What Are the Neural Correlates of Impaired Awareness of Social Cognition and Function in Dementia? A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
- Concept 1: Dementia (Dement* OR Alzheimer* OR AD OR DAT OR “Lewy body dementia” OR lewy bod* DLB OR “frontotemporal dementia” OR FTD OR “vascular dementia” OR VD OR “multi infarct dementia”);
- AND Concept 2: Social cognition (Social cogn* OR TOM OR “theory of mind” OR “emo* recogn*” OR empathy OR “emo* perception” OR social function* OR social behavi* OR social activit*);
- AND Concept 3: Awareness (Insight OR conscious* OR aware* OR “self-appraisal” OR “self-reflect” OR anosognosia);
- AND Concept 4: Neuroimaging (Neuroimaging OR imag* OR “magnetic resonance imaging” OR MRI OR “functional magnetic resonance imaging” OR fMRI OR “positron emission tomography” OR PET OR “single-photon emission computed tomography” OR SPECT OR “computed tomography” OR CT OR DTI OR “diffusion tensor imaging” OR DWI OR “diffusion-weighted imaging”).
2.2. Identification and Selection of Studies
2.3. Protocol and Registration
3. Results
3.1. Study Characteristics
3.2. Neural Correlates Identified via Structural Neuroimaging Studies
3.3. Neural Correlates Identified via Functional Neuroimaging Studies
3.4. Inferences of Neural Correlates Based on Group-Wise Comparison across Structural and Functional Studies
3.5. Comparison across Methodologies
4. Discussion
4.1. Limitations
4.2. Future Research and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budgett, J.; Brown, A.; Daley, S.; Page, T.E.; Banerjee, S.; Livingston, G.; Sommerlad, A. The Social Functioning in Dementia Scale (SF-DEM): Exploratory Factor Analysis and Psychometric Properties in Mild, Moderate, and Severe Dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.; Lough, S.; Stone, V.; Erzinclioglu, S.; Martin, L.; Baron-Cohen, S.; Hodges, J.R. Theory of Mind in Patients with Frontal Variant Frontotemporal Dementia and Alzheimer’s Disease: Theoretical and Practical Implications. Brain 2002, 125, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Kumfor, F.; Irish, M.; Hodges, J.R.; Piguet, O. Discrete Neural Correlates for the Recognition of Negative Emotions: Insights from Frontotemporal Dementia. PLoS ONE 2013, 8, 457. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying Neurocognitive Disorders: The DSM-5 Approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal Lobar Degeneration. A Consensus on Clinical Diagnostic Criteria. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef]
- Kumfor, F.; Miller, L.; Lah, S.; Hsieh, S.; Savage, S.; Hodges, J.R.; Piguet, O. Are You Really Angry? The Effect of Intensity on Facial Emotion Recognition in Frontotemporal Dementia. Soc. Neurosci. 2011, 6, 502–514. [Google Scholar] [CrossRef]
- Snowden, J.S.; Bathgate, D.; Varma, A.; Blackshaw, A.; Gibbons, Z.C.; Neary, D. Distinct Behavioural Profiles in Frontotemporal Dementia and Semantic Dementia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 323–332. [Google Scholar] [CrossRef]
- Kumfor, F.; Piguet, O. Emotion Recognition in the Dementias: Brain Correlates and Patient Implications. Neurodegener. Dis. Manag. 2013, 3, 277–288. [Google Scholar] [CrossRef]
