A Combined Image- and Coordinate-Based Meta-Analysis of Whole-Brain Voxel-Based Morphometry Studies Investigating Subjective Tinnitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Combined Image- and Coordinate-Based Meta-Analysis
2.6. Heterogeneity and Publication Bias
3. Results
3.1. Studies Included
3.2. SDM-PSI Meta-Analyses
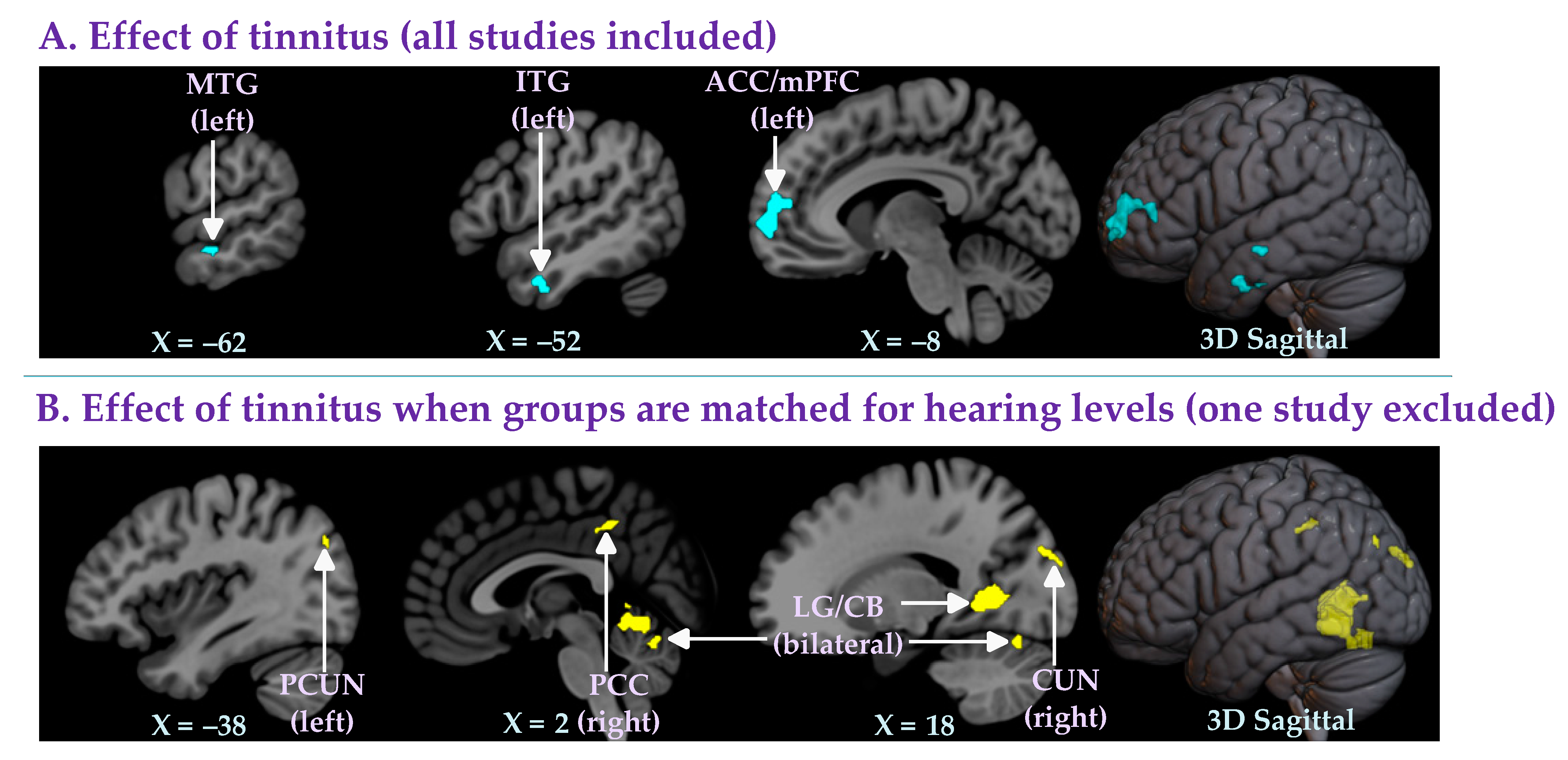
3.2.1. Effect of Tinnitus (All Studies Included)
3.2.2. Effect of Tinnitus When Groups Are Matched for Hearing Levels (One Study Excluded)
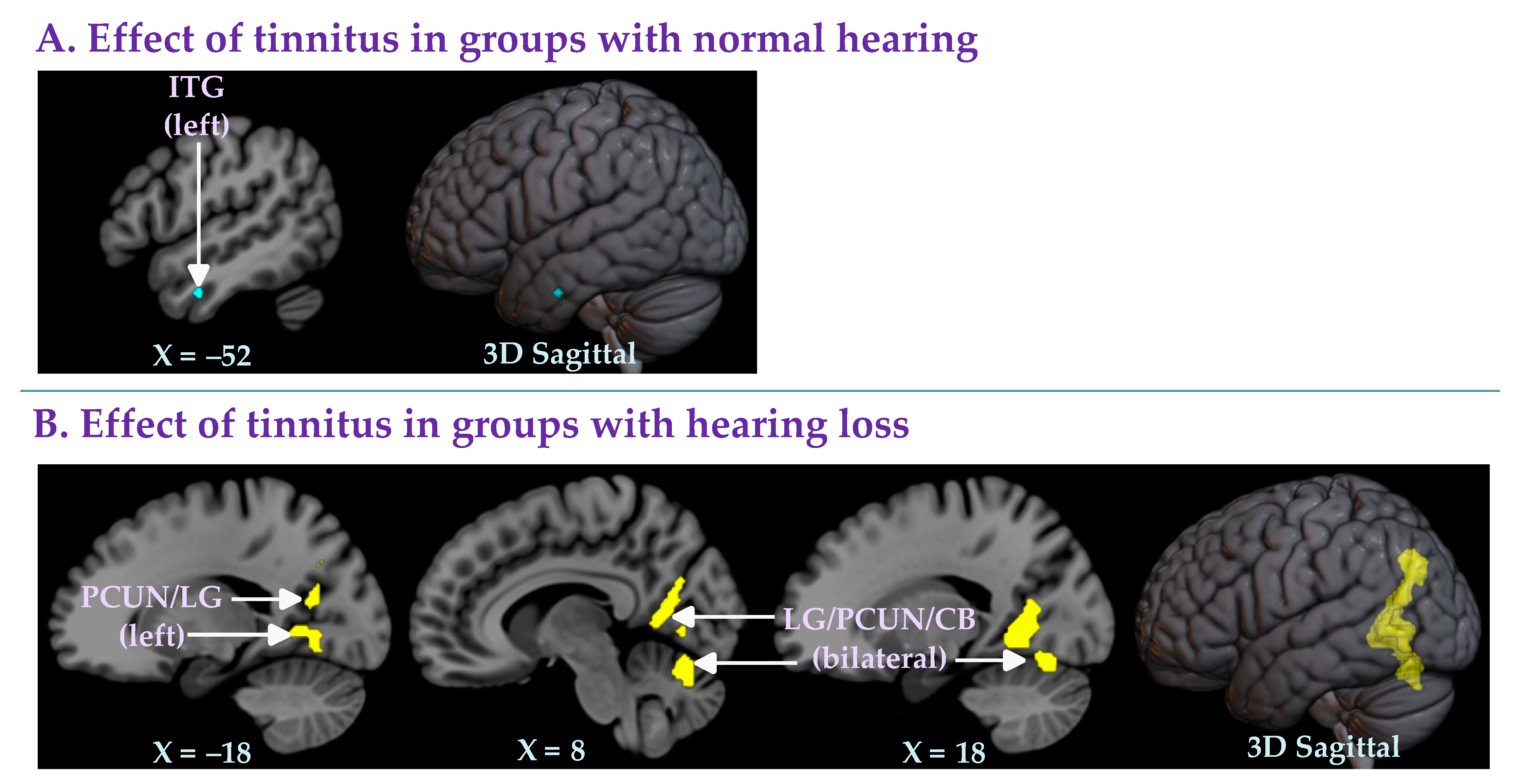
3.2.3. Effect of Tinnitus in Groups with Normal Hearing
3.2.4. Effect of Tinnitus in Groups with Hearing Loss
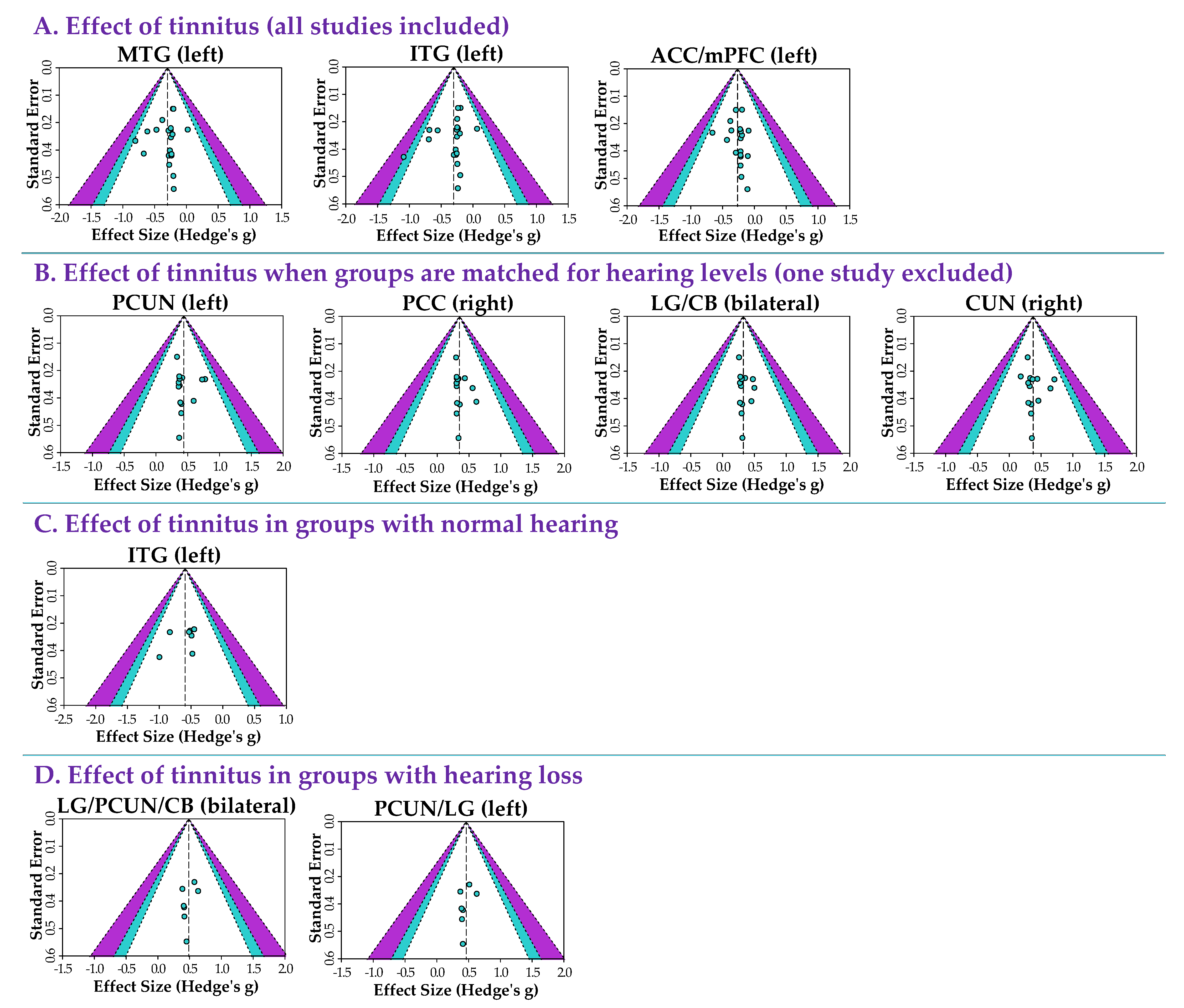
3.2.5. Heterogeneity and Publication Bias
4. Discussion
4.1. Effects of Matching Hearing Levels When Identifying Changes in Gray Matter Associated with Tinnitus
4.2. Brain Regions Associated with Tinnitus and Hearing Loss
4.3. Recommendations for Future VBM Studies of Tinnitus
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016, 337, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.M.; Lin, H.W.; Bhattacharyya, N. Tinnitus Epidemiology: Prevalence, Severity, Exposures And Treatment Patterns In The United States. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M.M.; Smit, A.L.; Brabers, A.E.M.; de Jong, J.D.; Stokroos, R.J.; Stegeman, I. Using Different Cutoffs to Define Tinnitus and Assess Its Prevalence—A Survey in the Dutch General Population. Front. Neurol. 2021, 12, 690192. [Google Scholar] [CrossRef] [PubMed]
- Schubert, N.M.A.; Rosmalen, J.G.M.; van Dijk, P.; Pyott, S.J. A retrospective cross-sectional study on tinnitus prevalence and disease associations in the Dutch population-based cohort Lifelines. Hear. Res. 2021, 411, 108355. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Lugo, A.; Akeroyd, M.A.; Schlee, W.; Gallus, S.; Hall, D.A. Tinnitus prevalence in Europe: A multi-country cross-sectional population study. Lancet Reg. Health–Eur. 2022, 12, 100250. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Edmondson-Jones, M.; Fortnum, H.; Dawes, P.; Middleton, H.; Munro, K.J.; Moore, D.R. The prevalence of tinnitus and the relationship with neuroticism in a middle-aged UK population. J. Psychosom. Res. 2014, 76, 56–60. [Google Scholar] [CrossRef]
- Adjamian, P.; Hall, D.A.; Palmer, A.R.; Allan, T.W.; Langers, D.R.M. Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci. Biobehav. Rev. 2014, 45, 119–133. [Google Scholar] [CrossRef]
- Elgoyhen, A.B.; Langguth, B.; De Ridder, D.; Vanneste, S. Tinnitus: Perspectives from human neuroimaging. Nat. Rev. Neurosci. 2015, 16, 632–642. [Google Scholar] [CrossRef]
- Scott-Wittenborn, N.; Karadaghy, O.A.; Piccirillo, J.F.; Peelle, J.E. A methodological assessment of studies that use voxel-based morphometry to study neural changes in tinnitus patients. Hear. Res. 2017, 355, 23–32. [Google Scholar] [CrossRef]
