A Novel Coupling Model of Physiological Degradation and Emotional State for Prediction of Alzheimer’s Disease Progression
Abstract
:1. Introduction
2. Related Work
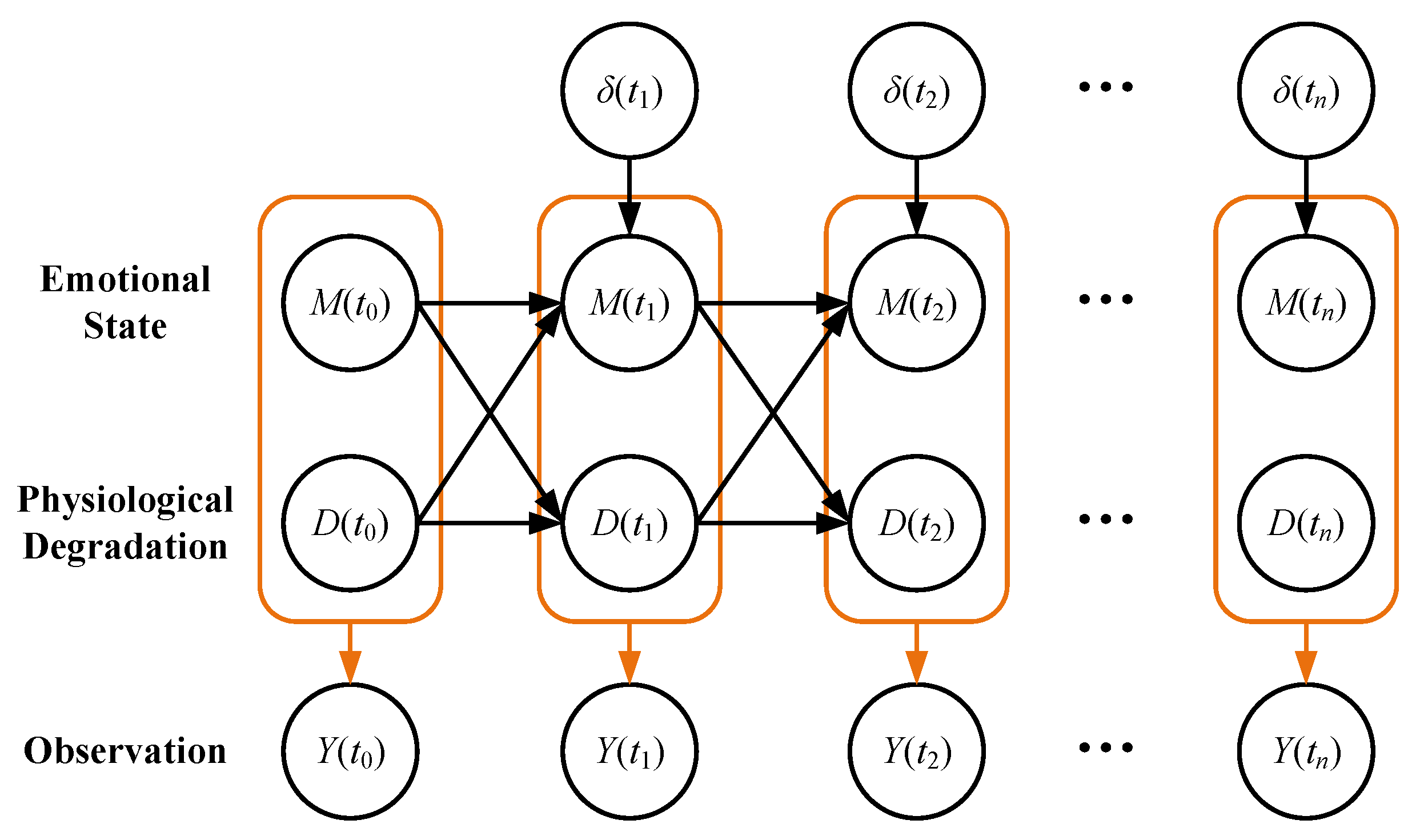
3. Methodology
3.1. Emotional State Transition Process
3.2. Physiological Degradation Process
3.3. Observation Model Based on Physiological Degradation and Emotional State
4. Experiments
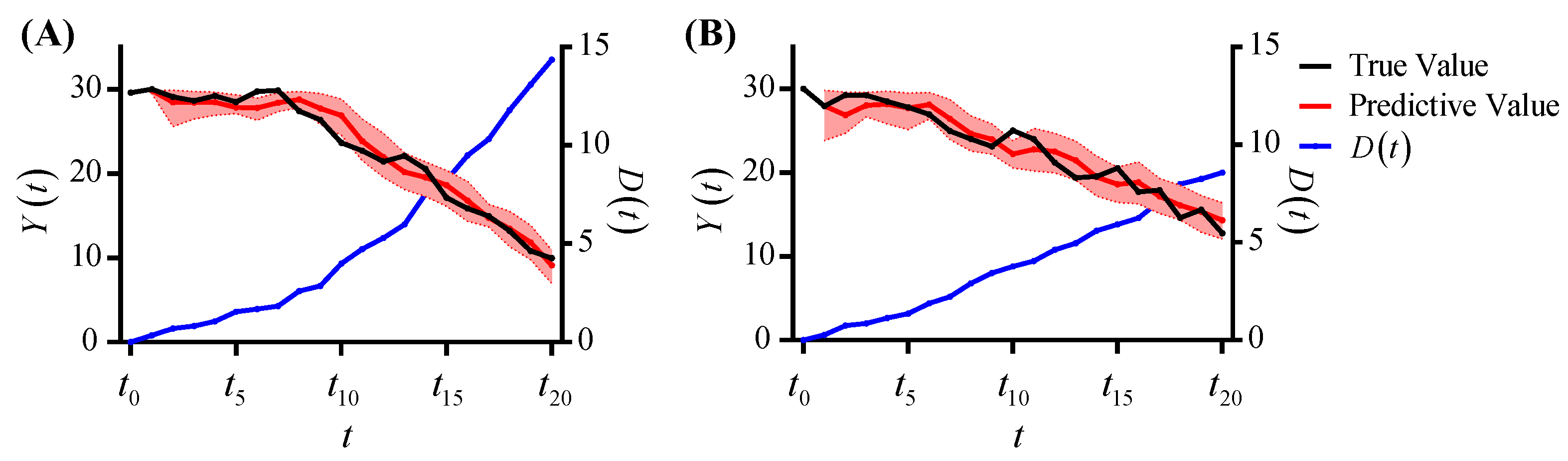
4.1. Experiment on Synthetic Data
4.1.1. Synthetic Data Generation
4.1.2. Synthetic Data Analysis
4.2. Prediction of Alzheimer’s Disease Progression
4.2.1. Data Preparation
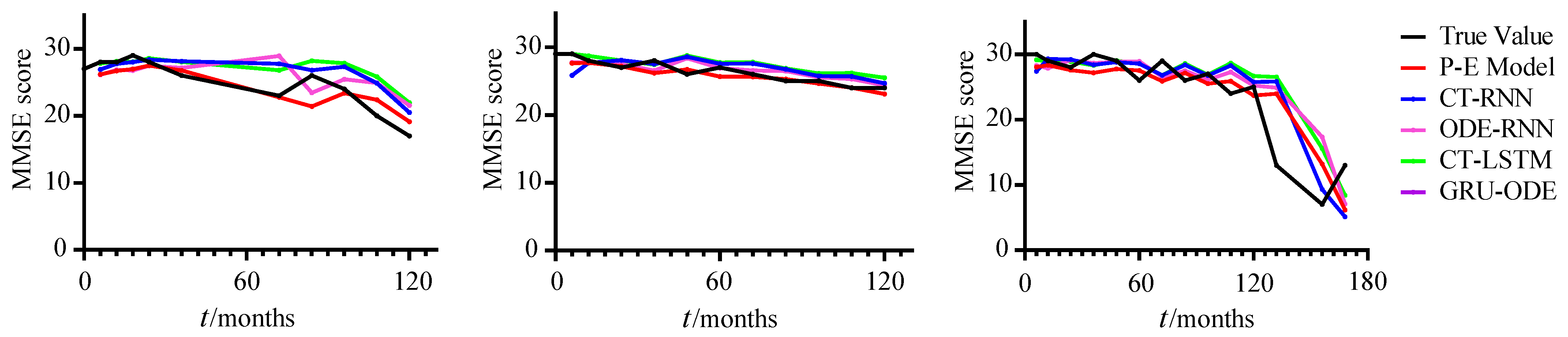
4.2.2. Alzheimer’s Disease Progression Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P-E model | Model of physiological degradation and emotional state |
| AD | Alzheimer’s disease |
| CN | Cognitive normal |
| MCI | Mild cognitive impairment |
| DPM | Disease progression model |
| RAVLT | Rey auditory verbal learning test |
| ADAS | Alzheimer’s disease assessment scale |
| APOE4 | Apolipoprotein E4 |
| MRI | Magnetic resonance imaging |
| FAQ | Functional assessment questionnaire |
| DIC | Deviance information criteria |
| MMSE | Mini-Mental state examination |
| LSTM | Long short-term memory |
| GRU | Gated recurrent unit |
| MCMC | Markov chain Monte Carlo |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative |
| RNN | Recurrent neural network |
| CT-RNN | Continuous-time recurrent neural network |
| ODE-RNN | Ordinary differential equation recurrent neural network |
| CT-LSTM | Continuous-time long short-term memory |
| GRU-ODE | Gated recurrent units with ordinary differential equation |
References
- Vickrey, B.G.; Mittman, B.S.; Connor, K.I.; Pearson, M.L.; Della Penna, R.D.; Ganiats, T.G.; DeMonte, R.W.; Chodosh, J.; Cui, X.; Vassar, S.; et al. The Effect of a Disease Management Intervention on Quality and Outcomes of Dementia Care. Ann. Intern. Med. 2006, 145, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Wang, C.; Tao, D. MRI-based