- Cosentino, S.; Zahodne, L.B.; Brandt, J.; Blacker, D.; Albert, M.; Dubois, B.; Stern, Y. Social Cognition in Alzheimer’s Disease: A Separate Construct Contributing to Dependence. Alzheimer’s Dement. 2014, 10, 818–826. [Google Scholar] [CrossRef]
- Henry, J.D.; Von Hippel, W.; Molenberghs, P.; Lee, T.; Sachdev, P.S. Clinical Assessment of Social Cognitive Function in Neurological Disorders. Nat. Rev. Neurol. 2016, 12, 28–39. [Google Scholar] [CrossRef]
- Heitz, C.; Noblet, V.; Phillipps, C.; Cretin, B.; Vogt, N.; Philippi, N.; Kemp, J.; De Petigny, X.; Bilger, M.; Demuynck, C.; et al. Cognitive and Affective Theory of Mind in Dementia with Lewy Bodies and Alzheimer’s Disease. Alzheimer’s Res. Ther. 2016, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, M.; Rankin, K.P.; Miller, B.L. Social Cognition. Contin. Lifelong Learn. Neurol. 2010, 16, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Duclos, H.; Desgranges, B.; Eustache, F.; Laisney, M. Impairment of Social Cognition in Neurological Diseases. Rev. Neurol. 2018, 174, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Adenzato, M.; Cavallo, M.; Enrici, I. Theory of Mind Ability in the Behavioural Variant of Frontotemporal Dementia: An Analysis of the Neural, Cognitive, and Social Levels. Neuropsychologia 2010, 48, 2–12. [Google Scholar] [CrossRef]
- Völlm, B.A.; Taylor, A.N.W.; Richardson, P.; Corcoran, R.; Stirling, J.; McKie, S.; Deakin, J.F.W.; Elliott, R. Neuronal Correlates of Theory of Mind and Empathy: A Functional Magnetic Resonance Imaging Study in a Nonverbal Task. Neuroimage 2006, 29, 90–98. [Google Scholar] [CrossRef]
- Fernandez-Duque, D.; Hodges, S.D.; Baird, J.A.; Black, S.E. Empathy in Frontotemporal Dementia and Alzheimer’s Disease. J. Clin. Exp. Neuropsychol. 2010, 32, 289–298. [Google Scholar] [CrossRef]
- Schnell, K.; Herpertz, S.C. Emotion Regulation and Social Cognition as Functional Targets of Mechanism-Based Psychotherapy in Major Depression with Comorbid Personality Pathology. J. Pers. Disord. 2018, 32, 12–35. [Google Scholar] [CrossRef]
- Yager, J.A.; Ehmann, T.S. Untangling Social Function and Social Cognition: A Review of Concepts and Measurement. Psychiatry 2006, 69, 47–68. [Google Scholar] [CrossRef]
- Tyrer, P.E.; Casey, P.E. Social Function in Psychiatry: The Hidden Axis of Classification Exposed; Wrightson Biomedical Publishing: New York, NY, USA, 1993. [Google Scholar]
- Wykes, T. Social Functioning in Psychiatry—The Hidden Axis of Classification Exposed; Tyrer, P., Casey, P., Eds.; Wrightson Biomedical: Petersfield, UK, 1993; p. 163. [Google Scholar]
- Sommerlad, A.; Singleton, D.; Jones, R.; Banerjee, S.; Livingston, G. Development of an Instrument to Assess Social Functioning in Dementia: The Social Functioning in Dementia Scale (SF-DEM). Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit. 2017, 7, 88–98. [Google Scholar] [CrossRef]
- McGlynn, S.M.; Schacter, D.L. Unawareness of Deficits in Neuropsychological Syndromes. J. Clin. Exp. Neuropsychol. Off. J. Int. Neuropsychol. Soc. 1989, 11, 143–205. [Google Scholar] [CrossRef]