- Simoes, J.P.; Daoud, E.; Shabbir, M.; Amanat, S.; Assouly, K.; Biswas, R.; Casolani, C.; Dode, A.; Enzler, F.; Jacquemin, L.; et al. Multidisciplinary Tinnitus Research: Challenges and Future Directions from the Perspective of Early Stage Researchers. Front. Aging Neurosci. 2021, 13, 647285. [Google Scholar] [CrossRef]
- Vanneste, S.; Heyning, P.V.D.; Ridder, D.D. Tinnitus: A Large VBM-EEG Correlational Study. PLoS ONE 2015, 10, e0115122. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Zimmerman, B.; Bido Medina, R.O.; Carpenter-Thompson, J.R.; Husain, F.T. Changes in gray and white matter in subgroups within the tinnitus population. Brain Res. 2018, 1679, 64–74. [Google Scholar] [CrossRef]
- Mennink, L.M.; Koops, E.A.; Langers, D.R.M.; Aalbers, M.W.; van Dijk, J.M.C.; van Dijk, P. Cerebellar Gray Matter Volume in Tinnitus. Front. Neurosci. 2022, 16, 862873. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, G.; Zhou, J.; Qu, Y.; Li, Z.; He, Z.; Yin, T.; Ma, P.; Sun, R.; Liang, F. A Multimodal Meta-Analysis of Structural and Functional Changes in the Brain of Tinnitus. Front. Hum. Neurosci. 2020, 14, 28. [Google Scholar] [CrossRef]
- Albajes-Eizagirre, A.; Solanes, A.; Vieta, E.; Radua, J. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage 2019, 186, 174–184. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Du, M.; Liu, J.; Chen, Z.; Huang, X.; Li, J.; Kuang, W.; Yang, Y.; Zhang, W.; Zhou, D.; Bi, F.; et al. Brain grey matter volume alterations in late-life depression. J. Psychiatry Neurosci. 2014, 39, 397–406. [Google Scholar] [CrossRef]
- Han, Q.; Hou, Y.; Shang, H. A Voxel-Wise Meta-Analysis of Gray Matter Abnormalities in Essential Tremor. Front. Neurol. 2018, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Albajes-Eizagirre, A.; Solanes, A.; Fullana, M.A.; Ioannidis, J.P.A.; Fusar-Poli, P.; Torrent, C.; Solé, B.; Bonnín, C.M.; Vieta, E.; Mataix-Cols, D.; et al. Meta-analysis of Voxel-Based Neuroimaging Studies using Seed-based d Mapping with Permutation of Subject Images (SDM-PSI). JoVE (J. Vis. Exp.) 2019, e59841. [Google Scholar] [CrossRef]
- Albajes-Eizagirre, A.; Solanes, A.; Radua, J. Meta-analysis of non-statistically significant unreported effects. Stat. Methods Med. Res. 2019, 28, 3741–3754. [Google Scholar] [CrossRef] [PubMed]
- Mühlau, M.; Rauschecker, J.P.; Oestreicher, E.; Gaser, C.; Röttinger, M.; Wohlschläger, A.M.; Simon, F.; Etgen, T.; Conrad, B.; Sander, D. Structural brain changes in tinnitus. Cereb. Cortex 2006, 16, 1283–1288. [Google Scholar] [CrossRef]
- Landgrebe, M.; Langguth, B.; Rosengarth, K.; Braun, S.; Koch, A.; Kleinjung, T.; May, A.; de Ridder, D.; Hajak, G. Structural brain changes in tinnitus: Grey matter decrease in auditory and non-auditory brain areas. Neuroimage 2009, 46, 213–218. [Google Scholar] [CrossRef]
- Husain, F.T.; Medina, R.E.; Davis, C.W.; Szymko-Bennett, Y.; Simonyan, K.; Pajor, N.M.; Horwitz, B. Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Res. 2011, 1369, 74–88. [Google Scholar] [CrossRef]