Alzheimer’s disease prediction via distilling the knowledge in multi-modal data. NeuroImage 2021, 244, 118586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, Z.; Han, X.; Li, H.; Tang, X. Prediction of Alzheimer’s Disease Progression Based on Magnetic Resonance Imaging. ACS Chem. Neurosci. 2021, 12, 4209–4223. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Liu, W.; Liu, H.; Shang, Q. Artificial Intelligence-based MRI Images for Brain in Prediction of Alzheimer’s Disease. J. Healthc. Eng. 2021, 2021, e8198552. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.S.; Garner, B.; Ooi, L. Understanding the Role of ApoE Fragments in Alzheimer’s Disease. Neurochem. Res. 2019, 44, 1297–1305. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimer’s Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- Sanz-Blasco, R.; Ruiz-Sánchez de León, J.M.; Ávila Villanueva, M.; Valentí-Soler, M.; Gómez-Ramírez, J.; Fernández-Blázquez, M.A. Transition from mild cognitive impairment to normal cognition: Determining the predictors of reversion with multi-state Markov models. Alzheimer’s Dement. 2022, 18, 1177–1185. [Google Scholar] [CrossRef]
- Liu, H.; Song, X.; Zhang, B. Varying-coefficient hidden Markov models with zero-effect regions. Comput. Stat. Data Anal. 2022, 173, 107482. [Google Scholar] [CrossRef]
- Williams, J.P.; Storlie, C.B.; Therneau, T.M.; Jr, C.R.J.; Hannig, J. A Bayesian Approach to Multistate Hidden Markov Models: Application to Dementia Progression. J. Am. Stat. Assoc. 2020, 115, 16–31. [Google Scholar] [CrossRef]
- Johansson, M.; Stomrud, E.; Lindberg, O.; Westman, E.; Johansson, P.M.; van Westen, D.; Mattsson, N.; Hansson, O. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol. Aging 2020, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Anlló, E.M.; Dauphin, S.; Fargeau, M.N.; Ingrand, P.; Gil, R. Music and emotion in Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Zhornitsky, S.; Chao, H.H.; van Dyck, C.H.; Li, C.S.R. Emotion Processing Dysfunction in Alzheimer’s Disease: An Overview of Behavioral Findings, Systems Neural Correlates, and Underlying Neural Biology. Am. J. Alzheimer’s Dis. Other Dementias® 2022, 37, 1–33. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimer’s Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef]
