Chronic Pain after Bone Fracture: Current Insights into Molecular Mechanisms and Therapeutic Strategies
Abstract
1. Introduction
2. CFP Preclinical Animal Model
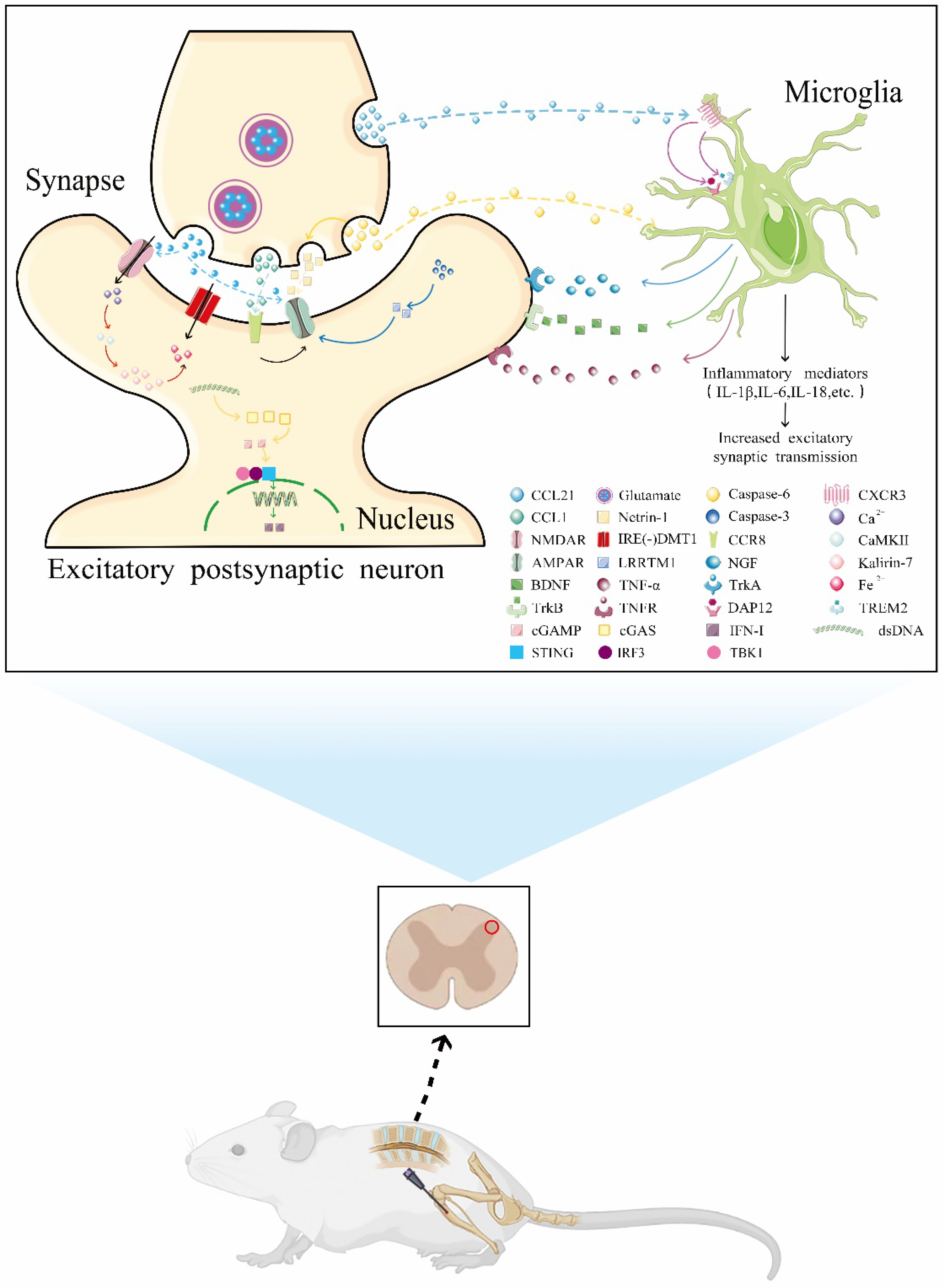
3. Molecules Involved in CFP and Their Mechanisms of Action
3.1. AMPA Receptor Cascades
3.1.1. Caspase-3
3.1.2. Caspase-6
3.2. NMDA Receptor Cascades
3.3. Neuroinflammation
3.3.1. The NF-κB Pathway
3.3.2. IL-6 Signaling
3.3.3. Chemokines
3.3.4. Kallikrein–Kinin System
3.3.5. Neurotrophins
4. Commonly Used Clinical Analgesics
4.1. NSAIDs
4.2. Opioids
4.3. NMDA Receptor Antagonist
4.4. Anti-Absorbers
4.4.1. Calcitonin
4.4.2. Bisphosphonates
4.5. Vitamin D
5. Emerging Analgesic Targets or Drugs
5.1. STING
5.2. DHA and SPMs
5.3. Artesunate
5.4. Others
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chen, W.; Lv, H.; Liu, S.; Liu, B.; Zhu, Y.; Chen, X.; Yang, G.; Liu, L.; Zhang, T.; Wang, H.; et al. National incidence of traumatic fractures in China: A retrospective survey of 512 187 individuals. Lancet Glob. Health 2017, 5, e807–e817. [Google Scholar] [CrossRef]
- Holmes, D. Non-union bone fracture: A quicker fix. Nature 2017, 550, S193. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, L.G.; Perugini, A.J.; Fehrenbacher, J.C.; White, F.A.; Kacena, M.A. Assessment, Quantification, and Management of Fracture Pain: From Animals to the Clinic. Curr. Osteoporos. Rep. 2020, 18, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Friesgaard, K.D.; Gromov, K.; Knudsen, L.F.; Brix, M.; Troelsen, A.; Nikolajsen, L. Persistent pain is common 1 year after ankle and wrist fracture surgery: A register-based questionnaire study. Br. J. Anaesth. 2016, 116, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.S.; Devereaux, P.J.; LeManach, Y.; Busse, J.W. Patient coping and expectations about recovery predict the development of chronic post-surgical pain after traumatic tibial fracture repair. Br. J. Anaesth. 2016, 117, 365–370. [Google Scholar] [CrossRef]
- Kim, C.-H.; Yang, J.Y.; Min, C.H.; Shon, H.-C.; Kim, J.W.; Lim, E.J. The effect of regional nerve block on perioperative delirium in hip fracture surgery for the elderly: A systematic review and meta-analysis of randomized controlled trials. Orthop. Traumatol. Surg. Res. 2022, 108, 103151. [Google Scholar] [CrossRef]
- Chater, T.E.; Goda, Y. The Shaping of AMPA Receptor Surface Distribution by Neuronal Activity. Front. Synaptic Neurosci. 2022, 14, 833782. [Google Scholar] [CrossRef]
- Mitchell, S.A.T.; Majuta, L.A.; Mantyh, P.W. New Insights in Understanding and Treating Bone Fracture Pain. Curr. Osteoporos. Rep. 2018, 16, 325–332. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Roth, J.H.; Richards, R.S. Pain and disability reported in the year following a distal radius fracture: A cohort study. BMC Musculoskelet. Disord. 2003, 4, 24. [Google Scholar] [CrossRef]
- Minville, V.; Laffosse, J.-M.; Fourcade, O.; Girolami, J.-P.; Tack, I. Mouse model of fracture pain. Anesthesiology 2008, 108, 467–472. [Google Scholar] [CrossRef]
- Freeman, K.T.; Koewler, N.J.; Jimenez-Andrade, J.M.; Buus, R.J.; Herrera, M.B.; Martin, C.D.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. A fracture pain model in the rat: Adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology 2008, 108, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Handool, K.O.; Ibrahim, S.M.; Kaka, U.; Omar, M.A.; Abu, J.; Yusoff, S.M.; Yusof, L.M. Optimization of a closed rat tibial fracture model. J. Exp. Orthop. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, V.L.; Quarta, M.; Paine, P.; Forman, T.E.; Pajarinen, J.; Takemura, Y.; Goodman, S.B.; Rando, T.; Clark, J.D. Angiotensin receptor blockade mimics the effect of exercise on recovery after orthopaedic trauma by decreasing pain and improving muscle regeneration. J. Physiol. 2020, 598, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.M.; Li, T.-K.; Li, J.; Ho, W.-T.; Chow, S.K.-H.; Leung, S.S.; Cheung, W.-H.; Ip, M. A systematic review on current osteosynthesis-associated infection animal fracture models. J. Orthop. Translat. 2020, 23, 8–20. [Google Scholar] [CrossRef]
- Garcia, P.; Herwerth, S.; Matthys, R.; Holstein, J.H.; Histing, T.; Menger, M.D.; Pohlemann, T. The LockingMouseNail--a new implant for standardized stable osteosynthesis in mice. J. Surg. Res. 2011, 169, 220–226. [Google Scholar] [CrossRef]
- Shen, H.; Gardner, A.M.; Vyas, J.; Ishida, R.; Tawfik, V.L. Modeling Complex Orthopedic Trauma in Rodents: Bone, Muscle and Nerve Injury and Healing. Front. Pharmacol. 2021, 11, 620485. [Google Scholar] [CrossRef]
- Magnusdottir, R.; Gohin, S.; ter Heegde, F.; Hopkinson, M.; McNally, I.; Fisher, A.; Upton, N.; Billinton, A.; Chenu, C. Fracture-induced pain-like behaviours in a femoral fracture mouse model. Osteoporos. Int. 2021, 32, 2347–2359. [Google Scholar] [CrossRef]