- Leaver, A.M.; Seydell-Greenwald, A.; Turesky, T.K.; Morgan, S.; Kim, H.J.; Rauschecker, J.P. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front. Syst. Neurosci. 2012, 6, 21. [Google Scholar] [CrossRef]
- Boyen, K.; Langers, D.R.M.; de Kleine, E.; van Dijk, P. Gray matter in the brain: Differences associated with tinnitus and hearing loss. Hear. Res. 2013, 295, 67–78. [Google Scholar] [CrossRef]
- Melcher, J.R.; Knudson, I.M.; Levine, R.A. Subcallosal brain structure: Correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear. Res. 2013, 295, 79–86. [Google Scholar] [CrossRef]
- Allan, T.W.; Besle, J.; Langers, D.R.M.; Davies, J.; Hall, D.A.; Palmer, A.R.; Adjamian, P. Neuroanatomical Alterations in Tinnitus Assessed with Magnetic Resonance Imaging. Front. Aging Neurosci. 2016, 8, 221. [Google Scholar] [CrossRef]
- Luan, Y.; Wang, C.; Jiao, Y.; Tang, T.; Zhang, J.; Teng, G.-J. Prefrontal-Temporal Pathway Mediates the Cross-Modal and Cognitive Reorganization in Sensorineural Hearing Loss With or Without Tinnitus: A Multimodal MRI Study. Front. Neurosci. 2019, 13, 222. [Google Scholar] [CrossRef] [Green Version]
- Besteher, B.; Gaser, C.; Ivanšić, D.; Guntinas-Lichius, O.; Dobel, C.; Nenadić, I. Chronic tinnitus and the limbic system: Reappraising brain structural effects of distress and affective symptoms. NeuroImage Clin. 2019, 24, 101976. [Google Scholar] [CrossRef] [PubMed]
- Koops, E.A.; de Kleine, E.; van Dijk, P. Gray matter declines with age and hearing loss, but is partially maintained in tinnitus. Sci. Rep. 2020, 10, 21801. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lv, H.; Wang, Z.; Liu, C.; Ren, P.; Zhang, P.; Chen, Q.; Liu, Y.; Zhao, P.; Gong, S.; et al. Neuroanatomical Alterations in Patients With Tinnitus Before and After Sound Therapy: A Voxel-Based Morphometry Study. Front. Neurosci. 2020, 14, 911. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, H.; Lee, J.Y.; Kim, J.H.; Lee, D.Y.; Mook-Jung, I.; Kim, Y.H.; Kim, Y.K. Effects of Chronic Tinnitus on Metabolic and Structural Changes in Subjects with Mild Cognitive Impairment. Front. Aging Neurosci. 2020, 12, 594282. [Google Scholar] [CrossRef]
- Wei, X.; Lv, H.; Chen, Q.; Wang, Z.; Liu, C.; Zhao, P.; Gong, S.; Yang, Z.; Wang, Z. Neuroanatomical Alterations in Patients with Tinnitus before and after Sound Therapy: A Combined VBM and SCN Study. Front. Hum. Neurosci. 2021, 14, 607452. [Google Scholar] [CrossRef]
- Chen, Q.; Lv, H.; Wang, Z.; Wei, X.; Zhao, P.; Yang, Z.; Gong, S.; Wang, Z. Brain Structural and Functional Reorganization in Tinnitus Patients without Hearing Loss after Sound Therapy: A Preliminary Longitudinal Study. Front. Neurosci. 2021, 15, 573858. [Google Scholar] [CrossRef]
