Sensory Modulation in Children with Developmental Coordination Disorder Compared to Autism Spectrum Disorder and Typically Developing Children
Abstract
:1. Introduction
1.1. Developmental Coordination Disorder (DCD)
1.2. Sensory Processing in Developmental Coordination Disorder
1.2.1. Sensory Discrimination
1.2.2. Sensory Modulation
1.2.3. Sensory Processing Patterns
1.3. The Relationship between Sensory Processing and Social Emotional Measures
1.3.1. Social Skills
1.3.2. Anxiety and Depression
1.3.3. Alexithymia
1.3.4. Empathy
1.4. The Relationship between Sensory Processing and Behavior
1.5. The Relationship between Sensory Processing and Motor Skills
1.6. Current Study
- Do children with DCD experience differences in sensory processing? How does this compare to ASD and TD peers? Based on our previous work [23], we predict children with DCD to fall between ASD and TD groups in sensory over-responsivity. We further expand our prior studies by investigating specific aspects of sensory modulation and determine how many children in each group meet clinical cutoffs.
- Do children with DCD differ from children with ASD and TD children on social emotional and behavioral measures? Based on our prior data [23,25], we expect children with DCD to fall between ASD and TD groups on social emotional measures, competencies, and empathic skills. We also explore differences in DCD that have previously not been explored in our prior data: anxiety as measured by the SCARED-P and repetitive behaviors.
- How does sensory over-responsivity in children with DCD correlate with social emotional, motor, and behavioral measures? Do these relationships differ in DCD as compared to ASD and TD children? We expect significant correlations between SOR and anxiety and the CBCL in all groups, significant correlations between SOR and motor difficulties in the ASD and DCD group, and significant correlations between SOR and social difficulties in the ASD group.
2. Materials and Methods
2.1. Participants
2.2. Assessments
2.2.1. Screening Measures
Wechsler Abbreviated Scale of Intelligence (WASI-II)
Autism Diagnostic Observation Schedule (ADOS)
Autism Diagnostic Interview-Revised (ADI-R)
Conners 3rd Edition ADHD Index—Parent (Conners 3AI)
2.2.2. Sensory Measures
Short Sensory Profile-2 (SSP-2)
Sensory Over-Responsivity Scale (SensOR)
2.2.3. Motor Measures
Movement Assessment Battery for Children Second Edition (MABC-2)
Developmental Coordination Disorder Questionnaire (DCDQ)
2.2.4. Social Emotional Measures
Social Responsiveness Scale, 2nd Edition (SRS-2)
A Developmental NEuroPSYchological Assessment—Second Edition (NEPSY-II)
Interpersonal Reactivity Index (IRI)
The Screen for Child Anxiety Related Emotional Disorders-Parent (SCARED-P)
Alexithymia Questionnaire for Children (AQC)
2.2.5. Behavioral Measures
Repetitive Behaviors Scale-Revised (RBS-R)
Child Behavior Checklist/6-18 (CBCL)
2.3. Analysis
2.3.1. Determining Sensory Sensitivities
2.3.2. Correlation Analyses
3. Results
3.1. Group Differences
3.1.1. Sensory Over-Responsivity (SensOR Inventory)
3.1.2. Sensory Response Patterns (SSP-2)
3.1.3. Anxiety
3.2. Correlation Analysis
4. Discussion
4.1. Group Differences
4.1.1. Sensory Modulation in DCD
4.1.2. Anxiety and Depression
4.1.3. Empathy
4.1.4. Alexithymia
4.1.5. Other Social Emotional Measures (CBCL Syndromes & NEPSY)
4.1.6. Behavior
4.2. Correlations
4.2.1. Social Skills
4.2.2. Anxiety and Depression
4.2.3. Empathy
4.2.4. Alexithymia
4.2.5. Other Social Emotional Measures (CBCL Syndromes & NEPSY)
4.2.6. Child Behavior Checklist
4.2.7. Repetitive Behaviors
4.2.8. Sensory Over-Responsivity and Motor Skills
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoen, S.A.; Miller, L.J.; Mulligan, S. Validity of the occupational performance scale of the Sensory Processing Three Dimensions measure. Am. J. Occup. Ther. 2021, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Nielsen, D.M.; Schoen, S.A. Attention deficit hyperactivity disorder and sensory modulation disorder: A comparison of behavior and physiology. Res. Dev. Disabil. 2012, 33, 804–818. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American psychiatric Association: Washington, DC, USA, 2013; Volume 5. [Google Scholar]
- Bar-Shalita, T.; Vatine, J.J.; Parush, S. Sensory modulation disorder: A risk factor for participation in daily life activities. Dev. Med. Child Neurol. 2008, 50, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-W.; Rodger, S.; Copley, J.; Branjerdporn, G.; Taggart, C. Sensory processing and its relationship with children’s daily life participation. Phys. Occup. Ther. Pediatrics 2016, 36, 73–87. [Google Scholar] [CrossRef]
- Jarus, T.; Lourie-Gelberg, Y.; Engel-Yeger, B.; Bart, O. Participation patterns of school-aged children with and without DCD. Res. Dev. Disabil. 2011, 32, 1323–1331. [Google Scholar] [CrossRef]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Schoen, S.A.; Mulligan, S.; Sullivan, J. Identification of sensory processing and integration symptom clusters: A preliminary study. Occup. Ther. Int. 2017, 2017, 2876080. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.J. Sensory Integration and Praxis Tests; Western Psychological Services: Los Angeles, CA, USA, 1989. [Google Scholar]
- May-Benson, T.A.; Cermak, S.A. Development of an assessment for ideational Praxis. Am. J. Occup. Ther. 2007, 61, 148–153. [Google Scholar] [CrossRef]
- Lane, S.J.; Mailloux, Z.; Schoen, S.; Bundy, A.; May-Benson, T.A.; Parham, L.D.; Smith Roley, S.; Schaaf, R.C. Neural foundations of ayres sensory integration®. Brain Sci. 2019, 9, 153. [Google Scholar] [CrossRef]