- Greger, I.H.; Mayer, M.L. Structural biology of glutamate receptor ion channels: Towards an understanding of mechanism. Curr. Opin. Struct. Biol. 2019, 57, 185–195. [Google Scholar] [CrossRef]
- Cui, W.; Li, Y.; Wang, Z.; Song, C.; Yu, Y.; Wang, G.; Li, J.; Wang, C.; Zhang, L. Spinal caspase-6 regulates AMPA receptor trafficking and dendritic spine plasticity through netrin-1 in postoperative pain after orthopedic surgery for tibial fracture in mice. Pain 2021, 162, 124–134. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Zhao, Q.; Li, Y.; Song, C.; Wang, C.; Yu, Y.; Wang, G. Spinal Protein Kinase Mζ Regulates α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptor Trafficking and Dendritic Spine Plasticity via Kalirin-7 in the Pathogenesis of Remifentanil-induced Postincisional Hyperalgesia in Rats. Anesthesiology 2018, 129, 173–186. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Li, Y.; Wang, Z.; Wang, G.; Yu, Y.; Song, C.; Cui, W. Spinal caspase-3 contributes to tibial fracture-associated postoperative allodynia via up-regulation of LRRTM1 expression in mice. Neurosci. Lett. 2020, 739, 135429. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, M.H.; Munn, R.G.K.; Fearnhead, H.O. Non-Canonical Roles of Apoptotic Caspases in the Nervous System. Front. Cell Dev. Biol. 2022, 10, 840023. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Vanderlinden, J.; Vints, K.; Ribeiro, L.F.; Vennekens, K.M.; Gounko, N.V.; Wierda, K.D.; de Wit, J. A Modular Organization of LRR Protein-Mediated Synaptic Adhesion Defines Synapse Identity. Neuron 2018, 99, 329–344.e7. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Miao, X.; Chen, J.; Sun, Y.; Liu, Z.; Tao, Y.; Yu, W. Down-regulation of GAP-43 by inhibition of caspases-3 in a rat model of neuropathic pain. Int. J. Clin. Exp. Pathol. 2012, 5, 948–955. [Google Scholar] [PubMed]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.-R. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci. Bull. 2018, 34, 98–108. [Google Scholar] [CrossRef]
- Berta, T.; Perrin, F.E.; Pertin, M.; Tonello, R.; Liu, Y.-C.; Chamessian, A.; Kato, A.C.; Ji, R.-R.; Decosterd, I. Gene Expression Profiling of Cutaneous Injured and Non-Injured Nociceptors in SNI Animal Model of Neuropathic Pain. Sci. Rep. 2017, 7, 9367. [Google Scholar] [CrossRef]
- Berta, T.; Lee, J.E.; Park, C.-K. Unconventional Role of Caspase-6 in Spinal Microglia Activation and Chronic Pain. Mediat. Inflamm. 2017, 2017, 9383184. [Google Scholar] [CrossRef]
- Berta, T.; Qadri, Y.J.; Chen, G.; Ji, R.-R. Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence. J. Dent. Res. 2016, 95, 1124–1131. [Google Scholar] [CrossRef]
- Berta, T.; Park, C.-K.; Xu, Z.-Z.; Xie, R.-G.; Liu, T.; Lü, N.; Liu, Y.-C.; Ji, R.-R. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-α secretion. J. Clin. Investig. 2014, 124, 1173–1186. [Google Scholar] [CrossRef]
- Dedek, A.; Hildebrand, M.E. Advances and Barriers in Understanding Presynaptic N-Methyl-D-Aspartate Receptors in Spinal Pain Processing. Front. Mol. Neurosci. 2022, 15, 864502. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Song, C.; Liu, H.; Li, Y.; Li, J.; Yu, Y.; Wang, G.; Cui, W. Spinal NR2B phosphorylation at Tyr1472 regulates IRE(-)DMT1-mediated iron accumulation and spine morphogenesis via kalirin-7 in tibial fracture-associated postoperative pain after orthopedic surgery in female mice. Reg. Anesth. Pain Med. 2021, 46, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shu, R.; Wang, H.; Yu, Y.; Wang, C.; Yang, M.; Wang, M.; Wang, G. Hydrogen-rich saline prevents remifentanil-induced hyperalgesia and inhibits MnSOD nitration via regulation of NR2B-containing NMDA receptor in rats. Neuroscience 2014, 280, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Dedek, A.; Xu, J.; Kandegedara, C.M.; Lorenzo, L.; Godin, A.G.; De Koninck, Y.; Lombroso, P.J.; Tsai, E.; Hildebrand, M.E. Loss of STEP61 couples disinhibition to N-methyl-d-aspartate receptor potentiation in rodent and human spinal pain processing. Brain 2019, 142, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tao, X.; Huang, P.; Lin, F.; Liu, Q.; Xu, L.; Xu, J.; Huang, Y. Corrigendum to ‘N-methyl-d-aspartate receptor subunit 2B on keratinocyte mediates peripheral and central sensitization in chronic post-ischemic pain in male rats’. Brain Behav. Immun. 2020, 87, 579–590, Erratum in Brain Behav Immun. 2020, 91, 799–800. [Google Scholar]
- Ren, Y.; Yang, M.; Wang, X.; Xu, B.; Xu, Z.; Su, B. ELAV-like RNA binding protein 1 regulates osteogenesis in diabetic osteoporosis: Involvement of divalent metal transporter 1. Mol. Cell. Endocrinol. 2022, 546, 111559. [Google Scholar] [CrossRef]
- Arita, A.; Kita, I.; Shinoda, S. Internalization and Decrease of Duodenal DMT1 Involved in Transient Suppression of Iron Uptake in Short-Acting Mucosal Block. Biol. Trace Elem. Res. 2022; in press. [Google Scholar] [CrossRef]