- Japee, S.; Holiday, K.; Satyshur, M.D.; Mukai, I.; Ungerleider, L.G. A role of right middle frontal gyrus in reorienting of attention: A case study. Front. Syst. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H.; Hurley, R.A.; Hayman, L.A.; Taber, K.H. Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. JNP 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Sun, W.; Tang, P.; Liang, Y.; Li, J.; Feng, J.; Zhang, N.; Lu, D.; He, J.; Chen, X. The anterior cingulate cortex directly enhances auditory cortical responses in air-puffing-facilitated flight behavior. Cell Rep. 2022, 38, 110506. [Google Scholar] [CrossRef]
- De Ridder, D.; Elgoyhen, A.B.; Romo, R.; Langguth, B. Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. USA 2011, 108, 8075–8080. [Google Scholar] [CrossRef] [Green Version]
- Haider, H.F.; Bojić, T.; Ribeiro, S.F.; Paço, J.; Hall, D.A.; Szczepek, A.J. Pathophysiology of Subjective Tinnitus: Triggers and Maintenance. Front Neurosci. 2018, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; De Ridder, D. The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front. Syst. Neurosci. 2012, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Schlee, W.; Weisz, N.; Bertrand, O.; Hartmann, T.; Elbert, T. Using Auditory Steady State Responses to Outline the Functional Connectivity in the Tinnitus Brain. PLoS ONE 2008, 3, e3720. [Google Scholar] [CrossRef]
- Vanneste, S.; Plazier, M.; de Heyning, P.V.; Ridder, D.D. Repetitive transcranial magnetic stimulation frequency dependent tinnitus improvement by double cone coil prefrontal stimulation. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1160–1164. [Google Scholar] [CrossRef]
- Patel, A.; Biso, G.M.N.R.; Fowler, J.B. Neuroanatomy, Temporal Lobe; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Diges, I.; Simón, F.; Cobo, P. Assessing Auditory Processing Deficits in Tinnitus and Hearing Impaired Patients with the Auditory Behavior Questionnaire. Front. Neurosci. 2017, 11, 187. [Google Scholar] [CrossRef]
- Ivansic, D.; Guntinas-Lichius, O.; Müller, B.; Volk, G.F.; Schneider, G.; Dobel, C. Impairments of Speech Comprehension in Patients with Tinnitus—A Review. Front. Aging Neurosci. 2017, 9, 224. [Google Scholar] [CrossRef]
- Liu, Y.W.; Wang, B.; Chen, B.; Galvin, J.J.; Fu, Q.-J. Tinnitus impairs segregation of competing speech in normal-hearing listeners. Sci. Rep. 2020, 10, 19851. [Google Scholar] [CrossRef]
- Araneda, R.; De Volder, A.G.; Deggouj, N.; Philippot, P.; Heeren, A.; Lacroix, E.; Decat, M.; Rombaux, P.; Renier, L. Altered top-down cognitive control and auditory processing in tinnitus: Evidences from auditory and visual spatial stroop. Restor. Neurol. Neurosci. 2015, 33, 67–80. [Google Scholar] [CrossRef]
- Hullett, P.W.; Hamilton, L.S.; Mesgarani, N.; Schreiner, C.E.; Chang, E.F. Human Superior Temporal Gyrus Organization of Spectrotemporal Modulation Tuning Derived from Speech Stimuli. J. Neurosci. 2016, 36, 2014–2026. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Lee, H.-H.; Chung, K.-C.; Chen, H.-C.; Shen, Y.-J.; Wu, J.-L. Relationships among speech perception, self-rated tinnitus loudness and disability in tinnitus patients with normal pure-tone thresholds of hearing. ORL J. Otorhinolaryngol. Relat. Spec. 2007, 69, 25–29. [Google Scholar] [CrossRef]