- Dunn, W. Sensory Profile 2; Pearson Assessments: Coushatta, LA, USA, 2014. [Google Scholar]
- Keating, J.; Gaffney, R.; Bramham, J.; Downes, M. Sensory modulation difficulties and assessment in children with attention deficit hyperactivity disorder: A systematic review. Eur. J. Dev. Psychol. 2022, 19, 110–144. [Google Scholar] [CrossRef]
- Pfeiffer, B.; Daly, B.P.; Nicholls, E.G.; Gullo, D.F. Assessing Sensory Processing Problems in Children With and Without Attention Deficit Hyperactivity Disorder. Phys. Occup. Ther. Pediatrics 2015, 35, 1–12. [Google Scholar] [CrossRef]
- Klein-Tasman, B.P.; Mervis, C.B. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev. Neuropsychol. 2003, 23, 269–290. [Google Scholar] [CrossRef]
- Niutanen, U.; Harra, T.; Lano, A.; Metsäranta, M. Systematic review of sensory processing in preterm children reveals abnormal sensory modulation, somatosensory processing and sensory-based motor processing. Acta Paediatr. 2020, 109, 45–55. [Google Scholar] [CrossRef]
- Glod, M.; Riby, D.M.; Rodgers, J. Sensory processing profiles and autistic symptoms as predictive factors in autism spectrum disorder and Williams syndrome. J. Intellect. Disabil. Res. 2020, 64, 657–665. [Google Scholar] [CrossRef]
- Hannant, P.; Cassidy, S.; Van de Weyer, R.; Mooncey, S. Sensory and motor differences in autism spectrum conditions and developmental coordination disorder in children. Hum. Mov. Sci. 2018, 58, 108–118. [Google Scholar] [CrossRef]
- Piek, J.P.; Dyck, M.J. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Hum. Mov. Sci. 2004, 23, 475–488. [Google Scholar] [CrossRef]
- Hen-Herbst, L.; Jirikowic, T.; Hsu, L.Y.; McCoy, S.W. Motor performance and sensory processing behaviors among children with fetal alcohol spectrum disorders compared to children with developmental coordination disorders. Res. Dev. Disabil. 2020, 103, 103680. [Google Scholar] [CrossRef]
- Blank, R.; Barnett, A.L.; Cairney, J.; Green, D.; Kirby, A.; Polatajko, H.; Rosenblum, S.; Smits-Engelsman, B.; Sugden, D.; Wilson, P.; et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 2019, 61, 242–285. [Google Scholar] [CrossRef]
- Chang, S.H.; Yu, N.Y. Comparison of motor praxis and performance in children with varying levels of developmental coordination disorder. Hum. Mov. Sci. 2016, 48, 7–14. [Google Scholar] [CrossRef]
- Harrison, L.A.; Kats, A.; Kilroy, E.; Butera, C.; Jayashankar, A.; Keles, U.; Aziz-Zadeh, L. Motor and sensory features successfully decode autism spectrum disorder and combine with the original RDoC framework to boost diagnostic classification. Sci. Rep. 2021, 11, 7839. [Google Scholar] [CrossRef]
- Bhat, A.N. Is Motor Impairment in Autism Spectrum Disorder Distinct From Developmental Coordination Disorder? A Report From the SPARK Study. Phys. Ther. 2020, 100, 633–644. [Google Scholar] [CrossRef]
- Kilroy, E.; Ring, P.; Hossain, A.; Nalbach, A.; Butera, C.; Harrison, L.; Jayashankar, A.; Vigen, C.; Aziz-Zadeh, L.; Cermak, S.A. Motor performance, praxis, and social skills in autism spectrum disorder and developmental coordination disorder. Autism Res. 2022, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Hirota, T.; Sakamoto, Y.; Adachi, M.; Takahashi, M.; Osato-Kaneda, A.; Kim, Y.S.; Leventhal, B.; Shui, A.; Kato, S.; et al. Prevalence and cumulative incidence of autism spectrum disorders and the patterns of co-occurring neurodevelopmental disorders in a total population sample of 5-year-old children. Mol. Autism 2020, 11, 35. [Google Scholar] [CrossRef]
- Lingam, R.; Hunt, L.; Golding, J.; Jongmans, M.; Emond, A. Prevalence of developmental coordination disorder using the DSM-IV at 7 years of age: A UK population-based study. Pediatrics 2009, 123, e693–e700. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.J. Patterns of perceptual-motor dysfunction in children: A factor analytic study. Percept. Mot. Ski. 1965, 20, 335–368. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.J. Cluster analyses of measures of sensory integration. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 1977, 31, 362–366. [Google Scholar]
- Cox, L.E.; Harris, E.C.; Auld, M.L.; Johnston, L.M. Impact of tactile function on upper limb motor function in children with Developmental Coordination Disorder. Res. Dev. Disabil. 2015, 45, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Elbasan, B.; Kayıhan, H.; Duzgun, I. Sensory integration and activities of daily living in children with developmental coordination disorder. Ital. J. Pediatrics 2012, 38, 14. [Google Scholar] [CrossRef]
- Johnston, J.S.; Ali, J.B.; Hill, E.L.; Bremner, A.J. Tactile localization performance in children with developmental coordination disorder (DCD) corresponds to their motor skill and not their cognitive ability. Hum. Mov. Sci. 2017, 53, 72–83. [Google Scholar] [CrossRef]
- Li, K.-Y.; Su, W.-J.; Fu, H.-W.; Pickett, K.A. Kinesthetic deficit in children with developmental coordination disorder. Res. Dev. Disabil. 2015, 38, 125–133. [Google Scholar] [CrossRef]
- Malloy-Miller, T.; Polatajko, H.; Anstett, B. Handwriting error patterns of children with mild motor difficulties. Can. J. Occup. Ther. 1995, 62, 258–267. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Holst-Wolf, J.M.; Tsai, C.-L.; Chen, F.-C.; Konczak, J. Haptic perception is altered in children with developmental coordination disorder. Neuropsychologia 2019, 127, 29–34. [Google Scholar] [CrossRef]
- AYRES, A.J. Development of the body scheme in children. Am. J. Occup. 1961, 15, 99–102. [Google Scholar]