- Xu, W.; Liu, W.; Yu, W. The involvement of iron responsive element (-) divalent metal transporter 1-mediated the spinal iron overload via CXCL10/CXCR3 pathway in neuropathic pain in rats. Neurosci. Lett. 2019, 694, 154–160. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, Y.; Zhang, J.; Hou, J.; Fang, J.; Shen, J.; Xu, C.; Huang, M.; Qiao, H.; An, S. Phencynonate hydrochloride exerts antidepressant effects by regulating the dendritic spine density and altering glutamate receptor expression. Behav. Pharmacol. 2021, 32, 660–672. [Google Scholar] [CrossRef]
- Kiraly, E.D.; Lemtiri-Chlieh, F.; Levine, E.S.; Mains, R.E.; Eipper, B.A. Kalirin binds the NR2B subunit of the NMDA receptor, altering its synaptic localization and function. J. Neurosci. 2011, 31, 12554–12565. [Google Scholar] [CrossRef]
- Apriawan, T.; Widjiati, W.; Utomo, D.N.; Al Fauzi, A.; Subagio, E.A.; Utomo, B.; Islam, A.A.; Bajamal, A.H.; Sudiana, I.K. Periosteum-induced ossification effect in skull defect through interleukin-8 and NF-κB pathway: An experimental study with Oryctolagus cuniculus rabbits. Surg. Neurol. Int. 2022, 13, 140. [Google Scholar] [CrossRef]
- Xue, S.; Cao, Z.-X.; Wang, J.-N.; Zhao, Q.-X.; Han, J.; Yang, W.-J.; Sun, T. Receptor-Interacting Protein Kinase 3 Inhibition Relieves Mechanical Allodynia and Suppresses NLRP3 Inflammasome and NF-κB in a Rat Model of Spinal Cord Injury. Front. Mol. Neurosci. 2022, 15, 861312. [Google Scholar] [CrossRef]
- Abu-Amer, Y. NF-κB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.C.; Rubenstein, W.J.; Lee, E.J.; Siu, A.L.; Morrison, R.S. TNF-α and sTNF-RII Are Associated with Pain Following Hip Fracture Surgery in Older Adults. Pain Med. 2018, 19, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Yang, H.-W.; Yang, T.-S.; Xie, W.; Hu, Z.-H. TNF-α-mediated peripheral and central inflammation are associated with increased incidence of PND in acute postoperative pain. BMC Anesthesiol. 2021, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Sabsovich, I.; Guo, T.-Z.; Zhao, R.; Kingery, W.S.; Clark, D.J. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain 2009, 144, 303–313. [Google Scholar] [CrossRef]
- Li, W.-W.; Guo, T.-Z.; Li, X.-Q.; Kingery, W.S.; Clark, D.J. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain 2010, 151, 843–852. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.-M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457, Erratum in Nat. Immunol. 2017, 18, 1271. [Google Scholar] [CrossRef]
- Li, W.-W.; Yang, Y.; Guo, T.-Z.; Sahbaie, P.; Shi, X.-Y.; Guang, Q.; Kingery, W.S.; Herzenberg, L.A.; Clark, J.D. IL-6 signaling mediates the germinal center response, IgM production and nociceptive sensitization in male mice after tibia fracture. Brain Behav. Immun. 2021, 94, 148–158. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Song, M. Clinical Observation of Ultrasound-Guided Nerve Block Anesthesia on Postoperative Pain Control of Fracture Patients. J. Healthc. Eng. 2022, 2022, 9510669. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.; Li, W.; Kingery, W.S.; Angst, M.S.; Curtin, C.M.; Clark, J.D. Changes resembling complex regional pain syndrome following surgery and immobilization. J. Pain 2013, 14, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Birklein, F.; Ibrahim, A.; Schlereth, T.; Kingery, W.S. The Rodent Tibia Fracture Model: A Critical Review and Comparison With the Complex Regional Pain Syndrome Literature. J. Pain. 2018, 19, 1102.e1–1102.e19. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, E.; Kahlenbach, J.; von Banchet, G.S.; König, C.; Schaible, H.-G.; Ebersberger, A. Spinal interleukin-6 is an amplifier of arthritic pain in the rat. Arthritis Rheum. 2012, 64, 2233–2242. [Google Scholar] [CrossRef]
- Malsch, P.; Andratsch, M.; Vogl, C.; Link, A.S.; Alzheimer, C.; Brierley, S.; Hughes, P.; Kress, M. Deletion of interleukin-6 signal transducer gp130 in small sensory neurons attenuates mechanonociception and down-regulates TRPA1 expression. J. Neurosci. 2014, 34, 9845–9856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Q.; Liu, Z.; Chen, S.-P.; Li, M.; Shahveranov, A.; Ye, D.-W.; Tian, Y.-K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflammation 2016, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Melemedjian, O.K.; Tillu, D.V.; Moy, J.K.; Asiedu, M.N.; Mandell, E.K.; Ghosh, S.; Dussor, G.; Price, T.J. Local translation and retrograde axonal transport of CREB regulates IL-6-induced nociceptive plasticity. Mol. Pain 2014, 10, 45. [Google Scholar] [CrossRef]
- Melemedjian, O.K.; Asiedu, M.N.; Tillu, D.V.; Peebles, K.A.; Yan, J.; Ertz, N.; Dussor, G.O.; Price, T.J. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J. Neurosci. 2010, 30, 15113–15123. [Google Scholar] [CrossRef]
