CysLT2R Antagonist HAMI 3379 Ameliorates Post-Stroke Depression through NLRP3 Inflammasome/Pyroptosis Pathway in Gerbils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocols
2.3. Establishing the tGCI Model
2.4. Restraint Stress
2.5. Intracerebroventricular Injection of Short Hairpin RNA (shRNA)-CysLT2R or Lentivirus (LV)-CysLT2R
2.6. Monitoring the Regional Cerebral Blood Flow (rCBF)
2.7. Behavioral Testing
2.8. Nissl Staining
2.9. TUNEL Assay
2.10. Preparation of the Cell Culture
2.11. OGD/Recovery (OGD/R) Model
2.12. Analysis of NLRP3 Inflammasome Activation in BV2 Cells
2.13. Quantitative PCR (Q-PCR)
2.14. Immunofluorescence Staining Analysis
2.15. Western Blot
2.16. Statistical Analysis
3. Results
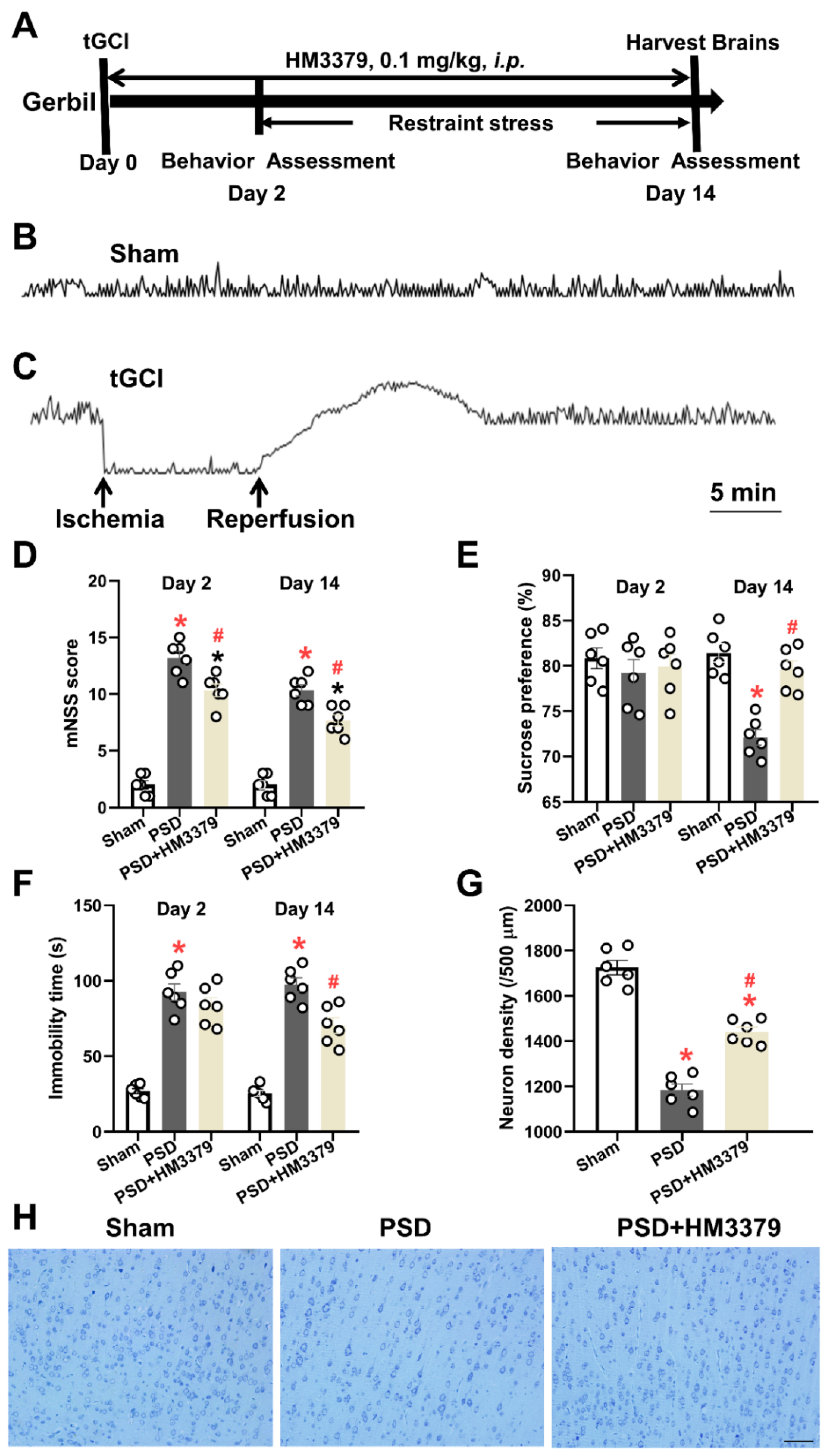
3.1. HM3379 Protected against Depression-like Behaviors and Neurological Injury in PSD Gerbils
3.2. HM3379 Inhibited Microglial Activation in PSD Gerbils
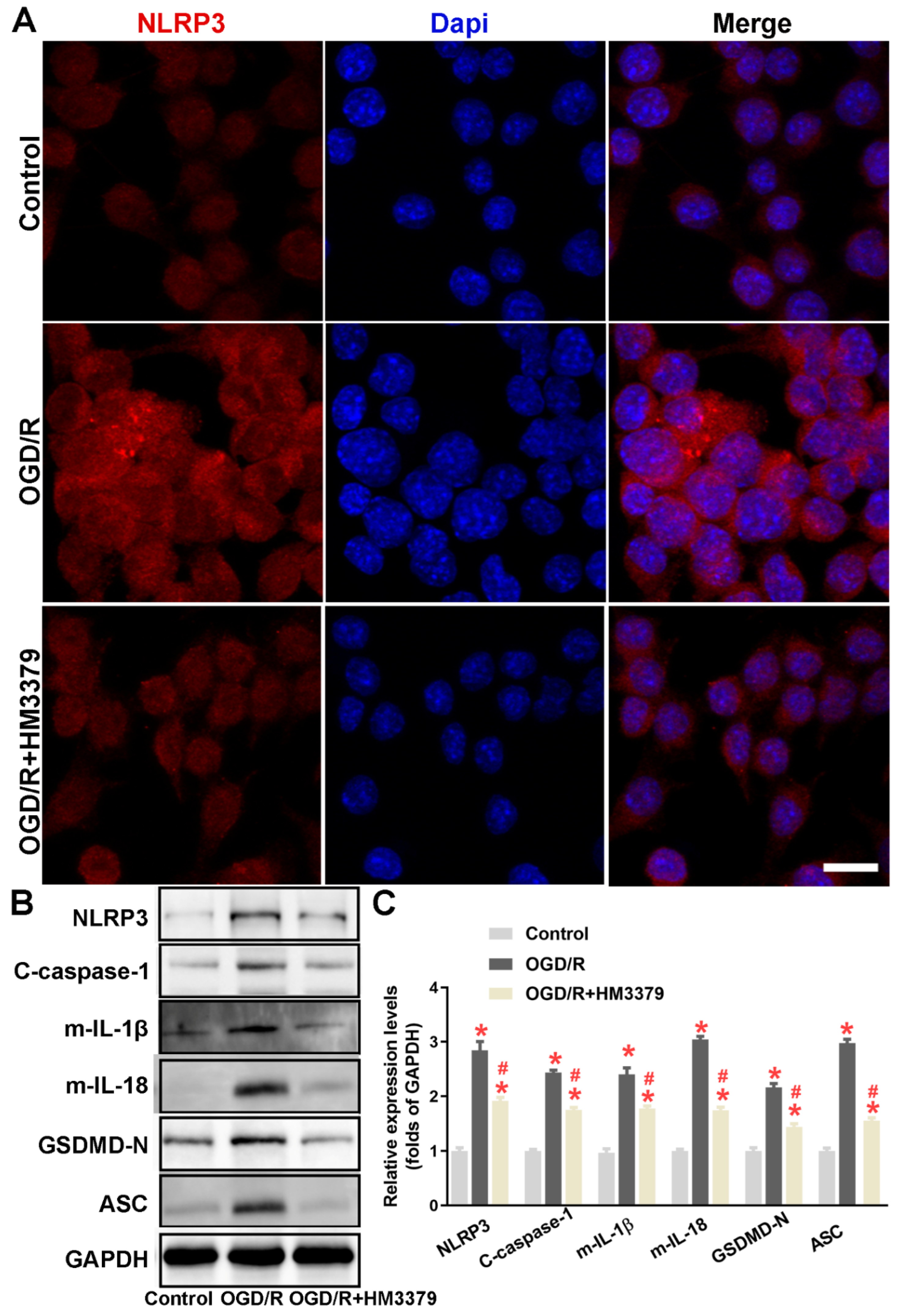
3.3. HM3379 Blocked PSD-Induced Activation of the NLRP3 Inflammasome and Pyroptosis in Gerbils
3.4. HM3379 Suppressed OGD/R-Induced NLRP3 Inflammasome Activation and Pyroptosis in BV2 Cells
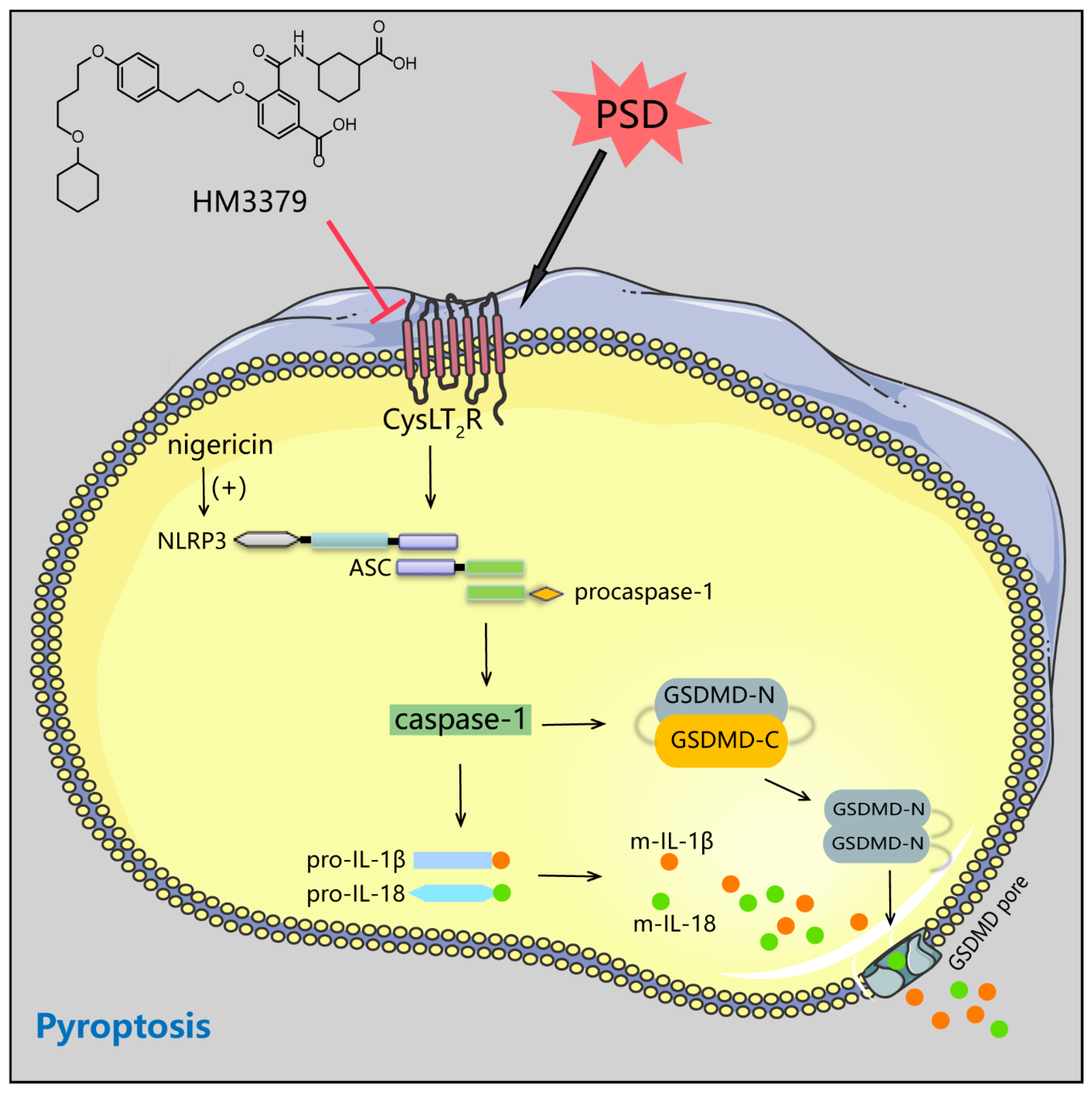
3.5. HM3379 Inhibited Pyroptosis through the NLRP3 Inflammasome Pathway in BV2 Cells
3.6. HM3379 Protected against PSD-Induced Depression-like Behaviors and Neurological Injury through CysLT2R in Gerbils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, J.H.; Jeon, J.; Kwon, H.; Cho, E.; Yun, J.; Lee, Y.C.; Ryu, J.H.; Park, S.J.; Cho, J.H.; Kim, D.H. Rubrofusarin Attenuates Chronic Restraint Stress-Induced Depressive Symptoms. Int. J. Mol. Sci. 2020, 21, 3454. [Google Scholar] [CrossRef]
- Siotto, M.; Germanotta, M.; Santoro, M.; Cipollini, V.; Guardati, G.; Papadopoulou, D.; Bray, E.; Mastrorosa, A.; Aprile, I. Serotonin Levels and Cognitive Recovery in Patients with Subacute Stroke after Rehabilitation Treatment. Brain Sci. 2021, 11, 642. [Google Scholar] [CrossRef]