- Mailloux, Z.; Mulligan, S.; Roley, S.S.; Blanche, E.; Cermak, S.; Coleman, G.G.; Bodison, S.; Lane, C.J. Verification and clarification of patterns of sensory integrative dysfunction. Am. J. Occup. Ther. 2011, 65, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, S. Patterns of sensory integration dysfunction: A confirmatory factor analysis. Am. J. Occup. Ther. 1998, 52, 819–828. [Google Scholar] [CrossRef]
- Mulligan, S. Cluster analysis of scores of children on the Sensory Integration and Praxis Tests. Occup. Ther. J. Res. 2000, 20, 256–270. [Google Scholar] [CrossRef]
- Roley, S.S.; Mailloux, Z.; Parham, L.D.; Schaaf, R.C.; Lane, C.J.; Cermak, S. Sensory integration and praxis patterns in children with autism. Am. J. Occup. Ther. 2015, 69, 6901220010p1–6901220010p8. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Casey, J. Developmental coordination disorders and sensory processing and integration: Incidence, associations and co-morbidities. Br. J. Occup. Ther. 2017, 80, 549–557. [Google Scholar] [CrossRef]
- Delgado-Lobete, L.; Pértega-Díaz, S.; Santos-del-Riego, S.; Montes-Montes, R. Sensory processing patterns in developmental coordination disorder, attention deficit hyperactivity disorder and typical development. Res. Dev. Disabil. 2020, 100, 103608. [Google Scholar] [CrossRef]
- Mikami, M.; Hirota, T.; Takahashi, M.; Adachi, M.; Saito, M.; Koeda, S.; Yoshida, K.; Sakamoto, Y.; Kato, S.; Nakamura, K. Atypical sensory processing profiles and their associations with motor problems in preschoolers with developmental coordination disorder. Child Psychiatry Hum. Dev. 2021, 52, 311–320. [Google Scholar] [CrossRef]
- Simpson, K.; Adams, D.; Alston-Knox, C.; Heussler, H.S.; Keen, D. Exploring the Sensory Profiles of Children on the Autism Spectrum Using the Short Sensory Profile-2 (SSP-2). J. Autism Dev. Disord. 2019, 49, 2069–2079. [Google Scholar] [CrossRef]
- Cosbey, J.; Johnston, S.S.; Dunn, M.L. Sensory processing disorders and social participation. Am. J. Occup. Ther. 2010, 64, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Hochhauser, M.; Engel-Yeger, B. Sensory processing abilities and their relation to participation in leisure activities among children with high-functioning autism spectrum disorder (HFASD). Res. Autism Spectr. Disord. 2010, 4, 746–754. [Google Scholar] [CrossRef]
- Miguel, H.; Sampaio, A.; Martínez-Regueiro, R.; Gómez-Guerrero, L.; López-Dóriga, C.G.; Gómez, S.; Carracedo, Á.; Fernández-Prieto, M. Touch processing and social behavior in ASD. J. Autism Dev. Disord. 2017, 47, 2425–2433. [Google Scholar] [CrossRef]
- Reynolds, S.; Bendixen, R.M.; Lawrence, T.; Lane, S.J. A pilot study examining activity participation, sensory responsiveness, and competence in children with high functioning autism spectrum disorder. J. Autism Dev. Disord. 2011, 41, 1496–1506. [Google Scholar] [CrossRef]
- Butera, C.; Ring, P.; Sideris, J.; Jayashankar, A.; Kilroy, E.; Harrison, L.; Cermak, S.; Aziz-Zadeh, L. Impact of Sensory Processing on School Performance Outcomes in High Functioning Individuals with Autism Spectrum Disorder. Mind Brain Educ. 2020, 14, 243–254. [Google Scholar] [CrossRef]
- Engel-Yeger, B.; Ziv-On, D. The relationship between sensory processing difficulties and leisure activity preference of children with different types of ADHD. Res. Dev. Disabil. 2011, 32, 1154–1162. [Google Scholar] [CrossRef]
- Cascio, C.J.; Woynaroski, T.; Baranek, G.T.; Wallace, M.T. Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Res. 2016, 9, 920–925. [Google Scholar] [CrossRef]
- Koenig, K.P.; Rudney, S.G. Performance challenges for children and adolescents with difficulty processing and integrating sensory information: A systematic review. Am. J. Occup. Ther. 2010, 64, 430–442. [Google Scholar] [CrossRef]
- Wagner, M.O.; Bös, K.; Jascenoka, J.; Jekauc, D.; Petermann, F. Peer problems mediate the relationship between developmental coordination disorder and behavioral problems in school-aged children. Res. Dev. Disabil. 2012, 33, 2072–2079. [Google Scholar] [CrossRef]
- Cairney, J.; Hay, J.A.; Veldhuizen, S.; Missiuna, C.; Faught, B.E. Developmental coordination disorder, sex, and activity deficit over time: A longitudinal analysis of participation trajectories in children with and without coordination difficulties. Dev. Med. Child Neurol. 2010, 52, e67–e72. [Google Scholar] [CrossRef]
- Green, D.; Baird, G.; Barnett, A.L.; Henderson, L.; Huber, J.; Henderson, S.E. The severity and nature of motor impairment in Asperger’s syndrome: A comparison with Specific Developmental Disorder of Motor Function. J. Child Psychol. Psychiatry 2002, 43, 655–668. [Google Scholar] [CrossRef]
- Magnuson, K.M.; Constantino, J.N. Characterization of depression in children with autism spectrum disorders. J. Dev. Behav. Pediatrics 2011, 32, 332–340. [Google Scholar] [CrossRef]
- Vasa, R.A.; Keefer, A.; McDonald, R.G.; Hunsche, M.C.; Kerns, C.M. A scoping review of anxiety in young children with autism spectrum disorder. Autism Res. 2020, 13, 2038–2057. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.L.; Campi, E.; Baranek, G.T. Associations among sensory hyperresponsiveness, restricted and repetitive behaviors, and anxiety in autism: An integrated systematic review. Res. Autism Spectr. Disord. 2021, 83, 101763. [Google Scholar] [CrossRef]
- Green, S.A.; Ben-Sasson, A. Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: Is there a causal relationship? J. Autism Dev. Disord. 2010, 40, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.; Leonard, H.C. Internalising symptoms in Developmental Coordination Disorder: The indirect effect of everyday executive function. Res. Dev. Disabil. 2021, 109, 103831. [Google Scholar] [CrossRef] [PubMed]