- Shi, X.; Guo, T.-Z.; Li, W.; Sahbaie, P.; Rice, K.C.; Sulima, A.; Clark, J.D.; Kingery, W.S. Exercise Reverses Nociceptive Sensitization, Upregulated Neuropeptide Signaling, Inflammatory Changes, Anxiety, and Memory Impairment in a Mouse Tibia Fracture Model. Anesthesiology 2018, 129, 557–575. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]
- Narazaki, M.; Kishimoto, T. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int. J. Mol. Sci. 2018, 19, 3528. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Thelen, M. Chemokines: Chemistry, Biochemistry and Biological Function. CHIMIA Int. J. Chem. 2016, 70, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Soler, D.; Chapman, T.R.; Poisson, L.R.; Wang, L.; Cote-Sierra, J.; Ryan, M.; McDonald, A.; Badola, S.; Fedyk, E.; Coyle, A.J.; et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J. Immunol. 2006, 177, 6940–6951. [Google Scholar] [CrossRef] [PubMed]
- Barsheshet, Y.; Wildbaum, G.; Levy, E.; Vitenshtein, A.; Akinseye, C.; Griggs, J.; Lira, S.A.; Karin, N. CCR8+ FOXp3+ Treg cells as master drivers of immune regulation. Proc. Natl. Acad. Sci. USA 2017, 114, 6086–6091. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, R.; Wang, X.; Li, Q.; Li, Y.; Jiao, Y.; Zhao, Q.; Guo, S.; Su, L.; Yu, Y.; et al. Spinal CCL1/CCR8 regulates phosphorylation of GluA1-containing AMPA receptor in postoperative pain after tibial fracture and orthopedic surgery in mice. Neurosci. Res. 2020, 154, 20–26. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, L.; Cao, S.; Hou, G.; Zhang, Q.; Ma, H.; Shi, B. Serum CCL21 as a Potential Biomarker for Cognitive Impairment in Spinal Cord Injury. Biomed. Res. Int. 2020, 2020, 6692802. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Zychowska, M.; Mika, J. Pharmacological blockade of CXCR3 by (±)-NBI-74330 reduces neuropathic pain and enhances opioid effectiveness—Evidence from in vivo and in vitro studies. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 3418–3437. [Google Scholar] [CrossRef]
- Honjoh, K.; Nakajima, H.; Hirai, T.; Watanabe, S.; Matsumine, A. Relationship of Inflammatory Cytokines From M1-Type Microglia/Macrophages at the Injured Site and Lumbar Enlargement With Neuropathic Pain After Spinal Cord Injury in the CCL21 Knockout (plt) Mouse. Front. Cell. Neurosci. 2019, 13, 525. [Google Scholar] [CrossRef]
- Konishi, H.; Kiyama, H. Non-pathological roles of microglial TREM2/DAP12: TREM2/DAP12 regulates the physiological functions of microglia from development to aging. Neurochem. Int. 2020, 141, 104878. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Zhang, H.; Wang, Y.; Gao, T.; Zhao, Y.; Wang, G.; Yu, Y.; Wang, C.; Li, Y. Artesunate Therapy Alleviates Fracture-Associated Chronic Pain After Orthopedic Surgery by Suppressing CCL21-Dependent TREM2/DAP12 Inflammatory Signaling in Mice. Front. Pharmacol. 2022, 13, 894963. [Google Scholar] [CrossRef]
- Rex, D.A.B.; Deepak, K.; Vaid, N.; Dagamajalu, S.; Kandasamy, R.K.; Flo, T.H.; Prasad, T.S.K. A modular map of Bradykinin-mediated inflammatory signaling network. J. Cell Commun. Signal. 2022, 16, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Bader, M. Kinin B1 receptors as a therapeutic target for inflammation. Expert Opin. Ther. Targets 2018, 22, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Minville, V.; Mouledous, L.; Jaafar, A.; Couture, R.; Brouchet, A.; Frances, B.; Tack, I.; Girolami, J.-P.; Gomez-Brouchet, A. Tibial post fracture pain is reduced in kinin receptors deficient mice and blunted by kinin receptor antagonists. J. Transl. Med. 2019, 17, 346. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.E.; Kroner, J.; Baur, S.; Schmid, F.; Walmsley, A.; Mottl, H.; Ignatius, A. Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. J. Orthop. Res. 2015, 33, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Guo, T.-Z.; Liang, D.; Shi, X.; Wei, T.; Kingery, W.S.; Clark, D.J. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain 2009, 147, 277–286. [Google Scholar] [CrossRef]
- Koewler, N.J.; Freeman, K.T.; Buus, R.J.; Herrera, M.B.; Jimenez-Andrade, J.M.; Ghilardi, J.R.; Peters, C.; Sullivan, L.J.; Kuskowski, M.A.; Lewis, J.L.; et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J. Bone Miner. Res. 2007, 22, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.T.; Evangelista, B.G.; Venega, R.A.; Seminowicz, D.A.; Chacur, M. Anti-NGF treatment can reduce chronic neuropathic pain by changing peripheral mediators and brain activity in rats. Behav. Pharmacol. 2019, 30, 79–88. [Google Scholar] [CrossRef]
- Majuta, L.A.; Longo, G.; Fealk, M.N.; McCaffrey, G.; Mantyh, P.W. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain 2015, 156, 157–165. [Google Scholar] [CrossRef]
- Bannwarth, B.; Kostine, M. Targeting nerve growth factor (NGF) for pain management: What does the future hold for NGF antagonists? Drugs 2014, 74, 619–626. [Google Scholar] [CrossRef]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012, 196, 775–788. [Google Scholar] [CrossRef]