- Conrad, C.D.; Stumpf, W.E. Direct visual input to the limbic system: Crossed retinal projections to the nucleus anterodorsalis thalami in the tree shrew. Exp. Brain Res. 1975, 23, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bogousslavsky, J.; Miklossy, J.; Deruaz, J.P.; Assal, G.; Regli, F. Lingual and fusiform gyri in visual processing: A clinico-pathologic study of superior altitudinal hemianopia. J. Neurol Neurosurg. Psychiatry 1987, 50, 607–614. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Fjell, A.M.; Dale, A.M.; Fischl, B.; Quinn, B.T.; Makris, N.; Salat, D.; Reinvang, I. Regional cortical thickness matters in recall after months more than minutes. NeuroImage 2006, 31, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Kalpouzos, G.; Chételat, G.; Baron, J.-C.; Landeau, B.; Mevel, K.; Godeau, C.; Barré, L.; Constans, J.-M.; Viader, F.; Eustache, F.; et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol. Aging 2009, 30, 112–124. [Google Scholar] [CrossRef]
- Jung, J.; Kang, J.; Won, E.; Nam, K.; Lee, M.-S.; Tae, W.S.; Ham, B.-J. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: A voxel-based morphometry study. J. Affect. Disord. 2014, 169, 179–187. [Google Scholar] [CrossRef]
- Hamza, Y.; Zeng, F.-G. Tinnitus Is Associated with Improved Cognitive Performance in Non-hispanic Elderly with Hearing Loss. Front. Neurosci. 2021, 15, 735950. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Carpenter-Thompson, J.; Husain, F.T. Connectivity of precuneus to the default mode and dorsal attention networks: A possible invariant marker of long-term tinnitus. Neuroimage Clin. 2017, 16, 196–204. [Google Scholar] [CrossRef]
- Khan, R.A.; Sutton, B.P.; Tai, Y.; Schmidt, S.A.; Shahsavarani, S.; Husain, F.T. A large-scale diffusion imaging study of tinnitus and hearing loss. Sci. Rep. 2021, 11, 23395. [Google Scholar] [CrossRef]
- Schecklmann, M.; Lehner, A.; Poeppl, T.B.; Kreuzer, P.M.; Rupprecht, R.; Rackl, J.; Burger, J.; Frank, E.; Hajak, G.; Langguth, B.; et al. Auditory cortex is implicated in tinnitus distress: A voxel-based morphometry study. Brain Struct. Funct. 2013, 218, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, A.N.V.; Salimi-Khorshidi, G.; Lai, M.-C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
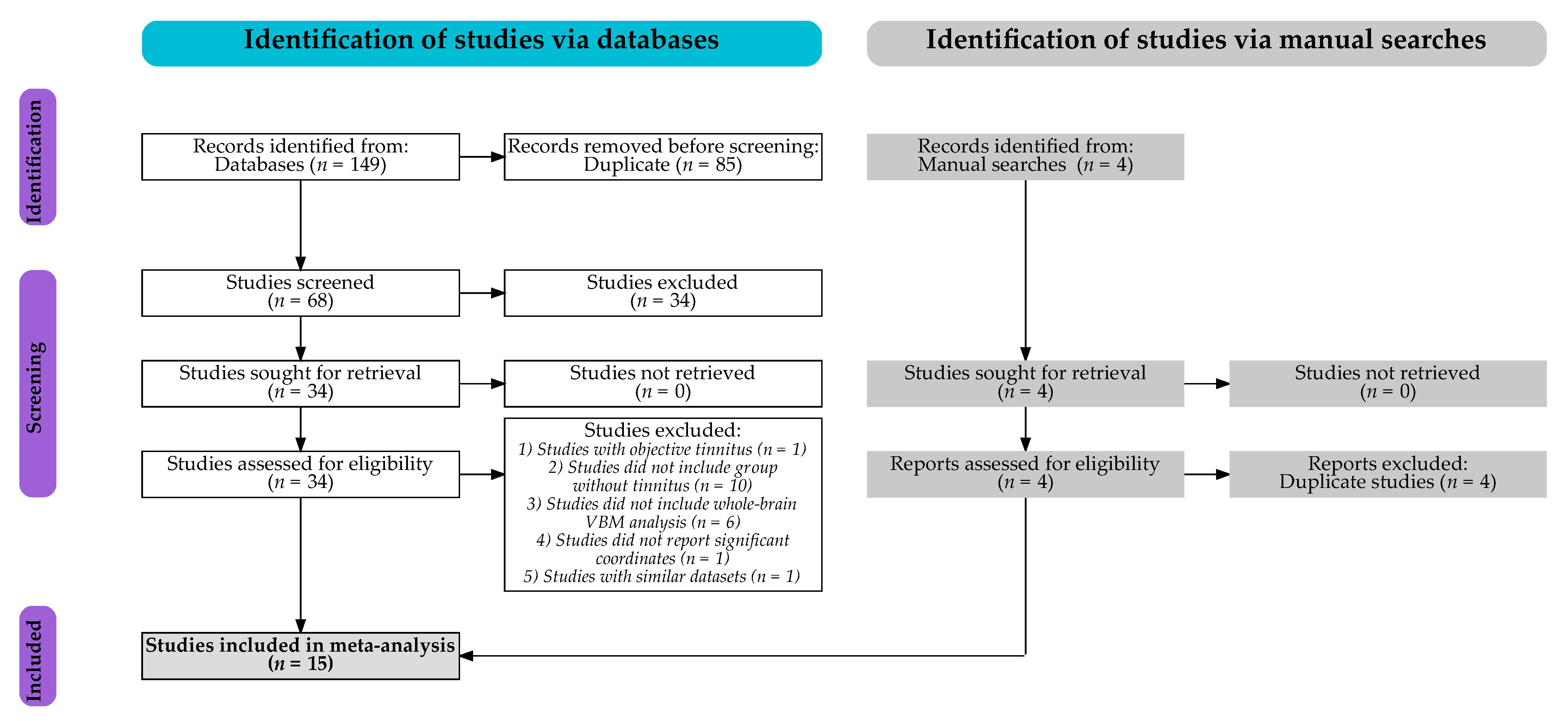
| Study | Groups | n (Females) | Mean Age (years) | Groups Matched for | Mean Tinnitus Duration (months) | Mean Tinnitus Severity Score | MRI Scanner | Smoothing FWHM | Significant Threshold | Whole-Brain VBM Results Associated with Tinnitus | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | HL | ||||||||||