- Zamboni, G.; Wilcock, G. Lack of Awareness of Symptoms in People with Dementia: The Structural and Functional Basis. Int. J. Geriatr. Psychiatry 2011, 26, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Nelis, S.M.; Clare, L.; Martyr, A.; Markova, I.; Roth, I.; Woods, R.T.; Whitaker, C.J.; Morris, R.G. Awareness of Social and Emotional Functioning in People with Early-Stage Dementia and Implications for Carers. Aging Ment. Health 2011, 15, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Chavoix, C.; Insausti, R. Self-Awareness and the Medial Temporal Lobe in Neurodegenerative Diseases. Neurosci. Biobehav. Rev. 2017, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Neira, C.; Tedde, A.; Coulthard, E.; Thai, N.J.; Pennington, C. Neural Correlates of Altered Insight in Frontotemporal Dementia: A Systematic Review. NeuroImage Clin. 2019, 24, 66. [Google Scholar] [CrossRef]
- Lacerda, I.B.; Sousa, M.F.B.; Santos, R.L.; Nogueira, M.M.L.; Dourado, M.C.N. Concepts and Objects of Awareness in Alzheimer´s Disease: An Updated Systematic Review. J. Bras. Psiquiatr. 2016, 65, 99–109. [Google Scholar] [CrossRef]
- Clare, L.; Markova, I.S.; Roth, I.; Morris, R.G. Awareness in Alzheimer’s Disease and Associated Dementias: Theoretical Framework and Clinical Implications. Aging Ment. Health 2011, 15, 936–944. [Google Scholar] [CrossRef]
- Takai, M.; Takahashi, M.; Iwamitsu, Y.; Ando, N.; Okazaki, S.; Nakajima, K.; Oishi, S.; Miyaoka, H. The Experience of Burnout among Home Caregivers of Patients with Dementia: Relations to Depression and Quality of Life. Arch. Gerontol. Geriatr. 2009, 49, e1. [Google Scholar] [CrossRef]
- Farina, N.; Page, T.E.; Daley, S.; Brown, A.; Bowling, A.; Basset, T.; Livingston, G.; Knapp, M.; Murray, J.; Banerjee, S. Factors Associated with the Quality of Life of Family Carers of People with Dementia: A Systematic Review. Alzheimer´s Dement. 2017, 13, 572–581. [Google Scholar] [CrossRef]
- Knapp, M.; Iemmi, V.; Romeo, R. Dementia Care Costs and Outcomes: A Systematic Review. Int. J. Geriatr. Psychiatry 2013, 28, 551–561. [Google Scholar] [CrossRef]
- Aalten, P.; Van Valen, E.; Clare, L.; Kenny, G.; Verhey, F. Awareness in Dementia: A Review of Clinical Correlates. Aging Ment. Health 2005, 9, 414–422. [Google Scholar] [CrossRef]
- Leicht, H.; Berwig, M.; Gertz, H.J. Anosognosia in Alzheimers Disease: The Role of Impairment Levels in Assessment of Insight across Domains. J. Int. Neuropsychol. Soc. 2010, 16, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Lau, H.C. How to Measure Metacognition. Front. Hum. Neurosci. 2014, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, M.S.; Ivry, R.B.; Mangun, G.R. Cognitive Neuroscience. The Biology of the Mind, 4th ed.; W. W. Norton & Company: New York, NY, USA, 2014. [Google Scholar]
- Kennedy, D.P.; Adolphs, R. The Social Brain in Psychiatric and Neurological Disorders. Trends Cogn. Sci. 2012, 16, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Cheng, W.; Feng, J. The Orbitofrontal Cortex: Reward, Emotion and Depression. Brain Commun. 2020, 2, 196. [Google Scholar] [CrossRef]