- Hempstead, B.L. Brain-Derived Neurotrophic Factor: Three Ligands, Many Actions. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 9–19. [Google Scholar] [PubMed]
- Zhang, M.-D.; Barde, S.; Yang, T.; Lei, B.; Eriksson, L.I.; Mathew, J.P.; Andreska, T.; Akassoglou, K.; Harkany, T.; Hökfelt, T.G.M.; et al. Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proc. Natl. Acad. Sci. USA 2016, 113, E6686–E6695. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Meeus, M.; Versijpt, J.; Moens, M.; Bos, I.; Knaepen, K.; Meeusen, R. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: A new therapeutic target? Expert. Opin. Ther. Targets 2015, 19, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Angthong, C.; Manuwong, S.; Singh, V.; Rajbhandari, P.; Veljkovic, A. Efficacy of intravenous perioperative parecoxib administration in the surgical fixation of unstable ankle fracture: A prospective, double-blinded, randomized, placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4779–4784. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Robson, J. The dangers of NSAIDs: Look both ways. Br. J. Gen. Pract. 2016, 66, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.-Y.; Shen, S.-H.; Yang, T.-Y.; Huang, T.-W.; Huang, K.-C. Non-steroidal anti-inflammatory drugs and the risk of a second hip fracture: A propensity-score matching study. BMC Musculoskelet. Disord. 2016, 17, 201. [Google Scholar] [CrossRef][Green Version]
- Kim, H.; Kim, D.-H.; Kim, D.M.; Kholinne, E.; Lee, E.-S.; Alzahrani, W.M.; Kim, J.W.; Jeon, I.-H.; Koh, K.H. Do Nonsteroidal Anti-Inflammatory or COX-2 Inhibitor Drugs Increase the Nonunion or Delayed Union Rates After Fracture Surgery? A Propensity-Score-Matched Study. J. Bone Jt. Surg. 2021, 103, 1402–1410. [Google Scholar] [CrossRef]
- DePeter, K.C.; Blumberg, S.M.; Becker, S.D.; Meltzer, J.A. Does the Use of Ibuprofen in Children with Extremity Fractures Increase their Risk for Bone Healing Complications? J. Emerg. Med. 2017, 52, 426–432. [Google Scholar] [CrossRef]
- Aliuskevicius, M.; Østgaard, S.E.; Rasmussen, S. No influence of ibuprofen on bone healing after Colles’ fracture—A randomized controlled clinical trial. Injury 2019, 50, 1309–1317. [Google Scholar] [CrossRef]
- Nuelle, J.A.; Coe, K.M.; Oliver, H.A.; Cook, J.L.; Hoernschemeyer, D.G.; Gupta, S.K. Effect of NSAID Use on Bone Healing in Pediatric Fractures: A Preliminary, Prospective, Randomized, Blinded Study. J. Pediatr. Orthop. 2020, 40, e683–e689. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Lequang, J.A.; Passik, S.; Coluzzi, F. Using opioid therapy for pain in clinically challenging situations: Questions for clinicians. Minerva Anestesiol. 2019, 85, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Dagenais-Beaulé, V.; Tourigny, J.-F.; Papillon-Ferland, L. Opioid Use and Pain Control in the Elderly After Elective or Urgent Orthopaedic Surgery: A Retrospective Cohort Study. Clin. Drug Investig. 2019, 39, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, J.; Sampson, C.; Jones, K.B.; Higgins, T.F. Postoperative opioid administration inhibits bone healing in an animal model. Clin. Orthop. Relat. Res. 2013, 471, 4076–4081. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.J.; Pratt, N.; Roughead, E.E. Risk of Hip Fracture in Older People Using Selective Serotonin Reuptake Inhibitors and Other Psychoactive Medicines Concurrently: A Matched Case-Control Study in Australia. Drugs-Real World Outcomes 2017, 4, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Taipale, H.; Hamina, A.; Karttunen, N.; Koponen, M.; Tanskanen, A.; Tiihonen, J.; Hartikainen, S.; Tolppanen, A.-M. Incident opioid use and risk of hip fracture among persons with Alzheimer disease: A nationwide matched cohort study. Pain 2019, 160, 417–423. [Google Scholar] [CrossRef]
- Schwarzer, A.; Kaisler, M.; Kipping, K.; Seybold, D.; Rausch, V.; Maier, C.; Vollert, J. Opioid intake prior to admission is not increased in elderly patients with low-energy fractures: A case-control study in a German hospital population. Eur. J. Pain 2018, 22, 1651–1661. [Google Scholar] [CrossRef]
- Li, L.; Setoguchi, S.; Cabral, H.; Jick, S. Opioid use for noncancer pain and risk of fracture in adults: A nested case-control study using the general practice research database. Am. J. Epidemiol. 2013, 178, 559–569. [Google Scholar] [CrossRef]
- Tolppanen, A.-M.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Hartikainen, S. Comparison of predictors of hip fracture and mortality after hip fracture in community-dwellers with and without Alzheimer’s disease-exposure-matched cohort study. BMC Geriatr. 2016, 16, 204. [Google Scholar] [CrossRef]