- Frank, D.; Kuts, R.; Tsenter, P.; Gruenbaum, B.F.; Grinshpun, Y.; Zvenigorodsky, V.; Shelef, I.; Natanel, D.; Brotfain, E.; Zlotnik, A.; et al. The effect of pyruvate on the development and progression of post-stroke depression: A new therapeutic approach. Neuropharmacology 2019, 155, 173–184. [Google Scholar] [CrossRef]
- Du, Y.; Liang, H.; Zhang, L.; Fu, F. Administration of Huperzine A exerts antidepressant-like activity in a rat model of post-stroke depression. Pharmacol. Biochem. Behav. 2017, 158, 32–38. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Sun, W.; Liu, X. The advances of post-stroke depression: 2021 update. J. Neurol. 2022, 269, 1236–1249. [Google Scholar] [CrossRef]
- Rahman, S.O.; Singh, R.K.; Hussain, S.; Akhtar, M.; Najmi, A.K. A novel therapeutic potential of cysteinyl leukotrienes and their receptors modulation in the neurological complications associated with Alzheimer’s disease. Eur. J. Pharmacol. 2019, 842, 208–220. [Google Scholar] [CrossRef]
- Shi, Q.J.; Wang, H.; Liu, Z.X.; Fang, S.H.; Song, X.M.; Lu, Y.B.; Wei, E.Q. HAMI 3379, a CysLT2R antagonist, dose- and time-dependently attenuates brain injury and inhibits microglial inflammation after focal cerebral ischemia in rats. Neuroscience 2015, 291, 53–69. [Google Scholar] [CrossRef]
- Shi, Q.J.; Xiao, L.; Zhao, B.; Zhang, X.; Wang, X.; Xu, D.; Wei, E.Q. Intracerebroventricular injection of HAMI 3379, a selective cysteinyl leukotriene receptor 2 antagonist, protects against acute brain injury after focal cerebral ischemia in rats. Brain Res. 2012, 1484, 57–67. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Wang, X.; Xu, D.; Chen, L.; Zhang, L.; Wei, E.Q. Cysteinyl leukotriene receptor 1 mediates LTD4-induced activation of mouse microglial cells in vitro. Acta Pharmacol. Sin. 2014, 35, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, X.; Xu, D.; Yu, S.; Shi, Q.; Zhang, L.; Wei, E.Q. HAMI 3379, a CysLT2 Receptor Antagonist, Attenuates Ischemia-Like Neuronal Injury