Patients with Methamphetamine Use Disorder Show Highly Utilized Proactive Inhibitory Control and Intact Reactive Inhibitory Control with Long-Term Abstinence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Questionnaires
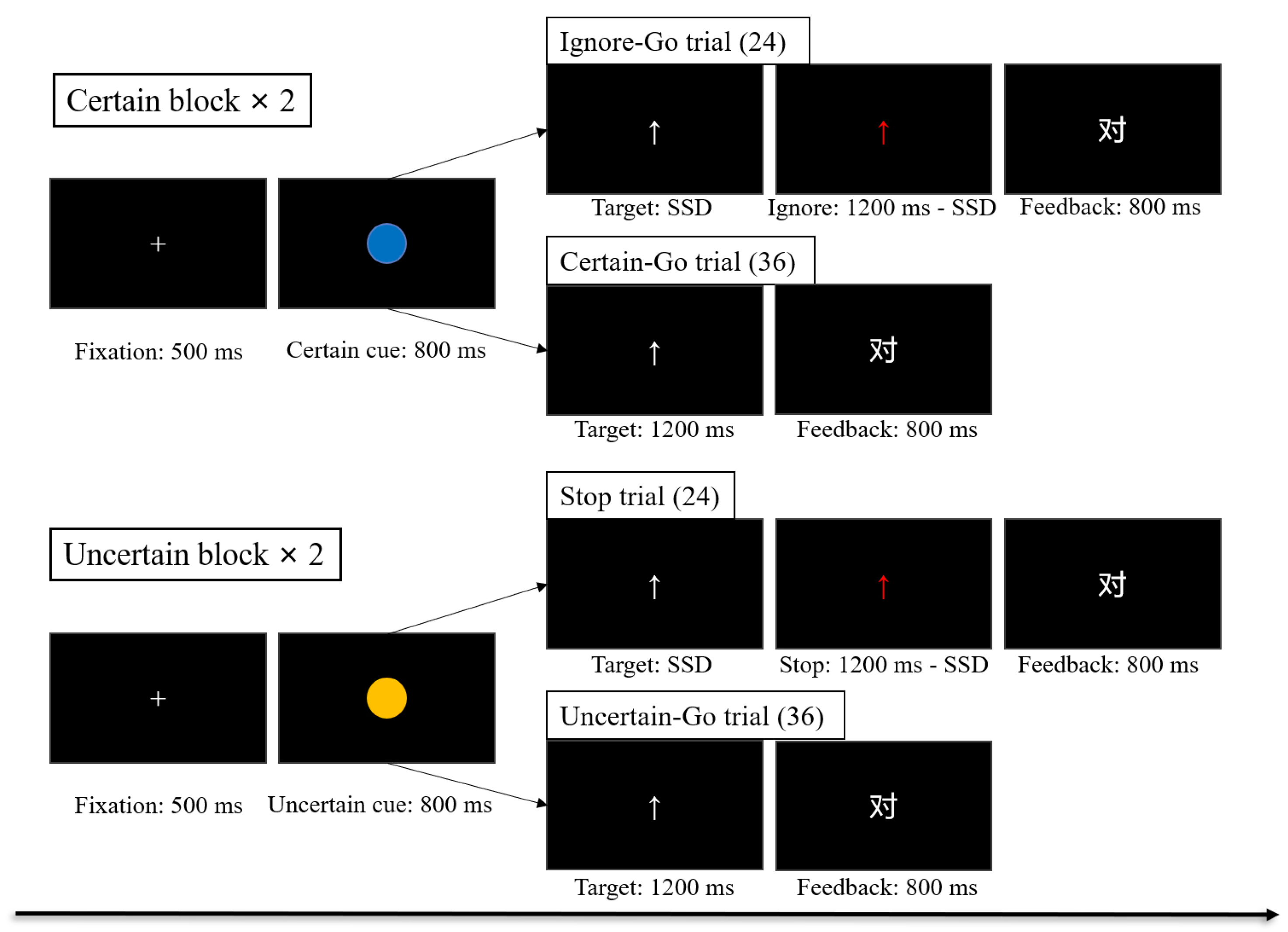
2.2.2. Task
2.2.3. Data Analysis
3. Results
3.1. Questionnaire Results
3.2. C-SST Results
3.3. Correlation Analysis Results
4. Discussion
4.1. MUD Patients with Long-Term Abstinence Utilized More Proactive Inhibitory Control than HCs
4.2. MUD Patients with Long-Term Abstinence Showed Intact Reactive Inhibition Control
4.3. Inhibitory Control of MUD Patients with Long-Term Abstinence Was Associated with Sensation Seeking
4.4. Limitations and Prospect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BDI-II | Beck Depression Inventory—version 2 |
| CI | Confidence interval |
| C-SST | Condition Stop-Signal Task |
| DSM-V | Diagnostic and Statistical Manual of Mental Disorders (5th edition) |
| FDR | False discovery rate |
| HCs | Healthy controls |
| MUD | Methamphetamine use disorder |
| PSS | Post-signal slowing |
| RT | Reaction time |
| SPSS | Statistical Package for the Social Sciences |
| SSD | Stop-signal delay |
| SSRT | Stop-signal reaction time |
| STAI-T | State-Trait Anxiety Inventory—T |
References
- Volkow, N.D.; Li, T.K. Drug addiction: The neurobiology of behaviour gone awry. Nat. Rev. Neurosci. 2004, 5, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, M.T. Drug Abuse as a Problem of Impaired Control: Current Approaches and Findings. Behav. Cogn. Neurosci. Rev. 2003, 2, 179–197. [Google Scholar] [CrossRef] [PubMed]
- World Drug Report 2021; Report; United Nations Office on Drug and Crime: Vienna, Austria, 2021.
- Li, Y.; Wang, P.; Bai, L.; Fu, W.; Wang, J. Current Situation analysis of New Drug Abusers in Yunnan Province-Surveillance Data From 2011–2013. Chin. J. Drug Depend. 2014, 23, 453. [Google Scholar] [CrossRef]
- O’Malley, G.F.; O’Malley, R. Amphetamines. In Report, Merck Manual for Health Care Professionals; Merck & Co., Inc.: Rahway, NJ, USA, 2012. [Google Scholar]
- Westfall, D.; Westfall, T. Miscellaneous Sympathomimetic Agonists. In Goodman & Gilman’s Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Zhao, X. Research on the New Drug Crime Incentives and Its Control Mechanisms. Citizens Law (Law Ed.) 2010, 10, 19–21. [Google Scholar]
- China Drug Situation Report 2020; Report; Office of the National Narcotics Control Commission: Beijing, China, 2021.
- Aron, A.R. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, F.; Stevens, T.; Chambers, C.D. Proactive and reactive stopping when distracted: An attentional account. J. Exp. Psychol. Hum. Percept. Perform. 2014, 40, 1295–1300. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. 2008, 137, 649–672. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, F.; Logan, G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008, 12, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Braver, T.S.; Gray, J.R.; Burgess, G.C. Explaining the Many Varieties of Working Memory Variation: Dual Mechanisms of Cognitive Control. In Variation in Working Memory; Oxford University Press: Oxford, UK, 2008; pp. 76–106. [Google Scholar] [CrossRef] [Green Version]