- Acurcio, F.A.; Moura, C.S.; Bernatsky, S.; Bessette, L.; Rahme, E. Opioid Use and Risk of Nonvertebral Fractures in Adults With Rheumatoid Arthritis: A Nested Case-Control Study Using Administrative Databases. Arthritis Rheumatol. 2016, 68, 83–91. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Baek, C.-W.; Kim, H.-J.; Kim, E.-J.; Byeon, G.-J. Remifentanil Negatively Regulates RANKL-Induced Osteoclast Differentiation and Bone Resorption by Inhibiting c-Fos/NFATc1 Expression. Tissue Eng. Regen. Med. 2018, 15, 333–340. [Google Scholar] [CrossRef]
- Yu, E.H.Y.; Tran, D.H.D.; Lam, S.W.; Irwin, M.G. Remifentanil tolerance and hyperalgesia: Short-term gain, long-term pain? Anaesthesia 2016, 71, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Kurian, R.; Raza, K.; Shanthanna, H. A systematic review and meta-analysis of memantine for the prevention or treatment of chronic pain. Eur. J. Pain 2019, 23, 1234–1250. [Google Scholar] [CrossRef]
- Jimenez, E.C. Peptide antagonists of NMDA receptors: Structure-activity relationships for potential therapeutics. Peptides 2022, 153, 170796. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ohue, M.; Nakajima, M.; Fujii, Y.; Miyauchi, A.; Takagi, Y. Comparison of the effects of elcatonin and risedronate on back and knee pain by electroalgometry using fall of skin impedance and quality of life assessment using SF-36. J. Bone Miner. Metab. 2011, 29, 588–597. [Google Scholar] [CrossRef]
- Karponis, A.; Rizou, S.; Pallis, D.; Zafeiris, C.P.; Georgiou, D.F.; Galanos, A.; Giannoulis, F.; Lyritis, G.P. Analgesic effect of nasal salmon calcitonin during the early post-fracture period of the distal radius fracture. J. Musculoskelet. Neuronal Interact. 2015, 15, 186–189. [Google Scholar] [PubMed]
- Knopp-Sihota, J.A.; Newburn-Cook, C.V.; Homik, J.; Cummings, G.G.; Voaklander, D. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic vertebral compression fractures: A systematic review and meta-analysis. Osteoporos. Int. 2012, 23, 17–38. [Google Scholar] [CrossRef]
- Foye, P.M.; Shupper, P.; Wendel, I. Coccyx fractures treated with intranasal calcitonin. Pain Physician 2014, 17, E229–E233. [Google Scholar] [CrossRef]
- Roy, A.; Thulasiraman, S.; Panneerselvam, E.; Doss, G.T.; Selvaraj, M.N.; Ganesh, S.K.; Raja, K.V.; Kangusamy, B. Evaluation of the efficacy of salmon calcitonin nasal spray on bone healing following open reduction and internal fixation of mandibular fractures—A randomized controlled trial. J. Cranio-Maxillofac. Surg. 2021, 49, 1151–1157. [Google Scholar] [CrossRef]
- Srinivasan, A.; Wong, F.K.; Karponis, D. Calcitonin: A useful old friend. J. Musculoskelet. Neuronal. Interact. 2020, 20, 600–609. [Google Scholar]
- Sun, L.-M.; Lin, M.-C.; Muo, C.-H.; Liang, J.-A.; Kao, C.-H. Calcitonin nasal spray and increased cancer risk: A population-based nested case-control study. J. Clin. Endocrinol. Metab. 2014, 99, 4259–4264. [Google Scholar] [CrossRef]
- Rogers, M.J.; Crockett, J.C.; Coxon, F.P.; Mönkkönen, J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011, 49, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.; Bale, H.; Gludovatz, B.; Wat, A.; Tang, S.; Wang, M.; Busse, B.; Zimmermann, E.; Schaible, E.; Allen, M.R.; et al. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone 2015, 81, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.A.; Gludovatz, B.; Riedel, C.; Luengo, E.A.; Saiyed, R.; Marty, E.; Lorich, D.G.; Lane, J.M.; Ritchie, R.O.; Busse, B.; et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc. Natl. Acad. Sci. USA 2017, 114, 8722–8727. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The Effect of Vitamin D Treatment On Nerve Growth Factor (NGF) Release from Hippocampal Neurons. Noro Psikiyatr Ars. 2014, 51, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.A.N.; Cui, X.; Hammond, L.; Eyles, D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018, 32, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, X.; Tang, J.; Kasiappan, R.; Jinwal, U.; Li, P.; Hann, S.; Nicosia, S.V.; Wu, J.; Zhang, X.; et al. The coupling of epidermal growth factor receptor down regulation by 1alpha,25-dihydroxyvitamin D3 to the hormone-induced cell cycle arrest at the G1-S checkpoint in ovarian cancer cells. Mol. Cell. Endocrinol. 2011, 338, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.; Simpson, J.A.; Kotowicz, M.; Young, D.; Nicholson, G. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822, Erratum in JAMA 2010, 303, 2357. [Google Scholar] [CrossRef] [PubMed]
- Misgar, R.A.; Sahu, D.; Bhat, M.H.; Wani, A.I.; Bashir, M.I. Vitamin D Toxicity: A Prospective Study from a Tertiary Care Centre in Kashmir Valley. Indian J. Endocrinol. Metab. 2019, 23, 363–366. [Google Scholar] [CrossRef]
- Chowdry, A.M.; Azad, H.; Najar, M.S.; Mir, I. Acute kidney injury due to overcorrection of hypovitaminosis D: A tertiary center experience in the Kashmir Valley of India. Saudi J. Kidney Dis. Transplant. 2017, 28, 1321–1329. [Google Scholar] [CrossRef]