| Mühlau et al. [23] | NH+T | 28 (15) | 40 | Yes | Yes | Yes | 53 ± 52 | TQ = 25 | 1.5T | 8 | FDR p < 0.05 voxel-level | ↑GM: NS |
| NH−T | 28 (15) | 39 | - | - | ↓GM: SCG | |||||||
| Landgrebe et al. [24] | NH+T | 28 (13) | 32.2 | Yes | Yes | Yes | 53.3 | TQ = 32.9 | 1.5T | 10 | FDR p < 0.05 voxel-level | ↑GM: NS |
| NH−T | 28 (13) | 31.2 | - | - | ↓GM: NS | |||||||
| Husain et al. [25] | HL+T | 8 (0) | 56.13 | Yes | Yes | No | NA | THI = 17.25 | 3T | 8 | Uncorrected p < 0.001 | ↑GM: NS |
| NH−T | 11 (0) | 48.09 | - | - | ↓GM: NS | |||||||
| HL+T | 8 (0) | 56.13 | Yes | Yes | Yes | NA | THI = 17.25 | ↑ GM: MFG, SFG | ||||
| HL−T | 7 (0) | 51.38 | ↓GM: NS | |||||||||
| Leaver et al. [26] | HL+T | 23 (11) | 47.4 | Yes | Yes | Yes | 149.9 | NA | 3T | 6 | Uncorrected p < 0.002 | ↑GM: NS |
| HL−T | 21 (13) | 49 | - | - | ↓GM: dmPFC, SMG, vmPFC | |||||||
| Boyen et al. [27] | HL+T | 31 (11) | 56 | No | NA | No | NA | THI = 29 | 3T | 8 | FWE p < 0.05 voxel-level | ↑GM: MTG, SMG, STG |
| NH−T | 24 (8) | 58 | - | - | ↓GM: HTH, OG, SFG | |||||||
| HL+T | 31 (11) | 56 | No | NA | Yes | NA | THI = 29 | ↑GM: NS | ||||
| HL−T | 16 (3) | 63 | - | - | ↓GM: NS | |||||||
| Melcher et al. [28] | NH+T | 24 (12) | 46.9 | Yes | Yes | Yes | NA | TRQ = 26.7 | 3T | 8 | Uncorrected p < 0.001 | ↑GM: NS |
| NH−T | 24 (12) | 45.8 | - | - | ↓GM: NS | |||||||
| Allan et al. [29] | NH+T/HL+T | 73 (30) | 58.38 | Yes | Yes | Yes | NA | NA | 1.5T, 3T | 10 | FWE p < 0.05 | ↑GM: NS |
| NH−T/HL−T | 55 (25) | 56.91 | - | - | ↓GM: NS | |||||||
| NH+T | 15 (9) | 47.6 | Yes | Yes | Yes | NA | NA | ↑GM: NS | ||||
| NH−T | 15 (9) | 50.2 | - | - | ↓GM: NS | |||||||
| Schmidt et al. [13] | HL+T | 15 (5) | 55.13 | NA | NA | No | NA | THI = 9.33 | 3T | 10 | Uncorrected p < 0.001 cluster-level | ↑GM: ACC, SFG |
| NH−T | 13 (6) | 52.93 | - | - | ↓GM: NS | |||||||
| HL+T | 15 (5) | 55.13 | NA | NA | Yes | NA | THI = 9.33 | ↑GM: NS | ||||
| HL−T | 13 (8) | 57.61 | - | - | ↓GM: NS | |||||||
| Luan et al. [30] | HL+T | 10 (5) | 52 | Yes | Yes | No | NA | NA | 3T | 8 | FDR p < 0.05 voxel-level | ↑GM: NS |
| NH−T | 35 (18) | 55.97 | - | - | ↓GM: NS | |||||||
| HL+T | 10 (5) | 52 | Yes | Yes | Yes | NA | NA | ↑GM: NS | ||||
| HL−T | 25 (9) | 55.48 | - | - | ↓GM: NS | |||||||
| Besteher et al. [31] | HL+T | 59 (27) | 50.6 | NA | NA | No | NA | TQ = 42.2 | 3T | 8 | Uncorrected p < 0.001 | ↑GM: NS |
| NH−T | 66 (35) | 45 | - | - | ↓GM: IPC, PHPC, PCUN | |||||||
| Koops et al. [32] | HL+T | 39 (6) | 59.2 | No | NA | No | 147.5 | THI = 35 | 3T | 8 | TFCE FWE p < 0.05 | ↑GM: NS |
| NH−T | 39 (21) | 45.7 | - | - | ↓GM: NS | |||||||
| HL+T | 39 (6) | 59.2 | Yes | NA | Yes | 147.5 | THI = 35 | ↑GM: LG | ||||
| HL−T | 22 (9) | 62.6 | - | - | ↓GM: NS | |||||||
| Wei et al. [33] | NH+T | 27 (15) | 46.4 | Yes | Yes | Yes | 23.4 - | THI = 40 - | 3T | 6 | FDR p < 0.05 cluster-level | ↑GM: NS |
| NH−T | 27 (15) | 46.6 | ↓GM: CN, TH | |||||||||
| Lee et al. [34] | HL+T | 12 (6) | 73.27 | Yes | Yes | Yes | 42 | THI = 38.5 | 3T | 8 | Uncorrected p < 0.005 | ↑GM: NS |