- Baines, L.; Field, M.; Christiansen, P.; Jones, A. The effect of alcohol cue exposure and acute intoxication on inhibitory control processes and ad libitum alcohol consumption. Psychopharmacology 2019, 236, 2187–2199. [Google Scholar] [CrossRef] [Green Version]
- Baines, L.; Field, M.; Christiansen, P.; Jones, A. Isolating Proactive Slowing from Reactive Inhibitory Control in Heavy Drinkers. Subst. Use Misuse 2020, 55, 167–173. [Google Scholar] [CrossRef]
- Hu, S.; Ide, J.S.; Zhang, S.; Sinha, R.; Li, C.S.R. Conflict anticipation in alcohol dependence—A model-based fMRI study of stop signal task. Neuroimage Clin. 2015, 8, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Brevers, D.; Bechara, A.; Kilts, C.D.; Antoniali, V.; Bruylant, A.; Verbanck, P.; Kornreich, C.; Noel, X. Competing Motivations: Proactive Response Inhibition Toward Addiction-Related Stimuli in Quitting-Motivated Individuals. J. Gambl. Stud. 2018, 34, 785–806. [Google Scholar] [CrossRef] [PubMed]
- Guerin, A.A.; Bonomo, Y.; Lawrence, A.J.; Baune, B.T.; Nestler, E.J.; Rossell, S.L.; Kim, J.H. Cognition and Related Neural Findings on Methamphetamine Use Disorder: Insights and Treatment Implications From Schizophrenia Research. Front. Psychiatry 2019, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Pelletier, J.; Grot, S.; Hébert, C.; Barr, A.M.; Lecomte, T. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addict. Behav. 2018, 80, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Monterosso, J.R.; Aron, A.R.; Cordova, X.; Xu, J.; London, E.D. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005, 79, 273–277. [Google Scholar] [CrossRef]
- Tabibnia, G.; Monterosso, J.R.; Baicy, K.; Aron, A.R.; Poldrack, R.A.; Chakrapani, S.; Lee, B.; London, E.D. Different forms of self-control share a neurocognitive substrate. J. Neurosci. 2011, 31, 4805–4810. [Google Scholar] [CrossRef] [Green Version]
- Van der Plas, E.A.; Crone, E.A.; van den Wildenberg, W.P.; Tranel, D.; Bechara, A. Executive control deficits in substance-dependent individuals: A comparison of alcohol, cocaine, and methamphetamine and of men and women. J. Clin. Exp. Neuropsychol. 2009, 31, 706–719. [Google Scholar] [CrossRef]
- Bell, R.P.; Foxe, J.J.; Ross, L.A.; Garavan, H. Intact inhibitory control processes in abstinent drug abusers (I): A functional neuroimaging study in former cocaine addicts. Neuropharmacology 2014, 82, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Morie, K.P.; Garavan, H.; Bell, R.P.; De Sanctis, P.; Krakowski, M.I.; Foxe, J.J. Intact inhibitory control processes in abstinent drug abusers (II): A high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology 2014, 82, 151–160. [Google Scholar] [CrossRef]