- Woo, S.-R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.K.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842, Erratum in Immunity 2015, 42, 199. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Jiang, C.; Andriessen, A.S.; Wang, K.; Wang, Z.; Ding, H.; Zhao, J.; Luo, X.; Lee, M.S.; Lei, Y.L.; et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature 2021, 591, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; Van Der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Donnelly, C.R.; Jiang, C.; Liao, Y.; Luo, X.; Tao, X.; Bang, S.; McGinnis, A.; Lee, M.; Hilton, M.J.; et al. STING suppresses bone cancer pain via immune and neuronal modulation. Nat. Commun. 2021, 12, 4558. [Google Scholar] [CrossRef]
- Navratilova, E.; Porreca, F. Reward and motivation in pain and pain relief. Nat. Neurosci. 2014, 17, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Norling, L.V.; Headland, S.E.; Dalli, J.; Arnardottir, H.H.; Haworth, O.; Jones, H.R.; Irimia, D.; Serhan, C.N.; Perretti, M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 2016, 1, e85922. [Google Scholar] [CrossRef]
- Zhang, L.; Terrando, N.; Xu, Z.-Z.; Bang, S.; Jordt, S.-E.; Maixner, W.; Serhan, C.N.; Ji, R.-R. Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-operative Pain After Bone Fracture in Mice. Front. Pharmacol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Zou, Y.; Tuo, F.; Zhang, Z.; Guo, J.; Yuan, Y.; Zhang, H.; Xu, Z.; Pan, Z.; Tang, Y.; Deng, C.; et al. Safety and Efficacy of Adjunctive Therapy With Artesunate in the Treatment of Severe Malaria: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 596697. [Google Scholar] [CrossRef]
- Guruprasad, B.; Chaudhary, P.; Choedon, T.; Kumar, V.L. Artesunate Ameliorates Functional Limitations in Freund’s Complete Adjuvant-Induced Monoarthritis in Rat by Maintaining Oxidative Homeostasis and Inhibiting COX-2 Expression. Inflammation 2015, 38, 1028–1035. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Gao, T.; Zhang, H.; Li, J.; Wang, G.; Wang, C.; Li, Y. Artesunate Reduces Remifentanil-induced Hyperalgesia and Peroxiredoxin-3 Hyperacetylation via Modulating Spinal Metabotropic Glutamate Receptor 5 in Rats. Neuroscience 2022, 487, 88–98. [Google Scholar] [CrossRef]
| NSAIDs | References | Study Type | Study Population | Evaluation and Analysis Indicators | Results |
|---|---|---|---|---|---|
| Parecoxib | Angthong C et al., 2021 [85] | Prospective double-blinded randomized placebo-controlled trial | Unstable ankle fracture N = 40 Mean age: 49.3 ± 18.0 | Verbal numerical rating score (VNRS); verbal numerical rating percentage (VNRP) | Parecoxib (40 mg i.v.), although not providing good perioperative analgesia, may shorten the length of hospital stay |
| NSAIDs | Chuang PY et al., 2016 [87] | Propensity-score-matching study | Previous hip fracture N = 555 Mean age: >40 | Kaplan–Meier survival analysis | Patients with hip fractures taking NSAIDs had an increased risk of a second hip fracture, which was significantly related to the dose of NSAIDs taken, and older adults ≥60 years of age had a higher risk of a second fracture. |
| COX-2-selective NSAID | Kim H et al., 2021 [88] | Propensity-score-matched study | Patients treated with NSAIDs/COX-2 drugs after fracture surgery N = 8693 Mean age: ≥19 | Kaplan–Meier survival analysis | NSAIDs/COX-2 inhibitors for >3 weeks after orthopedic surgery for long bone fractures may lead to nonunion or delayed union. |
| Ibuprofen | DePeter KC et al., 2017 [89] | Retrospective study | Children with Extremity Fractures N = 808 Mean age: 6 months–17 years | X-rays | Perioperative or postoperative ibuprofen use in children with long bone fractures was not significantly associated with nonunion, delayed union, or bone redisplacement. |
| Ibuprofen | Aliskevicious M et al., 2019 [90] | Single-center, triple-blind, randomized clinical trial | Colles fracture N = 95 Mean age: 42–85 | Daily pain score (1–10); DASH score | Ibuprofen can provide good analgesia in patients with acute fractures while reducing the dose of opioids without serious orthopedic complications. |
| Ibuprofen | Nuelle JAV et al., 2020 [91] | Prospective randomized parallel single-blinded study | Children long bone fracture N = 95 Mean age: <16 (male); <14 (female) | Visual analog scale (VAS); X-rays | Ibuprofen does not inhibit the healing of long bone fractures in children and has no significant side effects, so it is recommended for the clinical treatment of children with fractures |
| Opioid Agonist | References | Study Type | Study Population | Evaluation and Analysis Indicators | Results |
|---|---|---|---|---|---|