- Zvěřová, M. Clinical aspects of Alzheimer’s disease. Clin. Biochem. 2019, 72, 3–6. [Google Scholar] [CrossRef]
- Meldolesi, J. Alzheimer’s disease: Key developments support promising perspectives for therapy. Pharmacol. Res. 2019, 146, 104316. [Google Scholar] [CrossRef]
- Mofrad, S.A.; Lundervold, A.J.; Vik, A.; Lundervold, A.S. Cognitive and MRI trajectories for prediction of Alzheimer’s disease. Sci. Rep. 2021, 11, 2122. [Google Scholar] [CrossRef]
- Lorenzi, M.; Filippone, M.; Frisoni, G.B.; Alexander, D.C.; Ourselin, S. Probabilistic disease progression modeling to characterize diagnostic uncertainty: Application to staging and prediction in Alzheimer’s disease. NeuroImage 2019, 190, 56–68. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, C.Y.; Maiti, T.; Li, Y.; Choi, J.; Bozoki, A.; Zhu, D.C. Analysis of conversion of Alzheimer’s disease using a multi-state Markov model. Stat. Methods Med. Res. 2019, 28, 2801–2819. [Google Scholar] [CrossRef]
- Kang, K.; Cai, J.; Song, X.; Zhu, H. Bayesian hidden Markov models for delineating the pathology of Alzheimer’s disease. Stat. Methods Med. Res. 2019, 28, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Van Der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B Stat. Methodol. 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Jung, W.; Jun, E.; Suk, H.I.; Alzheimer’s Disease Neuroimaging Initiative. Deep recurrent model for individualized prediction of Alzheimer’s disease progression. NeuroImage 2021, 237, 118143. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour Ghazi, M.; Nielsen, M.; Pai, A.; Cardoso, M.J.; Modat, M.; Ourselin, S.; Sørensen, L. Training recurrent neural networks robust to incomplete data: Application to Alzheimer’s disease progression modeling. Med. Image Anal. 2019, 53, 39–46. [Google Scholar] [CrossRef]