- Magid, V.; Maclean, M.G.; Colder, C.R. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addict. Behav. 2007, 32, 2046–2061. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Ryan, N.; Rubia, K.; Conrod, P.J. Response Inhibition and Reward Response Bias Mediate the Predictive Relationships Between Impulsivity and Sensation Seeking and Common and Unique Variance in Conduct Disorder and Substance Misuse. Alcohol. Clin. Exp. Res. 2011, 35, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Dervaux, A.; Baylé, F.J.; Laqueille, X.; Bourdel, M.C.; Le Borgne, M.H.; Olié, J.P.; Krebs, M.O. Is Substance Abuse in Schizophrenia Related to Impulsivity, Sensation Seeking, or Anhedonia? Am. J. Psychiatry 2001, 158, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, C.; Huang, J. Reliability and validity of the Chinese version of Beck Depression Inventory-II among depression patients. Chin. Ment. Health J. 2011, 25, 476–480. [Google Scholar]
- Zhang, Y.; He, Y. Handbook of Psychiatric Rating Scale, 2nd ed.; Hunan Science and Technology Press: Changsha, China, 2016. [Google Scholar]
- Wang, W.; Wu, Y.X.; Peng, Z.G.; Lu, S.W.; Yu, L.; Wang, G.P.; Fu, X.M.; Wang, Y.H. Test of sensation seeking in a Chinese sample. Personal. Individ. Differ. 2000, 28, 169–179. [Google Scholar] [CrossRef]
- Chikazoe, J.; Jimura, K.; Hirose, S.; Yamashita, K.; Miyashita, Y.; Konishi, S. Preparation to Inhibit a Response Complements Response Inhibition during Performance of a Stop-Signal Task. J. Neurosci. 2009, 29, 15870–15877. [Google Scholar] [CrossRef]
- Zheng, Y.; Mei, S.; Yi, W.; Li, Q.; Liu, X. Abnormal performance monitoring but intact response inhibition in sensation seeking. Psychophysiology 2019, 56, e13373. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Aron, A.R.; Band, G.P.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H.; et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 2019, 8, e46323. [Google Scholar] [CrossRef]
- Levitt, H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 1971, 49, 467–477. [Google Scholar] [CrossRef]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984, 10, 276–291. [Google Scholar] [CrossRef]
- Logan, G.D. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In Inhibitory Processes in Memory and Language; Book Section 5; Dagenbach, D., Carr, T., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 189–239. [Google Scholar]
- Li, C.S.R.; Milivojevic, V.; Kemp, K.; Hong, K.; Sinha, R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006, 85, 205–212. [Google Scholar] [CrossRef]
- Salo, R.; Fassbender, C.; Buonocore, M.H.; Ursu, S. Behavioral regulation in methamphetamine abusers: An fMRI study. Psychiatry Res. Neuroimaging 2013, 211, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigam, K.B.; Straub, L.K.; Zuniga, E.A.; Sami, A.; Cunningham, K.A.; Anastasio, N.C.; Moeller, F.G.; Bjork, J.M. Blunted prefrontal signature of proactive inhibitory control in cocaine use disorder. Drug Alcohol Depend. 2021, 218, 108402. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, J.; Vink, M.; Durston, S.; Zandbelt, B.B. Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. Neuroimage 2014, 103, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bartholdy, S.; Campbell, I.C.; Schmidt, U.; O’Daly, O.G. Proactive inhibition: An element of inhibitory control in eating disorders. Neurosci. Biobehav. Rev. 2016, 71, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.J. A debate on working memory and cognitive control: Can we learn about the treatment of substance use disorders from the neural correlates of anorexia nervosa? BMC Psychiatry 2016, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Houben, K.; Nederkoorn, C.; Wiers, R.W.; Jansen, A. Resisting temptation: Decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depend. 2011, 116, 132–136. [Google Scholar] [CrossRef]
