdt, V. Dysphagia, Dysarthria and Aphasia Following a First Acute Ischaemic Stroke: Incidence and Associated Factors. Eur. J. Neurol. 2020, 27, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.P.; Hopf, H.C.; Zorowka, P.G.; Fleischer, S.; Andreas, J. Dysarthria and Lacunar Stroke: Pathophysiologic Aspects. Neurology 1996, 47, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.P.; Wicht, S.; Vukurevic, G.; Fitzek, C.; Fitzek, S.; Stoeter, P.; Massinger, C.; Hopf, H.C. Dysarthria in Acute Ischemic Stroke: Lesion Topography, Clinicoradiologic Correlation, and Etiology. Neurology 2001, 56, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Kageyama, Y. Clinical Anatomic Study of Pure Dysarthria. Stroke 1991, 22, 809–812. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S. Pure Dysarthria, Isolated Facial Paresis, or Dysarthria-Facial Paresis Syndrome. Stroke 1994, 25, 1994–1998. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Kwon, S.U.; Lee, T.G. Pure Dysarthria Due to Small Cortical Stroke. Neurology 2003, 60, 1178–1180. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamada, T.; Torii, T.; Yoshimura, T.; Takase, K.; Togao, O.; Wakata, Y.; Hiwatashi, A.; Nakashima, N.; Kira, J.; et al. Pure Dysarthria and Dysarthria-Facial Paresis Syndrome Due to Internal Capsule and/or Corona Radiata Infarction. BMC Neurol. 2015, 15, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, I.; Baba, M.; Narita, S.; Matsunaga, M.; Takebe, K. Pure dysarthria due to anterior internal capsule and/or corona radiata infarction: A report of five cases. J. Neurol. Neurosurg. Psychiatry 1986, 49, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Beliavsky, A.; Perry, J.J.; Dowlatshahi, D.; Wasserman, J.; Sivilotti, M.L.; Sutherland, J.; Worster, A.; Emond, M.; Stotts, G.; Jin, A.Y.; et al. Acute Isolated Dysarthria Is Associated With a High Risk of Stroke. Cerebrovasc. Dis. Extra 2014, 4, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.W.; Ha, S.O.; Kim, J.H.; Yang, W.S.; Park, Y.S. Experience of a Neuro-emergency Expert in the Emergency Department During One Year of the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 9416. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, L.R. Ataxia in Patients With Brain Infarcts and Hemorrhages. Handb. Clin. Neurol. 2012, 103, 147–160. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).