by Inhibiting Microglial Activation. J. Pharmacol. Exp. Ther. 2013, 346, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Fang, S.; Cai, B.; Huang, X.; Zhang, X.; Lu, Y.; Wei, E.Q. ERK/Egr-1 signaling pathway is involved in CysLT2 receptor-mediated IL-8 production in HEK293 cells. Eur. J. Cell Biol. 2014, 93, 278–288. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Martínez-Alonso, E.; Chioua, M.; Escobar-Peso, A.; Gonzalo-Gobernado, R.; Montaner, J.; Marco-Contelles, J.; Alcazar, A. Quinolinyl Nitrone RP19 Induces Neuroprotection after Transient Brain Ischemia. ACS Chem. Neurosci. 2017, 8, 2202–2213. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Li, C.; Zhang, S.; Zheng, W.; Wei, E.; Zhang, L. CysLT 2 receptor mediates lipopolysaccharide-induced microglial inflammation and consequent neurotoxicity in vitro. Brain Res. 2015, 1624, 433–445. [Google Scholar] [CrossRef]
- Rubin, E.; Pippione, A.C.; Boyko, M.; Einaudi, G.; Sainas, S.; Collino, M.; Cifani, C.; Lolli, M.L.; Abu-Freha, N.; Kaplanski, J.; et al. A New NF-κB Inhibitor, MEDS-23, Reduces the Severity of Adverse Post-Ischemic Stroke Outcomes in Rats. Brain Sci. 2021, 12, 35. [Google Scholar] [CrossRef]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef]
- Espinosa-Garcia, C.; Atif, F.; Yousuf, S.; Sayeed, I.; Neigh, G.N.; Stein, D.G. Progesterone Attenuates Stress-Induced NLRP3 Inflammasome Activation and Enhances Autophagy Following Ischemic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3740. [Google Scholar] [CrossRef]
- Qiu, Z.; Lei, S.; Zhao, B.; Wu, Y.; Su, W.; Liu, M.; Meng, Q.T.; Zhou, B.; Leng, Y.; Xia, Z.Y. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Y.; Chen, N.; Wang, H. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int. Immunopharmacol. 2019, 67, 458–464. [Google Scholar] [CrossRef]
- Xu, P.; Hong, Y.; Xie, Y.; Yuan, K.; Li, J.; Sun, R.; Zhang, X.H.; Shi, X.L.; Li, R.R.; Wu, J.N.; et al. TREM-1 Exacerbates Neuroinflammatory Injury via NLRP3 Inflammasome-Mediated Pyroptosis in Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 2020, 12, 643–659. [Google Scholar] [CrossRef]
