Elucidating the Neurobiologic Etiology of Comorbid PTSD and Substance Use Disorders
Abstract
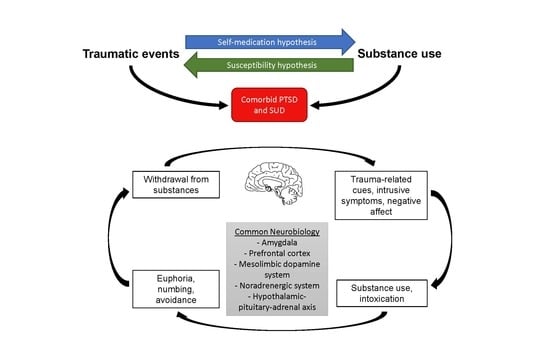
1. Introduction
2. Overlapping Clinical Symptomatology
3. Common Neurobiologic Etiology
4. Lessons from Translational and Clinical Research
5. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition DSM-5; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Copeland, W.E.; Keeler, G.; Angold, A.; Costello, E.J. Traumatic events and posttraumatic stress in childhood. Arch. Gen. Psychiatry 2007, 64, 577–584. [Google Scholar] [CrossRef]
- Saunders, B.E.; Adams, Z.W. Epidemiology of traumatic experiences in childhood. Child. Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 167–184; vii. [Google Scholar] [CrossRef]
- De Bellis, M.D. Developmental traumatology: A contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology 2002, 27, 155–170. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Administration on Children, Youth and Families, Children’s Bureau. In Childhood Maltreatment 2020; U.S. Department of Health and Human Services: Washington, DC, USA, 2022. [Google Scholar]
- Wildeman, C.; Emanuel, N.; Leventhal, J.M.; Putnam-Hornstein, E.; Waldfogel, J.; Lee, H. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014, 168, 706–713. [Google Scholar] [CrossRef]
- Simmons, S.; Suarez, L. Substance Abuse and Trauma. Child. Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 723–734. [Google Scholar] [CrossRef]
- Carliner, H.; Keyes, K.M.; McLaughlin, K.A.; Meyers, J.L.; Dunn, E.C.; Martins, S.S. Childhood Trauma and Illicit Drug Use in Adolescence: A Population-Based National Comorbidity Survey Replication-Adolescent Supplement Study. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 701–708. [Google Scholar] [CrossRef]
- Kilpatrick, D.G.; Ruggiero, K.J.; Acierno, R.; Saunders, B.E.; Resnick, H.S.; Best, C.L. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. J. Consult. Clin. Psychol. 2003, 71, 692–700. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Kilpatrick, D.G.; Resnick, H.S.; Milanak, M.E.; Miller, M.W.; Keyes, K.M.; Friedman, M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress 2013, 26, 537–547. [Google Scholar] [CrossRef]
- Norman, S.B.; Myers, U.S.; Wilkins, K.C.; Goldsmith, A.A.; Hristova, V.; Huang, Z.; McCullough, K.C.; Robinson, S.K. Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology 2012, 62, 542–551. [Google Scholar] [CrossRef]
- Danielson, C.K.; Adams, Z.; McCart, M.R.; Chapman, J.E.; Sheidow, A.J.; Walker, J.; Smalling, A.; de Arellano, M.A. Safety and Efficacy of Exposure-Based Risk Reduction Through Family Therapy for Co-occurring Substance Use Problems and Posttraumatic Stress Disorder Symptoms Among Adolescents: A Randomized Clinical Trial. JAMA Psychiatry 2020, 77, 574–586. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Weiner, J.L. Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Genes Brain Behav. 2017, 16, 15–43. [Google Scholar] [CrossRef]
- Bountress, K.E.; Cusack, S.E.; Sheerin, C.M.; Hawn, S.; Dick, D.M.; Kendler, K.S.; Amstadter, A.B. Alcohol consumption, interpersonal trauma, and drinking to cope with trauma-related distress: An auto-regressive, cross-lagged model. Psychol. Addict. Behav. 2019, 33, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.N.; Weiner, J.L. The neural, behavioral, and epidemiological underpinnings of comorbid alcohol use disorder and post-traumatic stress disorder. Int. Rev. Neurobiol. 2021, 157, 69–142. [Google Scholar]
- Hawn, S.E.; Cusack, S.E.; Amstadter, A.B. A Systematic Review of the Self-Medication Hypothesis in the Context of Posttraumatic Stress Disorder and Comorbid Problematic Alcohol Use. J. Trauma Stress 2020, 33, 699–708. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef]