- Tolliver, B.K.; Price, K.L.; Baker, N.L.; LaRowe, S.D.; Simpson, A.N.; McRae-Clark, A.L.; Saladin, M.E.; DeSantis, S.M.; Chapman, E.; Garrett, M.; et al. Impaired Cognitive Performance in Subjects with Methamphetamine Dependence during Exposure to Neutral versus Methamphetamine-Related Cues. Am. J. Drug Alcohol Abus. 2012, 38, 251–259. [Google Scholar] [CrossRef]
- Guerin, A.A.; Drummond, K.D.; Bonomo, Y.; Lawrence, A.J.; Rossell, S.L.; Kim, J.H. Assessing methamphetamine-related cue reactivity in people with methamphetamine use disorder relative to controls. Addict. Behav. 2021, 123, 107075. [Google Scholar] [CrossRef]
- Weafer, J.; Fillmore, M.T. Low-Dose Alcohol Effects on Measures of Inhibitory Control, Delay Discounting, and Risk-Taking. Curr. Addict. Rep. 2016, 3, 75–84. [Google Scholar] [CrossRef]
- Campbell, A.E.; Chambers, C.D.; Allen, C.P.G.; Hedge, C.; Sumner, P. Impairment of manual but not saccadic response inhibition following acute alcohol intoxication. Drug Alcohol Depend. 2017, 181, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Ruiz, M.J.; van den Wildenberg, W.P.; Bajo, M.T.; Hommel, B. Long-term effects of chronic khat use: Impaired inhibitory control. Front. Psychol. 2010, 1, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, R.; Lin, J.; Miles, S.; Kydd, R.; Russell, B. Striatal Volume Increases in Active Methamphetamine-Dependent Individuals and Correlation with Cognitive Performance. Brain Sci. 2012, 2, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Bardo, M.T.; Williams, Y.; Dwoskin, L.P.; Moynahan, S.E.; Perry, I.B.; Martin, C.A. The Sensation Seeking Trait and Substance Use: Research Findings and Clinical Implications. Curr. Psychiatry Rev. 2007, 3, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Zuckerman, M. Sensation Seeking and Risky Behavior; American Psychological Association: Washington, DC, USA, 2007. [Google Scholar]
- Noel, X.; Brevers, D.; Bechara, A.; Hanak, C.; Kornreich, C.; Verbanck, P.; Le Bon, O. Neurocognitive Determinants of Novelty and Sensation-Seeking in Individuals with Alcoholism. Alcohol Alcohol. 2011, 46, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, J.J.; Thompson-Lake, D.G.Y.; Cooper, K.; Verrico, C.D.; Newton, T.F.; De La Garza, R. A comparison of impulsivity, depressive symptoms, lifetime stress and sensation seeking in healthy controls versus participants with cocaine or methamphetamine use disorders. J. Psychopharmacol. 2014, 29, 50–56. [Google Scholar] [CrossRef]