- Lee, G.; Nho, K.; Kang, B.; Sohn, K.A.; Kim, D. Predicting Alzheimer’s disease progression using multi-modal deep learning approach. Sci. Rep. 2019, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Association, A. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Benoit, J.S.; Chan, W.; Piller, L.; Doody, R. Longitudinal Sensitivity of Alzheimer’s Disease Severity Staging. Am. J. Alzheimer’s Dis. Other Dementias 2020, 35, 1–8. [Google Scholar] [CrossRef]
- Vehtari, A.; Gelman, A.; Simpson, D.; Carpenter, B.; Bürkner, P.C. Rank-Normalization, Folding, and Localization: An Improved Rˆ for Assessing Convergence of MCMC (with Discussion). Bayesian Anal. 2021, 16, 667–718. [Google Scholar] [CrossRef]
- Mozer, M.C.; Kazakov, D.; Lindsey, R.V. Discrete Event, Continuous Time RNNs. arXiv 2017, arXiv:1710.04110. [Google Scholar]
- Rubanova, Y.; Chen, R.T.Q.; Duvenaud, D.K. Latent Ordinary Differential Equations for Irregularly-Sampled Time Series. In Advances in Neural Information Processing Systems; Curran Associates: New York, NY, USA, 2019; Volume 32. [Google Scholar]
- Mei, H.; Eisner, J.M. The Neural Hawkes Process: A Neurally Self-Modulating Multivariate Point Process. In Advances in Neural Information Processing Systems; Curran Associates: New York, NY, USA, 2017; Volume 30. [Google Scholar]
- De Brouwer, E.; Simm, J.; Arany, A.; Moreau, Y. GRU-ODE-Bayes: Continuous Modeling of Sporadically-Observed Time Series. In Advances in Neural Information Processing Systems; Curran Associates: New York, NY, USA, 2019; Volume 32. [Google Scholar]


| 1 | 5/4 | 3/3 | 0.1/−0.1 | 0.05 | 0 | 3 | 0.5 | 10 | 10 | 30 | −1 | −0.5 | 1 |
| 2 | 5/4 | 3/3 | 0.1/−0.1 | 0.05 | 0 | 3 | 0.5 | 5 | 10 | 30 | −1 | −0.5 | 1 |
| 3 | 5/3 | 3/3 | 0.1/−0.1 | 0.05 | 0 | 3 | 0.5 | 5 | 10 | 30 | −1 | −0.5 | 1 |
| 4 | 3/5 | 3/3 | 0.1/−0.1 | 0.05 | 0 | 3 | 0.5 | 5 | 10 | 30 | −1 | −0.5 | 1 |