- Draghi, T.T.G.; Cavalcante Neto, J.L.; Rohr, L.A.; Jelsma, L.D.; Tudella, E. Symptoms of anxiety and depression in children with developmental coordination disorder: A systematic review. J. De Pediatr. 2020, 96, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.; Jijon, A.M.; Leonard, H.C. Research review: Internalising symptoms in developmental coordination disorder: A systematic review and meta-analysis. J. Child Psychol. Psychiatry 2019, 60, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.L.; Hill, E.L. Anxiety profiles in children with and without developmental coordination disorder. Res. Dev. Disabil. 2011, 32, 1253–1259. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Wade, T.J.; Veldhuizen, S.; Missiuna, C.; Timmons, B.; Cairney, J. Emotional and Behavioral Problems in 4-and 5-year old children with and without motor delays. Front. Pediatrics 2019, 7, 474. [Google Scholar] [CrossRef]
- Liss, M.; Mailloux, J.; Erchull, M.J. The relationships between sensory processing sensitivity, alexithymia, autism, depression, and anxiety. Personal. Individ. Differ. 2008, 45, 255–259. [Google Scholar] [CrossRef]
- Neal, J.A.; Edelmann, R.J.; Glachan, M. Behavioural inhibition and symptoms of anxiety and depression: Is there a specific relationship with social phobia? Br. J. Clin. Psychol. 2002, 41, 361–374. [Google Scholar] [CrossRef]
- Harrison, L.A.; Kats, A.; Williams, M.E.; Aziz-Zadeh, L. The importance of sensory processing in mental health: A proposed addition to the research domain criteria (RDoC) and suggestions for RDoC 2.0. Front. Psychol. 2019, 10, 103. [Google Scholar] [CrossRef]
- Hill, E.; Berthoz, S.; Frith, U. Brief report: Cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J. Autism Dev. Disord. 2004, 34, 229–235. [Google Scholar] [CrossRef]
- Milosavljevic, B.; Carter Leno, V.; Simonoff, E.; Baird, G.; Pickles, A.; Jones, C.R.; Erskine, C.; Charman, T.; Happé, F. Alexithymia in adolescents with autism spectrum disorder: Its relationship to internalising difficulties, sensory modulation and social cognition. J. Autism Dev. Disord. 2016, 46, 1354–1367. [Google Scholar] [CrossRef]
- Serafini, G.; Gonda, X.; Canepa, G.; Pompili, M.; Rihmer, Z.; Amore, M.; Engel-Yeger, B. Extreme sensory processing patterns show a complex association with depression, and impulsivity, alexithymia, and hopelessness. J. Affect. Disord. 2017, 210, 249–257. [Google Scholar] [CrossRef]
- Jakobson, L.S.; Rigby, S.N. Alexithymia and Sensory Processing Sensitivity: Areas of Overlap and Links to Sensory Processing Styles. Front. Psychol. 2021, 12, 583786. [Google Scholar] [CrossRef]
- Salimi, H.; Alipour, G.; Miri, V.; Kermanshahi, F. Investigation of the correlation between sensory processing sensitivity and alexithymia with tendency to addiction in dormitory resident female students of Qazvin University of Medical Sciences, Iran. Qom Univ. Med. Sci. J. 2017, 11, 68–78. [Google Scholar]
- Butera, C.D.; Harrison, L.; Kilroy, E.; Jayashankar, A.; Shipkova, M.; Pruyser, A.; Aziz-Zadeh, L. Relationships between alexithymia, interoception, and emotional empathy in autism spectrum disorder. Autism 2022, 13623613221111310. [Google Scholar] [CrossRef]
- Heydrich, L.; Walker, F.; Blättler, L.; Herbelin, B.; Blanke, O.; Aspell, J.E. Interoception and empathy impact perspective taking. Front. Psychol. 2021, 11, 599429. [Google Scholar] [CrossRef]
- Stoica, T.; Depue, B. Shared characteristics of intrinsic connectivity networks underlying interoceptive awareness and empathy. Front. Hum. Neurosci. 2020, 14, 571070. [Google Scholar] [CrossRef]
- Mul, C.-L.; Stagg, S.D.; Herbelin, B.; Aspell, J.E. The feeling of me feeling for you: Interoception, alexithymia and empathy in autism. J. Autism Dev. Disord. 2018, 48, 2953–2967. [Google Scholar] [CrossRef]
- Yang, H.-X.; Hu, H.-X.; Zhang, Y.-J.; Wang, Y.; Lui, S.S.; Chan, R.C. A network analysis of interoception, self-awareness, empathy, alexithymia, and autistic traits. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 199–209. [Google Scholar] [CrossRef]
- Acevedo, B.P.; Aron, E.N.; Aron, A.; Sangster, M.D.; Collins, N.; Brown, L.L. The highly sensitive brain: An fMRI study of sensory processing sensitivity and response to others’ emotions. Brain Behav. 2014, 4, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Aron, E.N.; Aron, A. Sensory-Processing Sensitivity and Its Relation to Introversion and Emotionality. J. Personal. Soc. Psychol. 1997, 73, 345–368. [Google Scholar] [CrossRef]
- Schaefer, M.; Kevekordes, M.-C.; Sommer, H.; Gärtner, M. Of Orchids and Dandelions: Empathy but Not Sensory Processing Sensitivity Is Associated with Tactile Discrimination Abilities. Brain Sci. 2022, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, T.; Miller, L.J.; Schoen, S.A.; Jo Brout, J.; Sullivan, J.; Baron-Cohen, S. Sensory reactivity, empathizing and systemizing in autism spectrum conditions and sensory processing disorder. Dev. Cogn. Neurosci. 2018, 29, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Rodgers, J.; McConachie, H. Restricted and repetitive behaviours, sensory processing and cognitive style in children with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 635–642. [Google Scholar] [CrossRef]
- Foss-Feig, J.H.; Heacock, J.L.; Cascio, C.J. Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Res. Autism Spectr. Disord. 2012, 6, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, T. Sensory Processing and Motor Skill Performance in Elementary School Children with Autism Spectrum Disorder. Percept. Mot. Ski. 2013, 116, 197–209. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.; Jones, R.S.P. Sensory-perceptual abnormalities in autism: A case for more research? J. Autism Dev. Disord. 1997, 27, 283–293. [Google Scholar] [CrossRef] [PubMed]