- Fillmore, M.T.; Ostling, E.W.; Martin, C.A.; Kelly, T.H. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend. 2009, 100, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.; Wegmann, E.; Stark, R.; Müller, A.; Wölfling, K.; Robbins, T.W.; Potenza, M.N. The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neurosci. Biobehav. Rev. 2019, 104, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Yang, Z.; Dai, W.; Zheng, Y.; Sun, Y.; Liu, X. Dysfunctional cognitive control and reward processing in adolescents with Internet gaming disorder. Psychophysiology 2019, 57, e13469. [Google Scholar] [CrossRef]
- Corbin, W.R.; Papova, A.; Morean, M.E.; O’Malley, S.S.; Krishnan-Sarin, S.; Abi-Dargham, A.; Anticevic, A.; Pearlson, G.; Petrakis, I.; Pittman, B.P.; et al. Integrating Acquired Preparedness and Dual Process Models of Risk for Heavy Drinking and Related Problems. Psychol. Addict. Behav. 2015, 29, 864–874. [Google Scholar] [CrossRef]
- Zandbelt, B.B.; Bloemendaal, M.; Neggers, S.F.W.; Kahn, R.S.; Vink, M. Expectations and violations: Delineating the neural network of proactive inhibitory control. Hum. Brain Mapp. 2013, 34, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.D.; Garavan, H.; Bellgrove, M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009, 33, 631–646. [Google Scholar] [CrossRef]
- Dluzen, D.E.; Liu, B. Gender differences in methamphetamine use and responses: A review. Gend. Med. 2008, 5, 24–35. [Google Scholar] [CrossRef]
- Simpson, J.L.; Grant, K.M.; Daly, P.M.; Kelley, S.G.; Carlo, G.; Bevins, R.A. Psychological Burden and Gender Differences in Methamphetamine-Dependent Individuals in Treatment. J. Psychoact. Drugs 2016, 48, 261. [Google Scholar] [CrossRef] [PubMed]
- Corsi, K.F.; Garver-Apgar, C.; Booth, R.E. Gender differences in HIV risk and mental health among methamphetamine users. Drug Alcohol Depend. 2014, 140, e39. [Google Scholar] [CrossRef]
- Smith, J.L.; Iredale, J.M.; Mattick, R.P. Sex differences in the relationship between heavy alcohol use, inhibition and performance monitoring: Disconnect between behavioural and brain functional measures. Psychiatry Res.-Neuroimaging 2016, 254, 103–111. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, S.; Hu, S.; Bednarski, S.R.; Erdman, E.; Farr, O.M.; Hong, K.I.; Sinha, R.; Mazure, C.M.; Li, C.S.R. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain 2013, 136, 1231–1244. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhong, N.; Su, H.; Ruan, X.; Bao, J.; Zhang, L.; Du, J.; Xu, D.; Ding, R.; Xiao, K.; et al. Strengths, weaknesses, opportunities and threats (SWOT) analysis of reinitiation into methamphetamine abusers: Qualitative findings from an exploration of methamphetamine abusers in Shanghai, China. Gen. Psychiatry 2019, 32, e100062. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Mamy, J.; Gao, P.; Xiao, S. From Abstinence to Relapse: A Preliminary Qualitative Study of Drug Users in a Compulsory Drug Rehabilitation Center in Changsha, China. PLoS ONE 2015, 10, e0130711. [Google Scholar] [CrossRef]
- Yang, L.; Xu, J.; Cao, H.; Geng, Y. The Application of Computerized Psychology Intervention in Substance Addiction. J. Psychol. Sci. 2017, 40, 746–752. [Google Scholar] [CrossRef]
- Carroll, K.M.; Kiluk, B.D. Cognitive behavioral interventions for alcohol and drug use disorders: Through the stage model and back again. Psychol. Addict. Behav. 2017, 31, 847–861. [Google Scholar] [CrossRef] [PubMed]