- Hallam, B.; Chan, J.; Gonzalez Costafreda, S.; Bhome, R.; Huntley, J. What Are the Neural Correlates of Meta-Cognition and Anosognosia in Alzheimer’s Disease? A Systematic Review. Neurobiol. Aging 2020, 94, 250–264. [Google Scholar] [CrossRef]
- Schoo, L.A.; Van Zandvoort, M.J.E.; Biessels, G.J.; Kappelle, L.J.; Postma, A. Insight in Cognition: Self-Awareness of Performance across Cognitive Domains. Appl. Neuropsychol. 2013, 20, 95–102. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (Prisma-p) 2015: Elaboration and Explanation. BMJ 2015, 349, 1–25. [Google Scholar] [CrossRef]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A Process for Systematically Reviewing the Literature: Providing the Research Evidence for Public Health Nursing Interventions. Worldviews Evid. Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef]
- McLachlan, E.; Rai, S.; Al-Shihabi, A.; Huntley, J.; Burgess, N.; Howard, R.; Reeves, S. Neuroimaging Correlates of False Memory in ’Alzheimer’s Disease: A Preliminary Systematic Review. Psychiatry Res. Neuroimaging 2020, 296. [Google Scholar] [CrossRef]
- Hornberger, M.; Yew, B.; Gilardoni, S.; Mioshi, E.; Gleichgerrcht, E.; Manes, F.; Hodges, J.R. Ventromedial-Frontopolar Prefrontal Cortex Atrophy Correlates with Insight Loss in Frontotemporal Dementia and Alzheimer ’ s Disease. Hum. Brain Mapp. 2014, 35, 616–626. [Google Scholar] [CrossRef]
- Hutchings, R.; Hodges, J.R.; Piguet, O.; Kumfor, F. Why Should I Care? Dimensions of Socio-Emotional Cognition in Younger-Onset Dementia. J. Alzheimer’s Dis. 2015, 48, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Mychack, P.; Rosen, H.; Miller, B.L. Novel Applications of Social – Personality Measures to the Study of Dementia. Neurocase 2001, 7, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Parthimos, T.P.; Ph, D.; Karavasilis, E.; Ph, D.; Rankin, K.P.; Ph, D.; Seimenis, I.; Ph, D.; Leftherioti, K.; Sc, M.; et al. The Neural Correlates of Impaired Self-Monitoring Among Individuals With Neurodegenerative Dementias. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Shany-ur, T.; Lin, N.; Rosen, H.J.; Sollberger, M.; Miller, B.L.; Rankin, K.P. Self-Awareness in Neurodegenerative Disease Relies on Neural Structures Mediating Reward-Driven Attention. Brain 2014, 137, 2368–2381. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, M.; Rosen, H.J.; Shany-ur, T.; Ullah, J.; Stanley, C.M.; Laluz, V.; Weiner, M.W.; Wilson, S.M.; Miller, B.L.; Rankin, K.P. Neural Substrates of Socioemotional Self-Awareness in Neurodegenerative Disease. Brain Behav. 2014, 4, 201–214. [Google Scholar] [CrossRef]
- Ruby, P.; Schmidt, C.; Argembeau, A.D.; Collette, F.; Salmon, E. Social Mind Representation: Where Does It Fail in Frontotemporal Dementia? J. Cogn. Neurosci. 2007, 19, 671–683. [Google Scholar] [CrossRef]
- Duval, C.; Bejanin, A.; Piolino, P.; Laisney, M.; de La Sayette, V.; Belliard, S.; Eustache, F.; Desgranges, B. Theory of Mind Impairments in Patients with Semantic Dementia. Brain 2012, 135, 228–241. [Google Scholar] [CrossRef]
- Lennox, R.D.; Wolfe, R.N. Revision of the Self-Monitoring Scale. J. Pers. Soc. Psychol. 1984, 46, 1349–1364. [Google Scholar] [CrossRef]