- Strang, J.; Volkow, N.D.; Degenhardt, L.; Hickman, M.; Johnson, K.; Koob, G.F.; Marshall, B.D.L.; Tyndall, M.; Walsh, S.L. Opioid use disorder. Nat. Rev. Dis. Primers 2020, 6, 3. [Google Scholar] [CrossRef]
- Enman, N.M.; Zhang, Y.; Unterwald, E.M. Connecting the pathology of posttraumatic stress and substance use disorders: Monoamines and neuropeptides. Pharmacol. Biochem. Behav. 2014, 117, 61–69. [Google Scholar] [CrossRef]
- Danielson, C.K.; Hankin, B.L.; Badanes, L.S. Youth offspring of mothers with posttraumatic stress disorder have altered stress reactivity in response to a laboratory stressor. Psychoneuroendocrinology 2015, 53, 170–178. [Google Scholar] [CrossRef]
- Brady, K.T.; Sinha, R. Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. Am. J. Psychiatry 2005, 162, 1483–1493. [Google Scholar] [CrossRef]
- Goode, T.D.; Maren, S. Common neurocircuitry mediating drug and fear relapse in preclinical models. Psychopharmacology 2019, 236, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Steinman, M.Q.; Kirson, D.; Wolfe, S.A.; Khom, S.; D’Ambrosio, S.R.; Spierling Bagsic, S.R.; Bajo, M.; Vlkolinsky, R.; Hoang, N.K.; Singhal, A.; et al. Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders. Mol. Psychiatry 2021, 26, 3093–3107. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.L.; Nacewicz, B.M.; Sutterer, M.J.; Cayo, A.A.; Schaefer, S.M.; Rudolph, K.D.; Shirtcliff, E.A.; Pollak, S.D.; Davidson, R.J. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol. Psychiatry 2015, 77, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Wrase, J.; Makris, N.; Braus, D.F.; Mann, K.; Smolka, M.N.; Kennedy, D.N.; Caviness, V.S.; Hodge, S.M.; Tang, L.; Albaugh, M.; et al. Amygdala volume associated with alcohol abuse relapse and craving. Am. J. Psychiatry 2008, 165, 1179–1184. [Google Scholar] [CrossRef]
- Rich, M.T.; Huang, Y.H.; Torregrossa, M.M. Calcineurin Promotes Neuroplastic Changes in the Amygdala Associated with Weakened Cocaine-Cue Memories. J. Neurosci. 2020, 40, 1344–1354. [Google Scholar] [CrossRef]
- Gonzalez-Acosta, C.A.; Rojas-Ceron, C.A.; Buritica, E. Functional Alterations and Cerebral Variations in Humans Exposed to Early Life Stress. Front. Public Health 2020, 8, 536188. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef]
- Sinha, R.; Lacadie, C.M.; Constable, R.T.; Seo, D. Dynamic neural activity during stress signals resilient coping. Proc. Natl. Acad. Sci. USA 2016, 113, 8837–8842. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Wrocklage, K.M.; Averill, C.L.; Akiki, T.; Schweinsburg, B.; Roy, A.; Martini, B.; Southwick, S.M.; Krystal, J.H.; Scott, J.C. Anterior hippocampal dysconnectivity in posttraumatic stress disorder: A dimensional and multimodal approach. Transl. Psychiatry 2017, 7, e1045. [Google Scholar] [CrossRef]
- Gass, J.T.; Chandler, L.J. The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Front. Psychiatry 2013, 4, 46. [Google Scholar] [CrossRef]
- Zambrano-Vazquez, L.; Levy, H.C.; Belleau, E.L.; Dworkin, E.R.; Howard Sharp, K.M.; Pittenger, S.L.; Schumacher, J.A.; Coffey, S.F. Using the research domain criteria framework to track domains of change in comorbid PTSD and SUD. Psychol. Trauma 2017, 9, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Brodnik, Z.D.; Black, E.M.; Espana, R.A. Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress. Neuropsychopharmacology 2020, 45, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.S.; Blum, K.; Febo, M.; Baron, D.; Modestino, E.J.; Elman, I.; Badgaiyan, R.D. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front. Biosci. 2018, 10, 309–325. [Google Scholar]