- Li, Y.; Long, W.; Gao, M.; Jiao, F.; Chen, Z.; Liu, M.; Yu, L. TREM2 Regulates High Glucose-Induced Microglial Inflammation via the NLRP3 Signaling Pathway. Brain Sci. 2021, 11, 896. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Ma, R.; Wu, M.; Zhang, Y. Tetrandrine alleviates cerebral ischemia/reperfusion injury by suppressing NLRP3 inflammasome activation via Sirt-1. PeerJ 2020, 8, e9042. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, F.; Gao, Y.; Lei, H. Bilateral common carotid artery occlusion induced brain lesions in rats: A longitudinal diffusion tensor imaging study. Magn. Reson. Imaging 2015, 33, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Yoo, K.; Lee, C.H.; Park, J.H.; Yan, B.C.; Kwon, S.; Seo, J.Y.; Cho, J.H.; Won, M. Comparison of Neurogenesis in the Dentate Gyrus Between the Adult and Aged Gerbil Following Transient Global Cerebral Ischemia. Neurochem. Res. 2012, 37, 802–810. [Google Scholar] [CrossRef]
- Caldwell, H.G.; Coombs, G.B.; Howe, C.A.; Hoiland, R.L.; Patrician, A.; Lucas, S.J.E.; Ainslie, P.N. Evidence for temperature-mediated regional increases in cerebral blood flow during exercise. J. Physiol. 2020, 598, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.S.; Kim, H.; Park, J.H.; Kim, M.J.; Shin, Y.I.; Choi, B.T.; Lee, S.Y.; Shin, H.K. Indoleamine 2,3-Dioxygenase-Dependent Neurotoxic Kynurenine Metabolism Contributes to Poststroke Depression Induced in Mice by Ischemic Stroke along with Spatial Restraint Stress. Oxid. Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Nury, T.; Millot, N.; Lizard, G. Evidence of a non-apoptotic mode of cell death in microglial BV-2 cells exposed to different concentrations of zinc oxide nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 12500–12520. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, N.; Zhang, Y.; Chen, H. Salvianolic Acids for Injection alleviates cerebral ischemia/reperfusion injury by switching M1/M2 phenotypes and inhibiting NLRP3 inflammasome/pyroptosis axis in microglia in vivo and in vitro. J. Ethnopharmacol. 2021, 270, 113776. [Google Scholar] [CrossRef]