- Christidi, F.; Migliaccio, R.; Santamaría-García, H.; Santangelo, G.; Trojsi, F. Social Cognition Dysfunctions in Neurodegenerative Diseases: Neuroanatomical Correlates and Clinical Implications. Behav. Neurol. 2018, 2018, 794. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Rolls, E.T. Neural Correlates of Rapid Reversal Learning in a Simple Model of Human Social Interaction. Neuroimage 2003, 20, 1371–1383. [Google Scholar] [CrossRef]
- Rolls, E.T. The Orbitofrontal Cortex and Emotion in Health and Disease, Including Depression. Neuropsychologia 2019, 128, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Hornak, J.; Bramham, J.; Rolls, E.T.; Morris, R.G.; O’Doherty, J.; Bullock, P.R.; Polkey, C.E. Changes in Emotion after Circumscribed Surgical Lesions of the Orbitofrontal and Cingulate Cortices. Brain 2003, 126, 1691–1712. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Oishi, K.; Hsu, J.; Lindquist, M.; Gottesman, R.F.; Jarso, S.; Crainiceanu, C.; Mori, S.; Hillis, A.E. Acute Lesions That Impair Affective Empathy. Brain 2013, 136, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R. What Does the Amygdala Contribute to Social Cognition? Ann. N.Y. Acad. Sci. 2010, 1191. [Google Scholar] [CrossRef]
- Reniers, R.L.E.P.; Corcoran, R.; Völlm, B.A.; Mashru, A.; Howard, R.; Liddle, P.F. Moral Decision-Making, ToM, Empathy and the Default Mode Network. Biol. Psychol. 2012, 90, 202–210. [Google Scholar] [CrossRef]
- Mondragón, J.D.; Maurits, N.M.; De Deyn, P.P. Functional Neural Correlates of Anosognosia in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. Neuropsychol. Rev. 2019, 29, 139–165. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann. N.Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Antoine, N.; Bahri, M.A.; Bastin, C.; Collette, F.; Phillips, C.; Balteau, E.; Genon, S.; Salmon, E. Anosognosia and Default Mode Subnetwork Dysfunction in Alzheimer’s Disease. Hum. Brain Mapp. 2019, 40, 5330–5340. [Google Scholar] [CrossRef]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-Mode Network Activity Distinguishes Alzheimer’s Disease from Healthy Aging: Evidence from Functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef]
- Lenzoni, S.; Morris, R.G.; Mograbi, D.C. The Petrified Self 10 Years After: Current Evidence for Mnemonic Anosognosia. Front. Psychol. 2020, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Markova, I.S.; Clare, L.; Wang, M.; Romero, B.; Kenny, G. Awareness in Dementia: Conceptual Issues. Aging Ment. Heal. 2005, 9, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Huang, C.C.; Lin, C.P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. Neuroimage 2020, 206, 116189. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Metcalfe, J.; Cary, M.S.; De Leon, J.; Karlawish, J. Memory Awareness Influences Everyday Decision Making Capacity about Medication Management in Alzheimer’s Disease. Int. J. Alzheimers. Dis. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Koltai, D.C.; Welsh-Bohmer, K.A.; Schmechel, D.E. Influence of Anosognosia on Treatment Outcome among Dementia Patients. Neuropschological Rehabil. 2001, 11, 455–475. [Google Scholar] [CrossRef]