- Itoi, K.; Sugimoto, N. The brainstem noradrenergic systems in stress, anxiety and depression. J. Neuroendocrinol. 2010, 22, 355–361. [Google Scholar] [CrossRef]
- Wemm, S.E.; Sinha, R. Drug-induced stress responses and addiction risk and relapse. Neurobiol. Stress 2019, 10, 100148. [Google Scholar] [CrossRef]
- Le Dorze, C.; Tassin, J.P.; Chauveau, F.; Gisquet-Verrier, P. Behavioral and Noradrenergic Sensitizations in Vulnerable Traumatized Rats Suggest Common Bases with Substance Use Disorders. Mol. Neurobiol. 2019, 56, 611–620. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Gold, P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992, 267, 1244–1252. [Google Scholar] [CrossRef]
- Rajbhandari, A.K.; Baldo, B.A.; Bakshi, V.P. Predator Stress-Induced CRF Release Causes Enduring Sensitization of Basolateral Amygdala Norepinephrine Systems that Promote PTSD-Like Startle Abnormalities. J. Neurosci. 2015, 35, 14270–14285. [Google Scholar] [CrossRef]
- Koob, G.F. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry 1999, 46, 1167–1180. [Google Scholar] [CrossRef]
- Snyder, A.E.; Silberman, Y. Corticotropin releasing factor and norepinephrine related circuitry changes in the bed nucleus of the stria terminalis in stress and alcohol and substance use disorders. Neuropharmacology 2021, 201, 108814. [Google Scholar] [CrossRef] [PubMed]
- Danielson, C.K.; Hahn, A.M.; Bountress, K.E.; Adams, Z.W.; Calhoun, C.; Amstadter, A.B.; Thomas, S. Associations of subjective and objective stress responses with interpersonal trauma, PTSD, stress-induced drinking, and drinking to cope in young adults. Psychol. Addict. Behav. 2021, 35, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Dalvie, S.; Cuzen, N.L.; Cardenas, V.; Fein, G.; Stein, D.J. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: A voxel-based morphometry study. Metab. Brain Dis. 2014, 29, 311–321. [Google Scholar] [CrossRef][Green Version]
- Yeh, C.L.; Levar, N.; Broos, H.C.; Dechert, A.; Potter, K.; Evins, A.E.; Gilman, J.M. White matter integrity differences associated with post-traumatic stress disorder are not normalized by concurrent marijuana use. Psychiatry Res. Neuroimaging 2020, 295, 111017. [Google Scholar] [CrossRef] [PubMed]
- Domen, P.; Michielse, S.; Peeters, S.; Viechtbauer, W.; van Os, J.; Marcelis, M.; for Genetic Risk and Outcome of Psychosis (G.R.O.U.P.). Childhood trauma- and cannabis-associated microstructural white matter changes in patients with psychotic disorder: A longitudinal family-based diffusion imaging study. Psychol. Med. 2019, 49, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Saladin, M.E.; Brady, K.T.; Dansky, B.S.; Kilpatrick, D.G. Understanding comorbidity between PTSD and substance use disorders: Two preliminary investigations. Addict. Behav. 1995, 20, 643–655. [Google Scholar] [CrossRef]
- Hadad, N.A.; Schwendt, M.; Knackstedt, L.A. Hypothalamic-pituitary-adrenal axis activity in post-traumatic stress disorder and cocaine use disorder. Stress 2020, 23, 638–650. [Google Scholar] [CrossRef]
- Gawrysiak, M.J.; Jagannathan, K.; Regier, P.; Suh, J.J.; Kampman, K.; Vickery, T.; Childress, A.R. Unseen scars: Cocaine patients with prior trauma evidence heightened resting state functional connectivity (RSFC) between the amygdala and limbic-striatal regions. Drug Alcohol. Depend. 2017, 180, 363–370. [Google Scholar] [CrossRef]
- Morris, V.L.; Huffman, L.G.; Naish, K.R.; Holshausen, K.; Oshri, A.; McKinnon, M.; Amlung, M. Impulsivity as a mediating factor in the association between posttraumatic stress disorder symptoms and substance use. Psychol. Trauma 2020, 12, 659–668. [Google Scholar] [CrossRef]