- May-Benson, T.A.; Koomar, J.A. Systematic review of the research evidence examining the effectiveness of interventions using a sensory integrative approach for children. Am. J. Occup. Ther. 2010, 64, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watling, R.; Hauer, S. Effectiveness of ayres sensory integration® and sensory-based interventions for people with autism spectrum disorder: A systematic review. Am. J. Occup. Ther. 2015, 69, 6905180030p1–6905180030p12. [Google Scholar] [CrossRef]
- Kilroy, E.; Harrison, L.; Butera, C.; Jayashankar, A.; Cermak, S.; Kaplan, J.; Williams, M.; Haranin, E.; Bookheimer, S.; Dapretto, M. Unique deficit in embodied simulation in autism: An fMRI study comparing autism and developmental coordination disorder. Hum. Brain Mapp. 2021, 42, 1532–1546. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2008, 39, 1–11. [Google Scholar] [CrossRef]
- Wechsler, D. Weshsler Abbreviated Scale of Intelligence, 2nd ed.; Movement assessment battery for children-2; Henderson, S.E., Sugden, D., Barnett, A.L., Eds; 2011. Available online: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Wechsler-Abbreviated-Scale-of-Intelligence-%7C-Second-Edition/p/100000593.html (accessed on 5 August 2021).
- Crovitz, H.F.; Zener, K. A group-test for assessing hand-and eye-dominance. Am. J. Psychol. 1962, 75, 271–276. [Google Scholar] [CrossRef]
- Martin, N.C.; Piek, J.P.; Hay, D. DCD and ADHD: A genetic study of their shared aetiology. Hum. Mov. Sci. 2006, 25, 110–124. [Google Scholar] [CrossRef]
- Lord, C.; DiLavore, P.; Risi, S.; Gotham, K.; Bishop, S. (ADOS-2) Autism Diagnostic Observation Schedule; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Avni, E.; Ben-Itzchak, E.; Zachor, D.A. The presence of comorbid ADHD and anxiety symptoms in autism spectrum disorder: Clinical presentation and predictors. Front. Psychiatry 2018, 9, 717. [Google Scholar] [CrossRef]
- Conners, C.K. Conners (Conners 3), 3rd ed.; Western Psychological Services: Los Angeles, CA, USA, 2008. [Google Scholar]
- Henderson, S.E.; Sugden, D.; Barnett, A.L. Movement Assessment Battery for Children-2; Pearson Assessments: Coushatta, LA, USA, 2007. [Google Scholar]
- Wilson, B.N.; Kaplan, B.J.; Crawford, S.G.; Campbell, A.; Dewey, D. Reliability and validity of a parent questionnaire on childhood motor skills. Am. J. Occup. Ther. 2000, 54, 484–493. [Google Scholar] [CrossRef]
- Wilson, B.N.; Crawford, S.G.; Green, D.; Roberts, G.; Aylott, A.; Kaplan, B.J. Psychometric properties of the revised developmental coordination disorder questionnaire. Phys. Occup. Ther. Pediatrics 2009, 29, 182–202. [Google Scholar] [CrossRef]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale: SRS-2; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Schoen, S.A.; Miller, L.J.; Green, K.E. Pilot study of the sensory over-responsivity scales: Assessment and inventory. Am. J. Occup. Ther. 2008, 62, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY II: Clinical and Interpretive Manual; Pearson Assessments: Coushatta, LA, USA, 2007. [Google Scholar]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Personal. Soc. Psychol. 1983, 44, 113. [Google Scholar] [CrossRef]
- Birmaher, B.; Brent, D.A.; Chiappetta, L.; Bridge, J.; Monga, S.; Baugher, M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 1230–1236. [Google Scholar] [CrossRef]
- Rieffe, C.; Oosterveld, P.; Terwogt, M.M. An alexithymia questionnaire for children: Factorial and concurrent validation results. Personal. Individ. Differ. 2006, 40, 123–133. [Google Scholar] [CrossRef]
- Bodfish, J.; Symons, F.; Lewis, M. The Repetitive Behavior Scales: A Test Manual; Western Carolina Center Research Reports; Western Carolina Center: Morganton, NC, USA, 1999. [Google Scholar]
- Achenbach, T.M. Manual for ASEBA School-Age Forms & Profiles; University of Vermont, Research Center for Children, Youth & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Van Hulle, C.A.; Schmidt, N.L.; Goldsmith, H.H. Is sensory over-responsivity distinguishable from childhood behavior problems? A phenotypic and genetic analysis. J. Child Psychol. Psychiatry 2012, 53, 64–72. [Google Scholar] [CrossRef]
- Litvack-Miller, W.; McDougall, D.; Romney, D.M. The structure of empathy during middle childhood and its relationship to prosocial behavior. Genet. Soc. Gen. Psychol. Monogr. 1997, 123, 303–325. [Google Scholar]
- Pfeifer, J.H.; Iacoboni, M.; Mazziotta, J.C.; Dapretto, M. Mirroring others’ emotions relates to empathy and interpersonal competence in children. Neuroimage 2008, 39, 2076–2085. [Google Scholar] [CrossRef]
- Behrens, B.; Swetlitz, C.; Pine, D.S.; Pagliaccio, D. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Informant Discrepancy, Measurement Invariance, and Test-Retest Reliability. Child Psychiatry Hum. Dev. 2019, 50, 473–482. [Google Scholar] [CrossRef]
- Gonzalez, A.; Weersing, V.R.; Warnick, E.; Scahill, L.; Woolston, J. Cross-ethnic measurement equivalence of the SCARED in an outpatient sample of African American and non-Hispanic White youths and parents. J. Clin. Child Adolesc. Psychol. 2012, 41, 361–369. [Google Scholar] [CrossRef]
- Birmaher, B.; Khetarpal, S.; Brent, D.; Cully, M.; Balach, L.; Kaufman, J.; Neer, S.M. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Loas, G.; Braun, S.; Delhaye, M.; Linkowski, P. The measurement of alexithymia in children and adolescents: Psychometric properties of the Alexithymia Questionnaire for Children and the twenty-item Toronto Alexithymia Scale in different non-clinical and clinical samples of children and adolescents. PLoS ONE 2017, 12, e0177982. [Google Scholar] [CrossRef] [PubMed]