- Turró-Garriga, O.; Garre-Olmo, J.; René-Ramirez, R.; Calvó-Perxas, L.; Gascón-Bayarri, J.; Conde-Sala, J.L. Consequences of Anosognosia on the Cost of Caregivers´care in Alzheimer´s Disease. J. Alzheimer’s Dis. 2016, 54, 1551–1560. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]

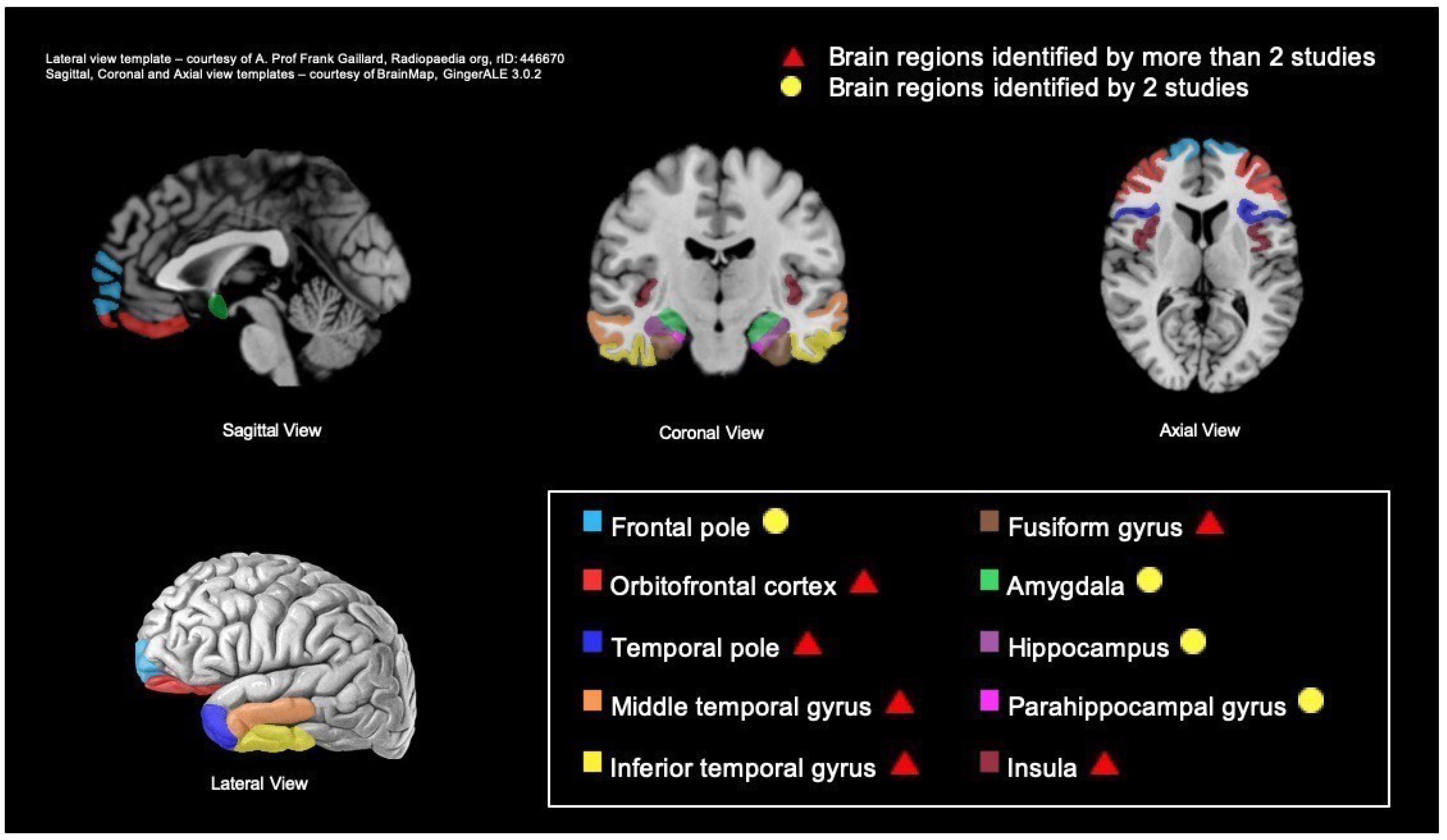
| Authors | Number of Patients and Type of Diagnosis | Mean Age of Patient (Standard Deviation (SD)) | Gender (M/F) | Mean MMSE Dementia Severity (SD) | Type of Scan | Awareness Measure | Quality Assessment (Score) | |
|---|---|---|---|---|---|---|---|---|
| Type | Measure | |||||||
| Duval et al. [50] | 15 SD | 64.27 (6.5) | 6/9 | n/a | MRI, PET | Patient–carer discrepancy score | Attribution of Intention Test, False Belief Test, Reading the Mind in the Eyes Test | Moderate (7) |
| Hornberger et al. [43] | 81 | MRI | Patient–carer discrepancy score | 28-item Insight Questionnaire | High (9) | |||
| 24 bvFTD | 63.13 (10.8) | 18/6 | 25.55 (3.7) | |||||
| 18 SD | 64.11 (9.2) | 13/5 | 24.06 (2.5) | |||||
| 13 PNFA | 63.64 (8.4) | 9/4 | 26.09 (2.9) | |||||
| 15 AD | 64.27 (9.1) | 12/3 | 22.07 (7.6) | |||||
| 11 LPA | 63.64 (8.4) | 4/7 | 22.18 (3.5) | |||||
| Hutchings et al. [44] | 41 | n/a | MRI | Patient–carer discrepancy score | Socio-emotional Questionnaire (SEQ) | Moderate (8) | ||
| 16 bvFTD | 64.05 (10.3) | 12/4 | ||||||
| 15 SD | 64.05 (5.9) | 8/7 | ||||||
| 10 AD | 66.25 (9.1) | 4/6 | ||||||
| Mychack et al. [45] | 4 | MRI | Patient–carer discrepancy score | Interpersonal Adjectives Scale (IAS), Interpersonal Measure of Psychopathy (IMP) | Low (2.5) | |||
| 1 SD | 68 | M | 23 | |||||
| 1 rtlvFTD | 52 | M | 30 | |||||
| 1 mild AD | 70 | F | 27 | |||||
| 1 moderate AD | 84 | F | 14 | |||||
| Parthimos et al. [46] | 77 39 AD 38 FTD (13bvFTD, 11 svPPA, 4 nfvPPA, 6 CBS, 4PSP) | n/a | 26/51 | n/a | MRI | Informant-rated score | Revised Self-monitoring Scale (RSMS) | High (10) |
| Ruby et al. [49] | 21 FTD | 64 (9) | 13/8 | n/a | PET | Patient–carer discrepancy score | Behaviour prediction Questionnaire, Personality assessment Questionnaire | Low (7) |
| Shany-ur et al. [47] | 78 | MRI | Patient–carer discrepancy score | Patient Competency Rating Scale (PCRS) | High (12) | |||
| 35 AD | 64.8 (8.6) | 17/18 | 23.4 (3.8) | |||||
| 21 bvFTD | 59.7 (7.2) | 12/9 | 24.9 (3.6) | |||||
| 7 rtvFTD | 61.9 (6.9) | 3/4 | 27 (1.2) | |||||
| 8 svPPA | 57.9 (6.6) | 4/4 | 21.8 (8.2) | |||||
| 7 nfvPPA | 66 (9.2) | 4/3 | 24.9 (6) | |||||
| Sollberger et al. [48] | 83 | MRI | Patient–carer discrepancy score | Interpersonal Reactivity index (IRI) | High (10) | |||
| 28 bvFTD | 62.4 (8.2) | 21/7 | 25.9 (4.7) | |||||
| 16 svPPA | 61.8 (6.7) | 10/6 | 25.3 (5.5) | |||||
| 4 nfvPPA | 62.0 (9.4) | 2/2 | 27.0 (3.6) | |||||
| 23 AD | 63.3 (10.3) | 15/8 | 19.9 (6.3) | |||||
| 12 CBS | 66.8 (9.2) | 4/8 | 22.6 (7.1) |
| Study | Domain | Assessment Method | Type of Scan | Coordinates (MNI) | Regions | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Structural studies | Awareness of emotion processing abilities | ||||||
| Hornberger et al. [43] | 28-item Insight Questionnaire Subscale • Emotion (Empathy) | MRI | −20 | 66 | 0 | L Frontal pole | |
| 22 | 64 | −6 | R Frontal pole | ||||