- Garke, M.A.; Isacsson, N.H.; Sorman, K.; Bjureberg, J.; Hellner, C.; Gratz, K.L.; Berghoff, C.R.; Sinha, R.; Tull, M.T.; Jayaram-Lindstrom, N. Emotion dysregulation across levels of substance use. Psychiatry Res. 2021, 296, 113662. [Google Scholar] [CrossRef]
- Rodriguez, L.; Read, J.P. Momentary emotional responding and emotion regulation in PTSD-related drinking urge. Exp. Clin. Psychopharmacol. 2020, 28, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Keeshin, B.R.; Bryant, B.J.; Gargaro, E.R. Emotional Dysregulation: A Trauma-Informed Approach. Child. Adolesc. Psychiatr. Clin. N. Am. 2021, 30, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.; Diguiseppi, G.; De Leon, J.; Prindle, J.; Sedano, A.; Rivera, D.; Henwood, B.; Rice, E. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol. Addict. Behav. 2019, 33, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Winningham, R.D.; Banks, D.E.; Buetlich, M.R.; Aalsma, M.C.; Zapolski, T.C.B. Substance use disorder and posttraumatic stress disorder symptomology on behavioral outcomes among juvenile justice youth. Am. J. Addict. 2019, 28, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Love, H.A.; Torgerson, C.N. Traumatic Experiences in Childhood and Adult Substance Use in a Nonclinical Sample: The Mediating Role of Arousal/Reactivity. J. Marital Fam. Ther. 2019, 45, 508–520. [Google Scholar] [CrossRef]
- Fortuna, L.R.; Porche, M.V.; Padilla, A. A treatment development study of a cognitive and mindfulness-based therapy for adolescents with co-occurring post-traumatic stress and substance use disorder. Psychol. Psychother. 2018, 91, 42–62. [Google Scholar] [CrossRef]
- Hahn, A.M.; Adams, Z.W.; Chapman, J.; McCart, M.R.; Sheidow, A.J.; de Arellano, M.A.; Danielson, C.K. Risk reduction through family therapy (RRFT): Protocol of a randomized controlled efficacy trial of an integrative treatment for co-occurring substance use problems and posttraumatic stress disorder symptoms in adolescents who have experienced interpersonal violence and other traumatic events. Contemp. Clin. Trials 2020, 93, 106012. [Google Scholar]
- Finkelhor, D.; Turner, H.; Ormrod, R.; Hamby, S.L. Violence, abuse, and crime exposure in a national sample of children and youth. Pediatrics 2009, 124, 1411–1423. [Google Scholar] [CrossRef]
- Finkelhor, D.; Turner, H.A.; Shattuck, A.; Hamby, S.L. Violence, crime, and abuse exposure in a national sample of children and youth: An update. JAMA Pediatr. 2013, 167, 614–621. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse. Trends & Statistics. Available online: https://www.drugabuse.gov/related-topics/trends-statistics (accessed on 22 August 2022).
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef]
- Anderson, K.N.; Radhakrishnan, L.; Lane, R.I.; Sheppard, M.; DeVies, J.; Azondekon, R.; Smith, A.R.; Bitsko, R.H.; Hartnett, K.P.; Lopes-Cardozo, B.; et al. Changes and Inequities in Adult Mental Health-Related Emergency Department Visits During the COVID-19 Pandemic in the US. JAMA Psychiatry 2022, 79, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Hansen, H. Evaluation of Increases in Drug Overdose Mortality Rates in the US by Race and Ethnicity Before and During the COVID-19 Pandemic. JAMA Psychiatry 2022, 79, 379–381. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. AAP-AACAP-CHA Declaration of a National Emergency in Child and Adolescent Mental Health. Available online: https://www.aap.org/en/advocacy/child-and-adolescent-healthy-mental-development/aap-aacap-cha-declaration-of-a-national-emergency-in-child-and-adolescent-mental-health/ (accessed on 22 August 2022).