| HL−T | 11 (4) | 74.83 | - | - | ↓GM: INS | |||||||
| Wei et al. [35] | NH+T | 33 (10) | 48.2 | Yes | Yes | Yes | NA | THI = 52.5 | 3T | 6 | FDR p < 0.05 cluster-level | ↑GM: NS |
| NH−T | 26 (11) | 47.3 | - | - | ↓GM: CAU, CAL, CUN, PHPC, STG | |||||||
| Chen et al. [36] | NH+T | 13 (7) | 42.23 | Yes | Yes | Yes | NA | THI = 53.38 | 3T | 8 | FWE p < 0.001 cluster-level | ↑GM: NS |
| NH−T | 18 (9) | 45.33 | - | - | ↓GM: MTG | |||||||
| Gray Matter Regions | MNI X, Y, Z | SDM Z | FWE p TFCE | Voxels | Hedge’s g Cluster | I2 Cluster | Egger’s p Cluster |
|---|---|---|---|---|---|---|---|
| A. Effect of tinnitus (all studies included) a | |||||||
| NH+T and HL+T > NH−T and HL−T | |||||||
| NS | - | - | - | - | - | - | - |
| NH+T and HL+T < NH−T and HL−T | |||||||
| Left anterior cingulate cortex/medial prefrontal cortex (ACC/mPFC) | −12, 44, 8 | −4.10 | 0.028 | 224 | −0.27 | 0 % | 0.787 |
| −8, 56, 10 | −4.01 | 0.018 | |||||
| −16, 66, −6 | −3.63 | 0.036 | |||||
| −8, 62, 0 | −3.60 | 0.026 | |||||
| Left inferior temporal gyrus (ITG) | −54, −10, −34 | −3.80 | 0.036 | 45 | −0.30 | 0 % | 0.392 |
| −54, −6, −34 | −3.69 | 0.036 | |||||
| −56, −16, −32 | −3.56 | 0.046 | |||||
| Left middle temporal gyrus (MTG) | −62, −16, −14 | −4.06 | 0.036 | 20 | −0.30 | 0 % | 0.614 |
| −60, −12, −12 | −3.85 | 0.038 | |||||
| B. Effect of tinnitus when groups are matched for hearing levels (one study excluded) b | |||||||
| NH+T and HL+T > NH−T and HL−T excluding study [31] | |||||||
| Right cuneus (CUN) | 16, −94, 24 | 4.16 | 0.016 | 67 | 0.38 | 0 % | 0.738 |
| 18, −88, 30 | 3.90 | 0.028 | |||||
| Bilateral lingual gyrus/cerebellum (LG/CB) | 20, −52, 2 | 4.39 | 0.002 | 948 | 0.33 | 0 % | 0.789 |
| −4, −56, −8 | 4.26 | 0.006 | |||||
| 12, −58, 0 | 3.96 | 0.004 | |||||
| 16, −72, −18 | 3.19 | 0.016 | |||||
| 4, −50, 0 | 3.15 | 0.012 | |||||
| 2, −66, −20 | 3.13 | 0.020 | |||||
| −2, −64, −20 | 3.09 | 0.020 | |||||
| 14, −72, −14 | 2.99 | 0.018 | |||||
| 8, −68, −16 | 2.98 | 0.020 | |||||
| 14, −72, 4 | 2.92 | 0.022 | |||||
| Right posterior cingulate gyrus (PCC) | 0, −42, 46 | 4.24 | 0.036 | 28 | 0.35 | 0 % | 0.724 |
| 0, −32, 42 | 3.80 | 0.038 | |||||
| Left precuneus (PCUN) | −38, −78, 14 | 4.70 | 0.038 | 12 | 0.44 | 0 % | 0.813 |
| NH+T and HL+T < NH−T and HL−T excluding study [31] | |||||||
| NS | - | - | - | - | - | - | - |
| Gray Matter Regions | MNI X, Y, Z | SDM Z | FWE p TFCE | Voxels | Hedge’s g Cluster | I2 Cluster | Egger’s p Cluster |
|---|---|---|---|---|---|---|---|
| A. Effect of tinnitus in groups with normal hearing a | |||||||
| NH+T > NH−T | |||||||
| NS | - | - | - | - | - | - | - |
| NH+T < NH−T | |||||||
| Left inferior temporal gyrus (ITG) | −52, −6, −30 | −4.12 | 0.042 | 12 | −0.59 | 0 % | 0.517 |
| B. Effect of tinnitus in groups with hearing loss b | |||||||
| HL+T > HL−T | |||||||