| −24 | −4 | −10 | L Amygdala | ||||
| −34 | −10 | −16 | L Hippocampus | ||||
| Shany-ur et al. [47] | Patient Competency Rating Scale (PCRS) Subscale • Emotion regulation (e.g., accepting criticism from others) | MRI | 38 | 24 | −10 | R Inferior frontal gyrus, orbital part | |
| 40 | 20 | −2 | R Insula, anterior | ||||
| 18 | 66 | 22 | R Superior frontal gyrus | ||||
| −30 | 26 | −26 | L Insula, anterior | ||||
| −25 | 28 | −16 | L Inferior frontal gyrus, orbital part | ||||
| Sollberger et al. [48] | Interpersonal reactivity index (IRI) | MRI | 60 | 6 | −34 | R Inferior temporal gyrus | |
| −30 | −36 | −14 | L Fusiform gyrus | ||||
| Awareness of social function | |||||||
| Hornberger et al. [43] | 28-item Insight Questionnaire Subscale • Socially appropriate interaction | MRI | −28 | −8 | −36 | L Parahippocampal Gyrus | |
| −16 | 10 | −18 | L Orbitofrontal Cortex | ||||
| −44 | −58 | −6 | L Temporal Gyrus | ||||
| −46 | −12 | 2 | L Insular Cortex | ||||
| 64 | −12 | −34 | R Temporal Gyrus | ||||
| 34 | −2 | −18 | R Amygdala | ||||
| 28 | −58 | 34 | R Occipital Cortex | ||||
| Parthimos et al. [46] | Revised Self-monitoring Scale (RSMS) Subscale • Self-presentation | MRI | 27 | 15 | −27 | ||
| R Insula | |||||||
| R Superior temporal pole | |||||||
| R Orbitofrontal gyrus | |||||||
| R Parahippocampal gyrus | |||||||
| R Rectus | |||||||
| R Olfactory | |||||||
| −16.5 | 9 | −16.5 | |||||
| L Insula | |||||||
| L Olfactory | |||||||
| L Rectus | |||||||
| L Orbitofrontal gyrus | |||||||
| −63 | −19.5 | −12 | L Middle temporal gyrus | ||||
| Shany-ur et al. [47] | PCRS Subscale • Social interpersonal functioning (e.g., participating in group activities) | MRI | 24 | 10 | −4 | R Putamen | |
| 28 | −12 | −40 | R Fusiform gyrus | ||||
| Functional study | Awareness of social function | ||||||
| Ruby et al. [49] | Behaviour Prediction Questionnaire • Anosognosia for behavioural change (S1-R3) | PET | −40 | 12 | −20 | L Superior part of the temporal pole | |
| −58 | −10 | −30 | L Inferior part of the temporal pole | ||||
| 56 | 18 | −14 | R Superior part of the temporal pole | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hengstschläger, A.; Sommerlad, A.; Huntley, J. What Are the Neural Correlates of Impaired Awareness of Social Cognition and Function in Dementia? A Systematic Review. Brain Sci. 2022, 12, 1136. https://doi.org/10.3390/brainsci12091136
Hengstschläger A, Sommerlad A, Huntley J. What Are the Neural Correlates of Impaired Awareness of Social Cognition and Function in Dementia? A Systematic Review. Brain Sciences. 2022; 12(9):1136. https://doi.org/10.3390/brainsci12091136
Chicago/Turabian StyleHengstschläger, Anna, Andrew Sommerlad, and Jonathan Huntley. 2022. "What Are the Neural Correlates of Impaired Awareness of Social Cognition and Function in Dementia? A Systematic Review" Brain Sciences 12, no. 9: 1136. https://doi.org/10.3390/brainsci12091136
APA StyleHengstschläger, A., Sommerlad, A., & Huntley, J. (2022). What Are the Neural Correlates of Impaired Awareness of Social Cognition and Function in Dementia? A Systematic Review. Brain Sciences, 12(9), 1136. https://doi.org/10.3390/brainsci12091136