| HC (N = 35, Female = 17) | MUD (N = 35, Female = 19) | t Value | p Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (years) | 29.43 | 6.66 | 27.34 | 6.95 | t (68) = 1.28 | 0.204 |
| Education (years) | 10.34 | 1.85 | 9.53 | 1.90 | t (68) = 1.82 | 0.073 |
| Duration of methamphetamine abstinence (days) | - | 264.89 | 167.35 | - | - | |
| Lifetime methamphetamine dosage (grams) | - | 265.57 | 428.87 | - | - | |
| DSM-5 (methamphetamine) | - | 8.43 | 2.05 | - | - | |
| HC (N = 35, Female = 17) | MUD (N = 35, Female = 17) | t Value | p Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | (df = 68) | ||
| BDI-II (depression) | 9.86 | 9.45 | 11.82 | 9.46 | −0.86 | 0.391 |
| STAI-T (trait anxiety) | 57.37 | 5.41 | 56.26 | 8.86 | 0.64 | 0.528 |
| Sensation Seeking Scale | 13.54 | 5.87 | 16.86 | 5.10 | −2.52 | 0.014 |
| Boredom Susceptibility | 1.97 | 1.56 | 2.20 | 1.43 | −0.64 | 0.525 |
| Disinhibition of Desire | 2.83 | 1.93 | 4.00 | 2.61 | −2.13 | 0.037 |
| Experience Seeking | 3.89 | 1.55 | 4.11 | 1.68 | −0.59 | 0.555 |
| Thrill and Adventure Seeking | 4.86 | 2.65 | 6.54 | 2.16 | −2.92 | 0.005 |
| HC (N = 35, Female = 17) | MUD (N = 35, Female = 17) | t Value | p Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | (df = 68) | ||
| Certain-Go RT (ms) | 428.37 | 56.17 | 415.71 | 41.22 | - | - |
| Ignore-Go RT (ms) | 435.54 | 50.87 | 425.97 | 41.28 | - | - |
| Uncertain-Go RT (ms) | 521.40 | 67.16 | 545.80 | 79.10 | - | - |
| Fail-to-stop Go RT (ms) | 459.43 | 50.42 | 468.34 | 63.80 | - | - |
| Baseline RT (ms) | 585.40 | 102.64 | 621.26 | 115.45 | −1.37 | 0.174 |
| Preparation cost (ms) | 92.97 | 50.54 | 130.14 | 52.68 | −3.01 | 0.004 |
| Critical SSD (ms) | 261.14 | 53.63 | 280.37 | 51.04 | −1.54 | 0.129 |
| SSRT (ms) | 260.20 | 43.18 | 265.37 | 56.11 | −0.43 | 0.667 |
| PSS effect (ms) | 44.31 | 33.25 | 36.43 | 37.51 | 0.93 | 0.355 |
| Certain-Go accuracy | 97.82% | 3.00% | 97.94% | 2.60% | - | - |
| Ignore-Go accuracy | 95.71% | 4.10% | 94.94% | 4.44% | - | - |
| Uncertain-Go accuracy | 97.50% | 2.63% | 97.14% | 3.43% | - | - |
| Stop accuracy | 49.94% | 4.89% | 51.25% | 5.61% | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Zhou, H.; Møller, A.; Wei, P.; Hu, K.; Feng, K.; Han, J.; Li, Q.; Liu, X. Patients with Methamphetamine Use Disorder Show Highly Utilized Proactive Inhibitory Control and Intact Reactive Inhibitory Control with Long-Term Abstinence. Brain Sci. 2022, 12, 974. https://doi.org/10.3390/brainsci12080974
Dai W, Zhou H, Møller A, Wei P, Hu K, Feng K, Han J, Li Q, Liu X. Patients with Methamphetamine Use Disorder Show Highly Utilized Proactive Inhibitory Control and Intact Reactive Inhibitory Control with Long-Term Abstinence. Brain Sciences. 2022; 12(8):974. https://doi.org/10.3390/brainsci12080974
Chicago/Turabian StyleDai, Weine, Hui Zhou, Arne Møller, Ping Wei, Kesong Hu, Kezhuang Feng, Jie Han, Qi Li, and Xun Liu. 2022. "Patients with Methamphetamine Use Disorder Show Highly Utilized Proactive Inhibitory Control and Intact Reactive Inhibitory Control with Long-Term Abstinence" Brain Sciences 12, no. 8: 974. https://doi.org/10.3390/brainsci12080974