- Hooker, J.L.; Dow, D.; Morgan, L.; Schatschneider, C.; Wetherby, A.M. Psychometric analysis of the repetitive behavior scale-revised using confirmatory factor analysis in children with autism. Autism Res. 2019, 12, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Schertz, H.H.; Odom, S.L.; Baggett, K.M.; Sideris, J.H. Parent-reported repetitive behavior in toddlers on the autism spectrum. J. Autism Dev. Disord. 2016, 46, 3308–3316. [Google Scholar] [CrossRef]
- Lam, K.S.; Aman, M.G. The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2007, 37, 855–866. [Google Scholar] [CrossRef]
- Mirenda, P.; Smith, I.M.; Vaillancourt, T.; Georgiades, S.; Duku, E.; Szatmari, P.; Bryson, S.; Fombonne, E.; Roberts, W.; Volden, J. Validating the repetitive behavior scale-revised in young children with autism spectrum disorder. J. Autism Dev. Disord. 2010, 40, 1521–1530. [Google Scholar] [CrossRef]
- Scahill, L.; Dimitropoulos, A.; McDougle, C.J.; Aman, M.G.; Feurer, I.D.; McCracken, J.T.; Tierney, E.; Pu, J.; White, S.; Lecavalier, L. Children’s Yale–Brown obsessive compulsive scale in autism Spectrum disorder: Component structure and correlates of symptom checklist. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 97–107. [Google Scholar] [CrossRef]
- Crijnen, A.A.; Achenbach, T.M.; Verhulst, F.C. Problems reported by parents of children in multiple cultures: The Child Behavior Checklist syndrome constructs. Am. J. Psychiatry 1999, 156, 569–574. [Google Scholar] [CrossRef]
- Aylward, G.; Stancin, T.; Wolraich, M.; Drotar, D.; Dworkin, P.; Perrin, E. Screening and assessment tools. In Measurement and Psychometric Considerations. Developmental Behavioral PediatricsEvidence and Practice; Elsiver: Philadelphia, PA, USA, 2008; pp. 123–129. [Google Scholar]
- Maust, D.; Cristancho, M.; Gray, L.; Rushing, S.; Tjoa, C.; Thase, M.E. Psychiatric rating scales. Handb. Clin. Neurol. 2012, 106, 227–237. [Google Scholar] [CrossRef]
- Bishop, D.V. Which neurodevelopmental disorders get researched and why? PLoS ONE 2010, 5, e15112. [Google Scholar] [CrossRef]
- Draghi, T.T.G.; Cavalcante Neto, J.L.; Tudella, E. Symptoms of anxiety and depression in schoolchildren with and without developmental coordination disorder. J. Health Psychol. 2021, 26, 1519–1527. [Google Scholar] [CrossRef]
- Lane, A.E. Practitioner Review: Effective management of functional difficulties associated with sensory symptoms in children and adolescents. J. Child Psychol. Psychiatry 2020, 61, 943–958. [Google Scholar] [CrossRef]
- Cummins, A.; Piek, J.P.; Dyck, M.J. Motor coordination, empathy, and social behaviour in school-aged children. Dev. Med. Child Neurol. 2005, 47, 437–442. [Google Scholar] [CrossRef]
- Kim, H.; Han, S. Does personal distress enhance empathic interaction or block it? Personal. Individ. Differ. 2018, 124, 77–83. [Google Scholar] [CrossRef]
- Sumner, E.; Leonard, H.C.; Hill, E.L. Overlapping Phenotypes in Autism Spectrum Disorder and Developmental Coordination Disorder: A Cross-Syndrome Comparison of Motor and Social Skills. J. Autism Dev. Disord. 2016, 46, 2609–2620. [Google Scholar] [CrossRef]
- Abrams, G.; Jayashankar, A.; Kilroy, E.; Butera, C.; Harrison, L.; Ring, P.; Cermak, S.; Aziz-Zadeh, L. Differences in praxis errors in autism spectrum disorder compared to developmental coordination disorder. J. Autism. Dev. Disord. 2022. Submitted. [Google Scholar]
- Bruce, V.; Campbell, R.N.; Doherty-Sneddon, G.; Langton, S.; McAuley, S.; Wright, R. Testing face processing skills in children. Br. J. Dev. Psychol. 2000, 18, 319–333. [Google Scholar] [CrossRef]
- Crane, L.; Sumner, E.; Hill, E.L. Emotional and behavioural problems in children with Developmental Coordination Disorder: Exploring parent and teacher reports. Res. Dev. Disabil. 2017, 70, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.L.; Sikora, D.M.; McCoy, R. Prevalence and risk factors of maladaptive behaviour in young children with Autistic Disorder. J. Intellect. Disabil. Res. 2008, 52, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kanioglou, A.; Tsorbatzoudis, H.; Barkoukis, V. Socialization and behavioral problems of elementary school pupils with developmental coordination disorder. Percept. Mot. Ski. 2005, 101, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Cordier, R.; Bundy, A.; Hocking, C.; Einfeld, S. Empathy in the Play of Children with Attention Deficit Hyperactivity Disorder. OTJR 2010, 30, 122–132. [Google Scholar] [CrossRef]
- Delgado-Lobete, L.; Montes-Montes, R.; Pértega-Díaz, S.; Santos-Del-Riego, S.; Hartman, E.; Schoemaker, M.M. Motor performance and daily participation in children with and without probable developmental coordination disorder. Dev. Med. Child Neurol. 2022, 64, 220–227. [Google Scholar] [CrossRef]
- Izadi-Najafabadi, S.; Ryan, N.; Ghafooripoor, G.; Gill, K.; Zwicker, J.G. Participation of children with developmental coordination disorder. Res. Dev. Disabil. 2019, 84, 75–84. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.H.; Lee, Y. Correlation between motor coordination skills and emotional and behavioral difficulties in children with and without developmental coordination disorder. Int. J. Environ. Res. Public Health 2020, 17, 7362. [Google Scholar] [CrossRef]
- Wigham, S.; Rodgers, J.; South, M.; McConachie, H.; Freeston, M. The Interplay Between Sensory Processing Abnormalities, Intolerance of Uncertainty, Anxiety and Restricted and Repetitive Behaviours in Autism Spectrum Disorder. J. Autism Dev. Disord. 2014, 45, 943–952. [Google Scholar] [CrossRef]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef]