| 5 | 3&5 | 3/3 | 0.1/−0.1 | 0.05 | 0 | 3 | 0.5 | 5 | 10 | 30 | −1 | −0.5 | 1 |
| Prior Distributions | Posterior Distributions | |||||
|---|---|---|---|---|---|---|
| Parameters | Mean | Standard Deviation | Parameters | Mean | Standard Deviation | R-Hat |
| 0.10 | 0.05 | 0.03 | 0.01 | 1.00 | ||
| 0.30 | 0.05 | 0.16 | 0.03 | 1.00 | ||
| −0.10 | 0.05 | −0.16 | 0.04 | 1.00 | ||
| 1.30 | 0.05 | 1.29 | 0.05 | 1.00 | ||
| 1.00 | 0.05 | 1.11 | 0.04 | 1.00 | ||
| 5.00 | 0.10 | 4.98 | 0.10 | 1.00 | ||
| 6.00 | 0.10 | 6.02 | 0.10 | 1.00 | ||
| 29.50 | 0.05 | 29.49 | 0.05 | 1.00 | ||
| −0.50 | 0.05 | −0.41 | 0.05 | 1.04 | ||
| −1.00 | 0.05 | −0.86 | 0.04 | 1.01 | ||
| 0.80 | 0.05 | 1.05 | 0.03 | 1.00 | ||
| Model | MSE |
|---|---|
| P-E Model (ours) | 7.137 ± 0.035 |
| CT-RNN | 14.752 ± 1.474 |
| ODE-RNN | 13.498 ± 0.278 |
| CT-LSTM | 22.369 ± 0.590 |
| GRU-ODE | 14.315 ± 1.706 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, S.; The Alzheimer’s Disease Neuroimaging Initiative. A Novel Coupling Model of Physiological Degradation and Emotional State for Prediction of Alzheimer’s Disease Progression. Brain Sci. 2022, 12, 1132. https://doi.org/10.3390/brainsci12091132
Yang J, Wang S, The Alzheimer’s Disease Neuroimaging Initiative. A Novel Coupling Model of Physiological Degradation and Emotional State for Prediction of Alzheimer’s Disease Progression. Brain Sciences. 2022; 12(9):1132. https://doi.org/10.3390/brainsci12091132
Chicago/Turabian StyleYang, Jiawei, Shaoping Wang, and The Alzheimer’s Disease Neuroimaging Initiative. 2022. "A Novel Coupling Model of Physiological Degradation and Emotional State for Prediction of Alzheimer’s Disease Progression" Brain Sciences 12, no. 9: 1132. https://doi.org/10.3390/brainsci12091132
APA StyleYang, J., Wang, S., & The Alzheimer’s Disease Neuroimaging Initiative. (2022). A Novel Coupling Model of Physiological Degradation and Emotional State for Prediction of Alzheimer’s Disease Progression. Brain Sciences, 12(9), 1132. https://doi.org/10.3390/brainsci12091132