- Ito, M.; Shichita, T.; Okada, M.; Komine, R.; Noguchi, Y.; Yoshimura, A.; Morita, R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 2015, 6, 7360. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.; Kim, D.H.; Lee, C.H.; Park, S.J.; Jung, J.W.; Ko, K.H.; Cheong, J.H.; Lee, S.H.; Ryu, J.H. Neuroprotective effect of forsythiaside against transient cerebral global ischemia in gerbil. Eur. J. Pharmacol. 2011, 660, 326–333. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, M.; Nan, X.; Zhao, H.; Gong, D. Differences in the number of oxytocin, vasopressin, and tyrosine hydroxylase cells in brain regions associated with mating among great, midday, and Mongolian gerbils. Brain Res. 2020, 1733, 146677. [Google Scholar] [CrossRef]
- Koro, R.; Hijioka, M.; Arata, M.; Kitamura, Y. Lipoxin A4 Receptor Stimulation Attenuates Neuroinflammation in a Mouse Model of Intracerebral Hemorrhage. Brain Sci. 2022, 12, 162. [Google Scholar]
- Savitz, S.I.; Schäbitz, W.R. Reviving neuroprotection using a new approach: Targeting postsynaptic density-95 to arrest glutamate excitotoxicity. Cerebrovasc. Dis. 2012, 43, 3411–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tater, P.; Pandey, S. Post-stroke Movement Disorders: Clinical Spectrum, Pathogenesis, and Management. Neurol. India 2021, 69, 272–283. [Google Scholar]
- Rachfalska, N.; Putowski, Z.; Krzych, Ł.J. Distant Organ Damage in Acute Brain Injury. Brain Sci. 2020, 10, 1019. [Google Scholar] [CrossRef]