- Conelea, C.A.; Carter, A.C.; Freeman, J.B. Sensory over-responsivity in a sample of children seeking treatment for anxiety. J. Dev. Behav. Pediatrics 2014, 35, 510–521. [Google Scholar] [CrossRef]
- Goldsmith, H.H.; Van Hulle, C.A.; Arneson, C.L.; Schreiber, J.E.; Gernsbacher, M.A. A population-based twin study of parentally reported tactile and auditory defensiveness in young children. J. Abnorm. Child Psychol. 2006, 34, 393–407. [Google Scholar] [CrossRef]
- Miller, L.T.; Missiuna, C.A.; Macnab, J.J.; Malloy-Miller, T.; Polatajko, H.J. Clinical description of children with developmental coordination disorder. Can. J. Occup. Ther. 2001, 68, 5–15. [Google Scholar] [CrossRef]
- Jones, A.P.; Happé, F.G.E.; Gilbert, F.; Burnett, S.; Viding, E. Feeling, caring, knowing: Different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. J. Child Psychol. Psychiatry 2010, 51, 1188–1197. [Google Scholar] [CrossRef]
- Meng, C.; Huo, C.; Ge, H.; Li, Z.; Hu, Y.; Meng, J. Processing of expressions by individuals with autistic traits: Empathy deficit or sensory hyper-reactivity? PLoS ONE 2021, 16, e0254207. [Google Scholar] [CrossRef]
- Liss, M.; Timmel, L.; Baxley, K.; Killingsworth, P. Sensory processing sensitivity and its relation to parental bonding, anxiety, and depression. Personal. Individ. Differ. 2005, 39, 1429–1439. [Google Scholar] [CrossRef]
- Dellapiazza, F.; Vernhet, C.; Blanc, N.; Miot, S.; Schmidt, R.; Baghdadli, A. Links between sensory processing, adaptive behaviours, and attention in children with autism spectrum disorder: A systematic review. Psychiatry Res. 2018, 270, 78–88. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Kanne, S.M.; Wodka, E.L. Physical aggression in children and adolescents with autism spectrum disorders. Res. Autism Spectr. Disord. 2013, 7, 455–465. [Google Scholar] [CrossRef]
- Van den Boogert, F.; Sizoo, B.; Spaan, P.; Tolstra, S.; Bouman, Y.H.A.; Hoogendijk, W.; Roza, S. Sensory processing and aggressive behavior in adults with autism spectrum disorder. Brain Sci. 2021, 11, 95. [Google Scholar] [CrossRef]
- Boyd, B.A.; Baranek, G.T.; Sideris, J.; Poe, M.D.; Watson, L.R.; Patten, E.; Miller, H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Res. 2010, 3, 78–87. [Google Scholar] [CrossRef]
- Zetler, N.K.; Cermak, S.A.; Engel-Yeger, B.; Baranek, G.; Gal, E. Association Between Sensory Features and High-Order Repetitive and Restricted Behaviors and Interests Among Children With Autism Spectrum Disorder. Am. J. Occup. Ther. 2022, 76, 7603205010. [Google Scholar] [CrossRef]
- Duerden, E.G.; Oatley, H.K.; Mak-Fan, K.M.; McGrath, P.A.; Taylor, M.J.; Szatmari, P.; Roberts, S.W. Risk Factors Associated with Self-Injurious Behaviors in Children and Adolescents with Autism Spectrum Disorders. J. Autism Dev. Disord. 2012, 42, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, Á.E.; Coote, S.; Robinson, K. Occupational therapy practice with children with developmental coordination disorder: An online qualitative vignette survey. Br. J. Occup. Ther. 2021, 84, 307–316. [Google Scholar] [CrossRef]


| DCD N = 26 | ASD N = 57 | TD N = 53 | DCD:TD | DCD:ASD | TD:ASD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p | p | p | Partial Eta Squared | |
| Sex (sum M) | 15 | - | 44 | - | 31 | - | 0.041 d | 0.041 d | 0.041 d | 0.198 d |
| Age | 11.75 | 2.31 | 11.89 | 2.29 | 11.75 | 2.13 | 1.00 | 1.00 | 0.974 | 0.001 |
| Full-Scale IQ | 109.69 | 17.13 | 107.51 | 16.82 | 118.28 | 13.74 | 0.083 | 1.00 | <0.001 | 0.094 |
| DCD/ASD > TD or TD > DCD/ASD | ||||||||||
| SSP-2 Bystander | 16.62 | 6.41 | 19.28 | 7.24 | 8.98 | 5.47 | <0.001 ** | 0.225 | <0.001 ** | 0.355 |
| DCDQ Total c | 45.36 | 11.81 | 47.47 | 9.98 | 74.32 | 7.95 | <0.001 *** | 1.00 | <0.001 ** | 0.658 |
| DCD between ASD and TD: ASD > DCD > TD or TD > DCD > ASD | ||||||||||
| SSP-2 Seeker | 12.65 | 4.70 | 16.61 | 6.38 | 8.00 | 4.78 | <0.001 ** | 0.015 * | <0.001 ** | 0.318 |
| SSP-2 Avoider | 17.65 | 6.70 | 25.23 | 7.15 | 11.28 | 6.06 | <0.001 ** | <0.001 ** | <0.001 ** | 0.463 |
| SSP-2 Sensor | 20.31 | 5.64 | 28.89 | 7.18 | 12.64 | 6.49 | <0.001 ** | <0.001 ** | <0.001 ** | 0.513 |
| CBCL Social Problems | 58.92 | 7.12 | 64.44 | 9.80 | 51.55 | 2.85 | <0.001 *** | 0.002 ** | <0.001 *** | 0.393 |
| CBCL Attention Problems | 58.23 | 5.72 | 65.25 | 11.78 | 51.34 | 2.24 | 0.002 ** | <0.001 *** | <0.001 *** | 0.379 |
| CBCL Thought Problems | 56.50 | 6.69 | 65.11 | 9.61 | 51.98 | 3.29 | 0.024 * | <0.001 *** | <0.001 *** | 0.400 |
| CBCL Total Competencies | 43.23 | 10.90 | 34.70 | 7.51 | 50.43 | 10.06 | 0.015 * | <0.001 *** | <0.001 *** | 0.323 |
| CBCL School Competencies | 45.27 | 8.27 | 40.32 | 8.00 | 52.94 | 3.47 | <0.001 *** | 0.004 ** | <0.001 *** | 0.380 |
| ASD > DCD/TD or DCD/TD > ASD | ||||||||||
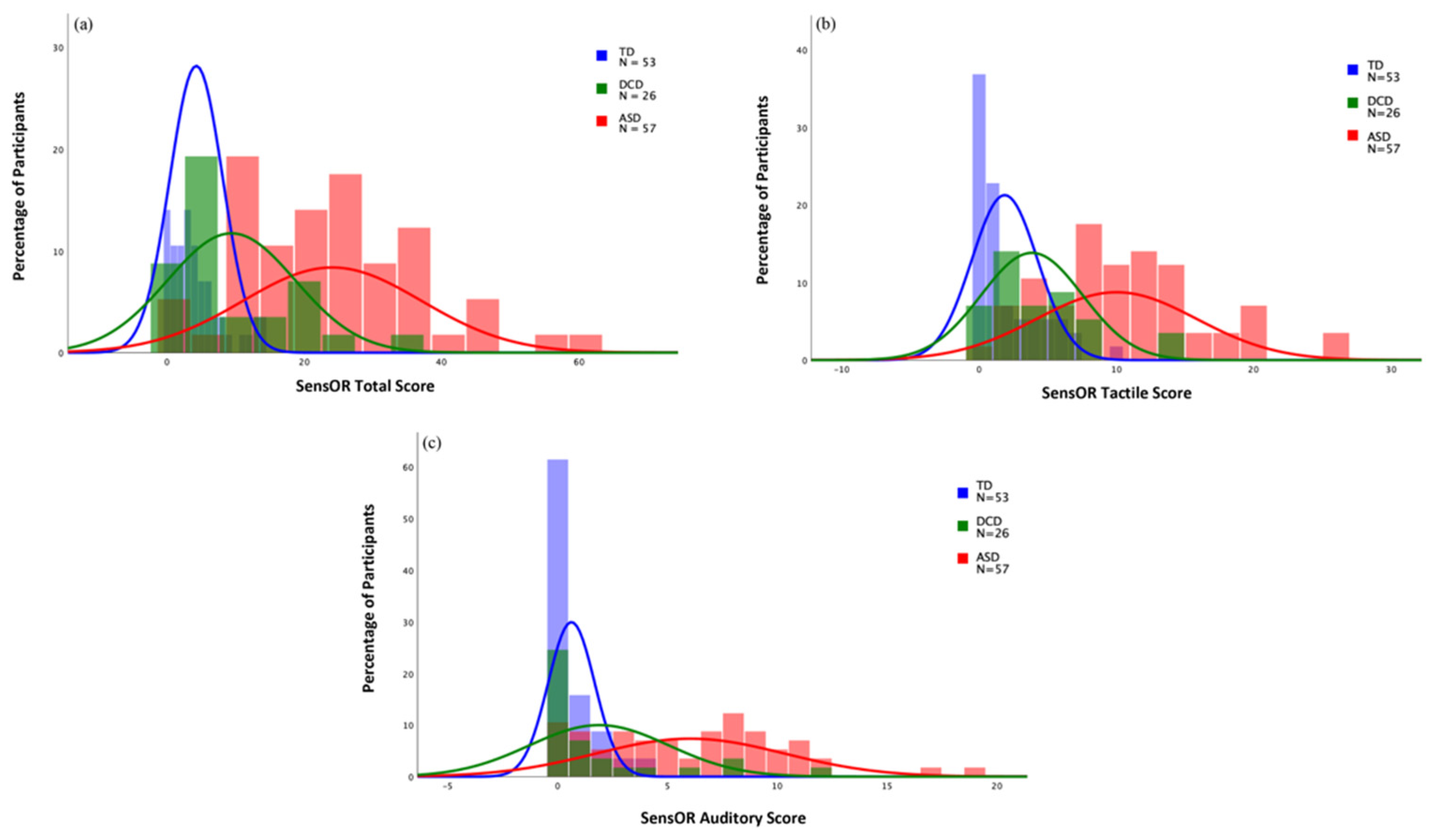
| SensOR Total | 9.38 | 9.31 | 24.07 | 13.04 | 4.26 | 3.87 | 0.128 | <0.001 *** | <0.001 *** | 0.441 |
| SensOR Tactile | 3.85 | 3.70 | 10.02 | 5.84 | 1.87 | 2.40 | 0.105 | <0.001 ** | <0.001 ** | 0.389 |
| SensOR Auditory | 1.88 | 3.18 | 6.04 | 4.31 | 0.62 | 1.06 | 0.141 | <0.001 ** | <0.001 ** | 0.360 |
| RBS Stereotyped | 1.12 | 1.45 | 3.51 | 2.89 | 0.08 | 0.33 | 0.121 | <0.001 *** | <0.001 *** | 0.354 |
| RBS Compulsive | 0.54 | 0.81 | 3.67 | 3.98 | 0.62 | 1.43 | 1.00 | <0.001 ** | <0.001 *** | 0.226 |
| RBS Ritualistic | 0.69 | 1.10 | 4.89 | 3.53 | 0.42 | 1.31 | 1.00 | <0.001 *** | <0.001 *** | 0.422 |
| RBS Sameness | 1.42 | 1.68 | 7.07 | 5.35 | 0.45 | 1.55 | 0.699 | <0.001 *** | <0.001 *** | 0.404 |
| RBS Restricted | 0.58 | 1.14 | 2.88 | 2.36 | 0.09 | 0.35 | 0.843 | <0.001 *** | <0.001 *** | 0.352 |