- Blanco, C.; Wall, M.M.; Lindquist, M.A.; Rodriguez-Fernandez, J.M.; Franco, S.; Wang, S.; Olfson, M. Generalizability of Neuroimaging Studies in 5 Common Psychiatric Disorders Based on the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J. Clin. Psychiatry 2016, 77, e1618–e1625. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.; Shah, R.; Nooner, K.B.; Nagel, B.J.; Tapert, S.F.; de Bellis, M.D.; Mishra, J. Impact of Childhood Trauma on Executive Function in Adolescence-Mediating Functional Brain Networks and Prediction of High-Risk Drinking. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 499–509. [Google Scholar] [CrossRef]
- Bjork, J.M.; Straub, L.K.; Provost, R.G.; Neale, M.C. The ABCD study of neurodevelopment: Identifying neurocircuit targets for prevention and treatment of adolescent substance abuse. Curr. Treat. Options Psychiatry 2017, 4, 196–209. [Google Scholar] [CrossRef]
- Jeong, H.J.; Durham, E.L.; Moore, T.M.; Dupont, R.M.; McDowell, M.; Cardenas-Iniguez, C.; Micciche, E.T.; Berman, M.G.; Lahey, B.B.; Kaczkurkin, A.N. The association between latent trauma and brain structure in children. Transl. Psychiatry 2021, 11, 240. [Google Scholar] [CrossRef]
- Lisdahl, K.M.; Sher, K.J.; Conway, K.P.; Gonzalez, R.; Feldstein Ewing, S.W.; Nixon, S.J.; Tapert, S.; Bartsch, H.; Goldstein, R.Z.; Heitzeg, M. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev. Cogn. Neurosci. 2018, 32, 80–96. [Google Scholar] [CrossRef]
- Girgenti, M.J.; Hare, B.D.; Ghosal, S.; Duman, R.S. Molecular and Cellular Effects of Traumatic Stress: Implications for PTSD. Curr. Psychiatry Rep. 2017, 19, 85. [Google Scholar] [CrossRef]
- Alexander, K.S.; Nalloor, R.; Bunting, K.M.; Vazdarjanova, A. Investigating Individual Pre-trauma Susceptibility to a PTSD-Like Phenotype in Animals. Front. Syst. Neurosci. 2019, 13, 85. [Google Scholar] [CrossRef]
- Chadwick, B.; Miller, M.L.; Hurd, Y.L. Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Front. Psychiatry 2013, 4, 129. [Google Scholar] [CrossRef]
- Goldfarb, M.G.; Brown, D.R. Diversifying participation: The rarity of reporting racial demographics in neuroimaging research. Neuroimage 2022, 254, 119122. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.L.; Calhoun, C.D.; Banks, D.E.; Halliday, C.A.; Hughes-Halbert, C.; Danielson, C.K. Making the "C-ACE" for a Culturally-Informed Adverse Childhood Experiences Framework to Understand the Pervasive Mental Health Impact of Racism on Black Youth. J. Child. Adolesc. Trauma 2021, 14, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.P.; Lotzin, A.; Schafer, I. A systematic review and meta-analysis of psychological interventions for comorbid post-traumatic stress disorder and substance use disorder. Eur. J. Psychotraumatol. 2022, 13, 2041831. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinckley, J.D.; Danielson, C.K. Elucidating the Neurobiologic Etiology of Comorbid PTSD and Substance Use Disorders. Brain Sci. 2022, 12, 1166. https://doi.org/10.3390/brainsci12091166
Hinckley JD, Danielson CK. Elucidating the Neurobiologic Etiology of Comorbid PTSD and Substance Use Disorders. Brain Sciences. 2022; 12(9):1166. https://doi.org/10.3390/brainsci12091166
Chicago/Turabian StyleHinckley, Jesse D., and Carla Kmett Danielson. 2022. "Elucidating the Neurobiologic Etiology of Comorbid PTSD and Substance Use Disorders" Brain Sciences 12, no. 9: 1166. https://doi.org/10.3390/brainsci12091166
APA StyleHinckley, J. D., & Danielson, C. K. (2022). Elucidating the Neurobiologic Etiology of Comorbid PTSD and Substance Use Disorders. Brain Sciences, 12(9), 1166. https://doi.org/10.3390/brainsci12091166