| Right lingual gyrus/precuneus/cerebellum (LG/PCUN/CB) | 18, −56, 0 | 4.57 | < 0.001 | 931 | 0.49 | 0 % | 0.735 |
| 18, −60, 10 | 3.56 | 0.008 | |||||
| 4, −68, −20 | 3.44 | 0.012 | |||||
| 8, −70, −18 | 3.38 | 0.012 | |||||
| 18, −70, −16 | 3.26 | 0.012 | |||||
| 8, −66, −24 | 3.00 | 0.016 | |||||
| 28, −66, −10 | 2.90 | 0.042 | |||||
| Left precuneus/lingual gyrus (PCUN/LG) | −14, −68, 38 | 3.66 | 0.012 | 466 | 0.46 | 0 % | 0.768 |
| −14, −68, −2 | 3.21 | 0.018 | |||||
| −16, −66, −8 | 2.98 | 0.020 | |||||
| −18, −58, 0 | 2.94 | 0.020 | |||||
| −18, −66, 18 | 2.85 | 0.020 | |||||
| −12, −58, 8 | 2.61 | 0.030 | |||||
| HL+T < HL−T | |||||||
| NS | - | - | - | - | - | - | - |
| Demographic Factors |
| Match groups for age, sex ratio, and hearing loss |
| Provide clear definitions for hearing loss (cause and duration) or provide audiograms for both ears |
| Report clinical information (anxiety, depression, hyperacusis, and tinnitus burden scores and tinnitus duration) |
| Methodological Factors |
| Provide clear description of the software, realignment algorithm, normalized template, resampling resolution, modulation, and smoothing kernel used during preprocessing |
| Correct results for multiple comparisons at the cluster or voxel level (FWE, FWE-TFCE, or FDR correction) |
| Control results for age and total intracranial volume |
| Report significant x, y, z-coordinates with t-values, z-values, and p-values |
| Data Sharing |
| Make unthresholded statistical images available, e.g., via NeuroVault website (https://neurovault.org/, accessed on 10 June 2022), or create a shared initiative to aggregate a large number of data sets with minimal requirements on T1 brain imaging protocols and questionnaires for controlling for potential variables of interest such as tinnitus duration, depression, and anxiety. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makani, P.; Thioux, M.; Pyott, S.J.; van Dijk, P. A Combined Image- and Coordinate-Based Meta-Analysis of Whole-Brain Voxel-Based Morphometry Studies Investigating Subjective Tinnitus. Brain Sci. 2022, 12, 1192. https://doi.org/10.3390/brainsci12091192
Makani P, Thioux M, Pyott SJ, van Dijk P. A Combined Image- and Coordinate-Based Meta-Analysis of Whole-Brain Voxel-Based Morphometry Studies Investigating Subjective Tinnitus. Brain Sciences. 2022; 12(9):1192. https://doi.org/10.3390/brainsci12091192
Chicago/Turabian StyleMakani, Punitkumar, Marc Thioux, Sonja J. Pyott, and Pim van Dijk. 2022. "A Combined Image- and Coordinate-Based Meta-Analysis of Whole-Brain Voxel-Based Morphometry Studies Investigating Subjective Tinnitus" Brain Sciences 12, no. 9: 1192. https://doi.org/10.3390/brainsci12091192
APA StyleMakani, P., Thioux, M., Pyott, S. J., & van Dijk, P. (2022). A Combined Image- and Coordinate-Based Meta-Analysis of Whole-Brain Voxel-Based Morphometry Studies Investigating Subjective Tinnitus. Brain Sciences, 12(9), 1192. https://doi.org/10.3390/brainsci12091192