| RBS Total | 5.46 | 4.51 | 24.67 | 17.45 | 2.11 | 5.25 | 0.710 | <0.001 *** | <0.001 *** | 0.423 |
| CBCL Anxious/Depressed | 53.69 | 6.28 | 61.91 | 10.65 | 52.42 | 4.42 | 1.00 | <0.001 *** | <0.001 *** | 0.254 |
| CBCL Withdrawn/Depressed | 54.96 | 6.70 | 62.70 | 10.19 | 51.70 | 2.64 | 0.153 | <0.001 *** | <0.001 *** | 0.311 |
| CBCL Somatic Complaints | 55.00 | 6.40 | 61.56 | 10.14 | 52.83 | 4.41 | 0.843 | <0.001 *** | <0.001 *** | 0.215 |
| CBCL Social Competencies | 45.92 | 10.03 | 36.38 | 8.87 | 49.09 | 8.93 | 0.772 | <0.001 *** | <0.001 *** | 0.246 |
| CBCL Aggressive Behavior | 53.00 | 4.29 | 57.46 | 9.16 | 50.68 | 1.76 | 0.307 | 0.007 ** | <0.001 *** | 0.201 |
| SCARED-P GAD | 3.58 | 4.38 | 6.67 | 4.58 | 2.25 | 2.73 | 0.395 | 0.002 ** | <0.001 *** | 0.219 |
| SCARED-P Social Anxiety | 2.38 | 2.80 | 5.74 | 4.03 | 2.64 | 3.12 | 1.00 | <0.001 *** | <0.001 *** | 0.190 |
| SCARED-P Separation Anxiety | 1.35 | 2.12 | 4.21 | 3.83 | 1.11 | 1.80 | 1.00 | <0.001 *** | <0.001 *** | 0.219 |
| SCARED-P School Avoidance | 0.65 | 1.16 | 1.40 | 1.90 | 0.36 | 0.762 | 1.00 | 0.046 * | <0.001 *** | 0.107 |
| SCARED-P Panic | 1.04 | 2.46 | 3.75 | 4.33 | 0.62 | 1.735 | 1.00 | <0.001 *** | <0.001 *** | 0.192 |
| SCARED-P Total b | 9.00 | 10.68 | 21.77 | 15.14 | 6.87 | 7.64 | 1.00 | <0.001 *** | <0.001 *** | 0.278 |
| NEPSY-ToM | 24.92 | 2.51 | 22.42 | 3.43 | 25.47 | 1.98 | 1.00 | <0.001 ** | <0.001 ** | 0.154 |
| Alexi-Describing Feelings | 0.62 | 0.39 | 1.01 | 0.43 | 0.73 | 0.48 | 1.00 | <0.001 ** | 0.002 * | 0.132 |
| Alexi-2-factor total | 6.88 | 4.19 | 9.72 | 4.65 | 7.11 | 4.68 | 1.00 | 0.022 * | 0.010 * | 0.083 |
| ASD > TD only | ||||||||||
| RBS Self-Injurious | 1.12 | 1.86 | 2.56 | 3.61 | 0.45 | 1.74 | 0.907 | 0.051 t | <0.001 *** | 0.119 |
| CBCL Rule-Breaking Behavior | 53.50 | 5.13 | 55.23 | 6.71 | 51.00 | 1.93 | 0.084 | 0.257 | <0.001 *** | 0.146 |
| IRI Personal Distress a | 13.42 | 5.0 | 14.59 | 4.69 | 12.40 | 5.15 | 1.00 | 0.432 | 0.021 * | 0.056 |
| TD > ASD only | ||||||||||
| CBCL Activities Competencies | 45.42 | 11.56 | 40.65 | 8.94 | 48.94 | 10.10 | 0.406 | 0.125 | <0.001 *** | 0.122 |
| No significant differences between groups | ||||||||||
| IRI Perspective Taking a | 14.73 | 4.40 | 12.79 | 5.58 | 14.81 | 4.70 | 1.00 | 0.156 | 0.088 | 0.046 |
| IRI Fantasy Scale a | 17.38 | 5.32 | 16.61 | 5.67 | 16.77 | 5.41 | 1.00 | 1.00 | 1.00 | 0.004 |
| IRI Empathetic Concern a | 18.23 | 4.55 | 16.63 | 5.26 | 17.72 | 4.73 | 1.00 | 0.809 | 1.00 | 0.009 |
| NEPSY-Affect Recognition | 27.88 | 3.04 | 26.26 | 3.43 | 28.25 | 2.94 | 1.00 | 0.378 | 0.140 | 0.036 |
| Alexi-Identifying Feelings | 0.54 | 0.41 | 0.67 | 0.46 | 0.49 | 0.39 | 1.00 | 0.571 | 0.138 | 0.032 |
| SCARED-P | SRS | IRI | NEPSY | RBS | DCDQ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Total | Total | PT | EC | FS | PD | AR | ToM | Stereo | SI | Comp | Rit | Same | Rest | Total | Total |
| DCD | 0.435 * | 0.395 t | 0.062 | −0.601 ** | −0.040 | −0.061 | 0.322 | 0.194 | 0.098 | 0.472 * | 0.117 | 0.321 | 0.575 ** | 0.189 | 0.570 ** | −0.433 * |
| ASD | 0.667 *** | 0.575 *** | −0.316 * | −0.022 | −0.008 | −0.050 | 0.088 | 0.103 | 0.371 ** | 0.560 *** | 0.485 *** | 0.561 *** | 0.586 *** | 0.386 ** | 0.631 *** | −0.249 |
| TD | 0.364 * | 0.184 | 0.042 | 0.157 | −0.103 | 0.085 | 0.012 | −0.085 | −0.018 | 0.068 | 0.103 | 0.220 | 0.233 | −0.041 | 0.167 | −0.270 |
| SCARED-P Subscores | Alexithymia | CBCL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | GAD | Panic | Soc Anx | Sep Anx | Sch Avoid | ID | Desc | 2 Fact | ActCom | SchCom | SocCom | Total Com | Anx/D | W/D | Som Cx | Soc Px | ThPx | AttPx | RB Bx | AgBx |
| DCD | 0.321 | 0.381 | 0.544 ** | 0.230 | 0.281 | 0.049 | 0.181 | 0.133 | −0.090 | 0.310 | 0.082 | 0.014 | 0.448 * | 0.584 ** | 0.039 | 0.238 | 0.245 | 0.121 | 0.256 | 0.403 t |
| ASD | 0.531 ** | 0.539 ** | 0.468 ** | 0.646 ** | 0.494 ** | 0.185 | 0.303 ** | 0.265 t | 0.157 | −0.249 | −064 | 0.021 | 0.506 *** | 0.454 *** | 0.503 *** | 0.598 *** | 0.613 *** | 0.505 *** | 0.364 ** | 0.299 * |
| TD | 0.240 | 0.314 | 0.218 | 0.469 *** | −0.027 | 0.100 | −0.169 | 0.026 | −0.010 | 0.026 | 0.060 | 0.019 | 0.364 ** | 0.418 ** | 0.252 | 0.268 | 0.064 | −0.014 | 0.089 | −0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringold, S.M.; McGuire, R.W.; Jayashankar, A.; Kilroy, E.; Butera, C.D.; Harrison, L.; Cermak, S.A.; Aziz-Zadeh, L. Sensory Modulation in Children with Developmental Coordination Disorder Compared to Autism Spectrum Disorder and Typically Developing Children. Brain Sci. 2022, 12, 1171. https://doi.org/10.3390/brainsci12091171
Ringold SM, McGuire RW, Jayashankar A, Kilroy E, Butera CD, Harrison L, Cermak SA, Aziz-Zadeh L. Sensory Modulation in Children with Developmental Coordination Disorder Compared to Autism Spectrum Disorder and Typically Developing Children. Brain Sciences. 2022; 12(9):1171. https://doi.org/10.3390/brainsci12091171
Chicago/Turabian StyleRingold, Sofronia M, Riley W McGuire, Aditya Jayashankar, Emily Kilroy, Christiana D Butera, Laura Harrison, Sharon A Cermak, and Lisa Aziz-Zadeh. 2022. "Sensory Modulation in Children with Developmental Coordination Disorder Compared to Autism Spectrum Disorder and Typically Developing Children" Brain Sciences 12, no. 9: 1171. https://doi.org/10.3390/brainsci12091171
APA StyleRingold, S. M., McGuire, R. W., Jayashankar, A., Kilroy, E., Butera, C. D., Harrison, L., Cermak, S. A., & Aziz-Zadeh, L. (2022). Sensory Modulation in Children with Developmental Coordination Disorder Compared to Autism Spectrum Disorder and Typically Developing Children. Brain Sciences, 12(9), 1171. https://doi.org/10.3390/brainsci12091171