| Opioids | Dagenais-Beaulé et al., 2019 [93] | Retrospective cohort study | After elective or urgent orthopedic surgery N = 250 Mean age: ≥65 | Confusion assessment method (CAM) score; digital pain rating scale (0–10) | Compared with the old group (>80 years old) and the young group (65–79 years old), it can be observed that the opioid consumption in the former group is lower within one week after surgery, but the side effects (disorder of consciousness, renal function damage, etc.) are significantly increased. |
| Opioids | Leach et al., 2017 [95] | Matched case-control study | Hip fracture N = 44,138 Mean age: >65 | Conditional logistic regression model | Initiated/continued opioid use in older adults (>65 years) increases the risk of hip fracture. |
| Weak opioids, buprenorophine, strong opioids | Taipale et al., 2019 [96] | MEDALZ cohort | Alzheimer’s patients N = 9500 Average age: 88 | Cox proportional hazard models | Long-term (>180 days) opioid use in patients with Alzheimer’s disease increases the risk of hip fracture, and this phenomenon is positively associated with opioid intensity. |
| Opioids | Schwarzer et al., 2018 [97] | Case-controlled study | Low-energy fractures N = 992 Mean age: >60 | Follow-up | Adverse events such as fatigue, gastrointestinal reactions, etc., are more common in patients with low-energy fractures taking opioids. |
| Opioids | Li L et al., 2013 [98] | Randomized risk-set sampling trial | Noncancer patients N = 1,700,000 Mean age: 18–80 | Conditional logistic regression model | Opioid use increases the risk of fractures in adults with noncancer pain, especially in the first few weeks of use, which is largely influenced by the acute central nervous system effects of opioids. |
| Opioids | Tolppanen AM et al., 2016 [99] | Exposure-matched cohort | Alzheimer’s patients N = 67,072 Mean age: 34–105 | Cox regression | Among non-AD patients in the control group, opioid use was strongly associated with higher fracture rates. |
| Opioids | Acurcio FA et al., 2016 [100] | Retrospective nested case-control study | rheumatoid arthritis (RA) N = 9769 Mean age: >20 | Follow-up | In RA patients, opioid use is associated with an increased incidence of nonvertebral fractures. |
| Calcitonin | References | Study Type | Study Population | Evaluation and Analysis Indicators | Results |
|---|---|---|---|---|---|
| Elcatonin vs. risedronate | Fujita T et al., 2011 [105] | Prospective randomized double-blind study | Chief complaint of back and/or knee pain N = 40 Mean age: >50 | Visual rating system (VRS) (0–100); electroalgometry (EAM) | Intramuscular injection of calcitonin showed good analgesic effect in both subjective (VRS) and objective (EAM) evaluation indicators. |
| Nasal salmon calcitonin | Karponis A et al., 2015 [106] | Prospective randomized double-blind study | Distal radius fracture N = 41 Mean age: >50 | Visual analogue scale (VAS) (0–10); follow-up | In patients with distal radius fractures, significant analgesic effects were seen 10 days after orthopedic surgery and persisted until 45 days after surgery. |
| Calcitonin | Knopp-Sihota JA et al., 2012 [107] | Systematic review and meta-analysis | recent and remote osteoporotic vertebral compression fractures (OVCF) N = 589 Mean age: >50 | Visual analogue scale (VAS) | The efficacy of calcitonin in the treatment of acute OVCF in elderly (>60 years old) patients is worthy of recognition, and its side effects are mild (mainly gastrointestinal reactions). |
| Salmon calcitonin nasal spray | Roy A et al., 2021 [109] | Randomized controlled trial | Mandibular fractures N = 14 Mean age: 20–40 | Visual analogue scale (VAS); Biochemical assessment; Radiological assessment (OPG) | Intranasal salmon calcitonin spray can inhibit postoperative pain after open reduction and internal fixation of fractures, increase plasma osteocalcin levels, and promote fracture healing. |
| Calcitonin nasal spray | Sun LM et al., 2014 [111] | Population-based nested case-control study | Osteoporosis N = 5652 | Follow-up | Women with osteoporosis have an increased risk of liver cancer after CNS use. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, H.; Li, N.; Li, J.; Zhang, L. Chronic Pain after Bone Fracture: Current Insights into Molecular Mechanisms and Therapeutic Strategies. Brain Sci. 2022, 12, 1056. https://doi.org/10.3390/brainsci12081056
Zhao Y, Zhang H, Li N, Li J, Zhang L. Chronic Pain after Bone Fracture: Current Insights into Molecular Mechanisms and Therapeutic Strategies. Brain Sciences. 2022; 12(8):1056. https://doi.org/10.3390/brainsci12081056
Chicago/Turabian StyleZhao, Yuying, Haoyue Zhang, Nan Li, Jing Li, and Linlin Zhang. 2022. "Chronic Pain after Bone Fracture: Current Insights into Molecular Mechanisms and Therapeutic Strategies" Brain Sciences 12, no. 8: 1056. https://doi.org/10.3390/brainsci12081056
APA StyleZhao, Y., Zhang, H., Li, N., Li, J., & Zhang, L. (2022). Chronic Pain after Bone Fracture: Current Insights into Molecular Mechanisms and Therapeutic Strategies. Brain Sciences, 12(8), 1056. https://doi.org/10.3390/brainsci12081056