- Yu, H.; Tang, M.H.; Zeng, Z.Y.; Huang, S.J.; Zheng, X.F.; Liu, Z.Y. Suppressive Effects of Gelsemine on Anxiety-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sci. 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Jiang, N.; Hu, Y.; Zhang, Y.; Wang, G.; Yin, M.; Ma, X.; Zhou, K.; Qi, J.; Yu, B.; et al. Ruscogenin Attenuates Cerebral Ischemia-Induced Blood-Brain Barrier Dysfunction by Suppressing TXNIP/NLRP3 Inflammasome Activation and the MAPK Pathway. Int. J. Mol. Sci. 2016, 17, 1418. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 2021, 47, 52. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Doitsh, G.; Galloway, N.L.K.; Geng, X.; Yang, Z.; Monroe, K.M.; Zepeda, O.; Hunt, P.W.; Hatano, H.; Sowinski, S.; Munoz-Arias, I.; et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2014, 505, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.M.; Karki, R.; Kanneganti, T. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sborgi, L.; Rühl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Muller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zhang, J.; Han, X.; Fang, J.; Zhou, S.; Lu, L.; Shi, Q.; Ying, H. CysLT2R Antagonist HAMI 3379 Ameliorates Post-Stroke Depression through NLRP3 Inflammasome/Pyroptosis Pathway in Gerbils. Brain Sci. 2022, 12, 976. https://doi.org/10.3390/brainsci12080976
Zhou L, Zhang J, Han X, Fang J, Zhou S, Lu L, Shi Q, Ying H. CysLT2R Antagonist HAMI 3379 Ameliorates Post-Stroke Depression through NLRP3 Inflammasome/Pyroptosis Pathway in Gerbils. Brain Sciences. 2022; 12(8):976. https://doi.org/10.3390/brainsci12080976
Chicago/Turabian StyleZhou, Li, Jiajia Zhang, Xue Han, Jie Fang, Shasang Zhou, Lingqun Lu, Qiaojuan Shi, and Huazhong Ying. 2022. "CysLT2R Antagonist HAMI 3379 Ameliorates Post-Stroke Depression through NLRP3 Inflammasome/Pyroptosis Pathway in Gerbils" Brain Sciences 12, no. 8: 976. https://doi.org/10.3390/brainsci12080976
APA StyleZhou, L., Zhang, J., Han, X., Fang, J., Zhou, S., Lu, L., Shi, Q., & Ying, H. (2022). CysLT2R Antagonist HAMI 3379 Ameliorates Post-Stroke Depression through NLRP3 Inflammasome/Pyroptosis Pathway in Gerbils. Brain Sciences, 12(8), 976. https://doi.org/10.3390/brainsci12080976




