Functional Coupling between the Fronto-Parietal Network and Default Mode Network Is Associated with Balanced Time Perspective
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Cognitive Measures
2.3. Brain Imaging Data Acquisition
2.4. Regions of Interest (ROI)
2.5. Brain Imaging Data Pre-Processing
2.6. Voxel-Wise Resting-State Functional Connectivity (rsFC) Analysis
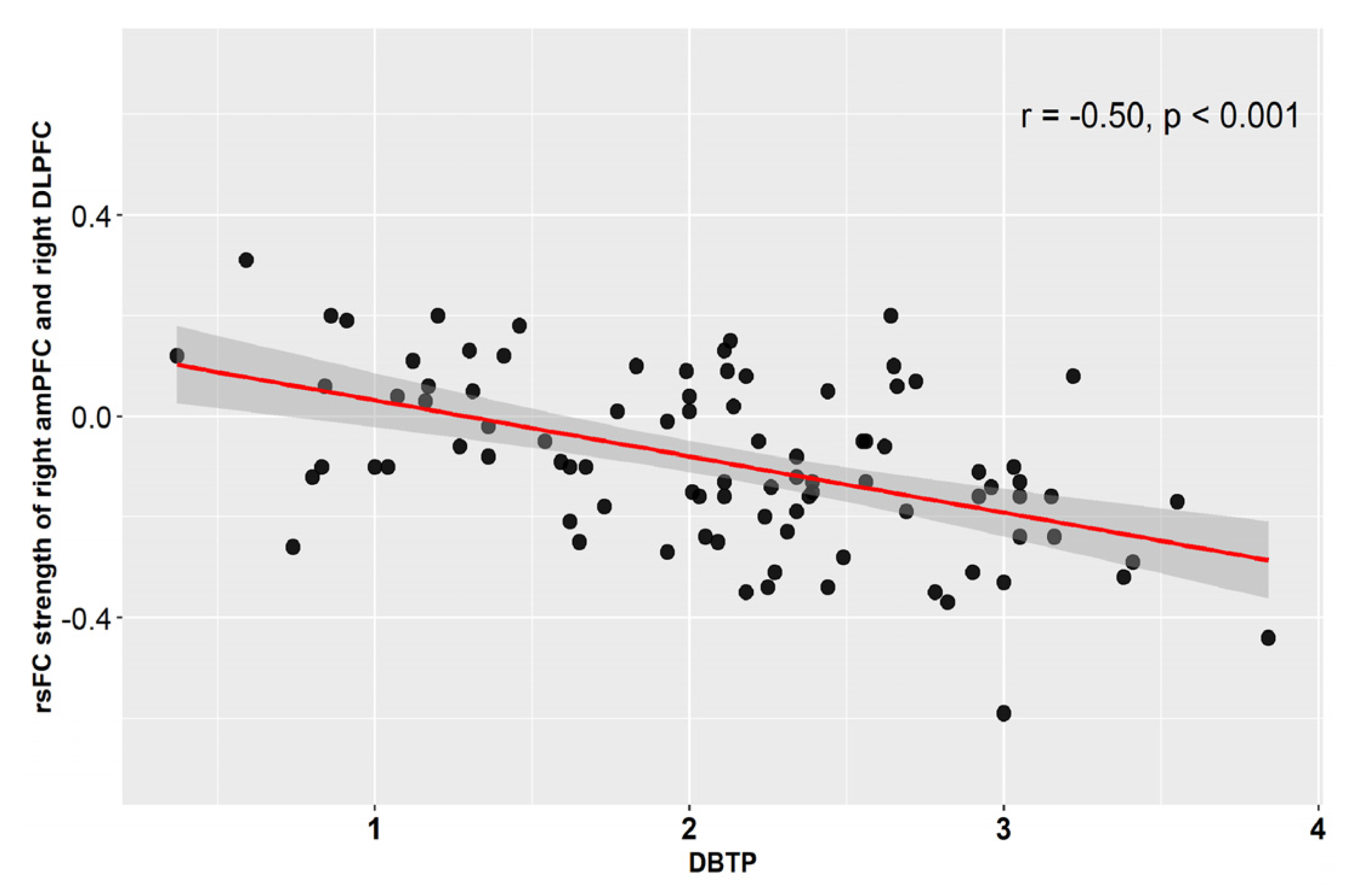
3. Results
3.1. Descriptive Information of DBTP
3.2. Correlations between Voxel-Wise rsFC and DBTP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boniwell, I.; Zimbardo, P.G. Balancing Time Perspective in Pursuit of Optimal Functioning. In Positive Psychology in Practice; John Wiley & Sons, Inc.: New York, NY, USA, 2015; pp. 165–178. [Google Scholar]
- Zimbardo, P.G.; Boyd, J.N. Putting time in perspective: A valid, reliable individual-differences metric. J. Personal. Soc. Psychol. 1999, 77, 1271–1288. [Google Scholar] [CrossRef]
- Tseferidi, S.-I.; Griva, F.; Anagnostopoulos, F. Time to get happy: Associations of time perspective with indicators of well-being. Psychol. Health Med. 2017, 22, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Boniwell, I.; Zimbardo, P.G. Time to find the right balance. Psychologist 2003, 16, 129–131. [Google Scholar]
- Chen, T.; Liu, L.L.; Cui, J.; Qin, X.; Shi, H.; Irish, M.; Wang, Y. Balanced Time Perspective and Life Satisfaction: The Mediating Role of “Temporal Negative Affect”. J. Happiness Stud. 2021, 22, 2563–2574. [Google Scholar] [CrossRef]
- Jankowski, K.S.; Zajenkowski, M.; Stolarski, M. What Are the Optimal Levels of Time Perspectives? Deviation from the Balanced Time Perspective-Revisited (DBTP-r). Psychol. Belg. 2020, 60, 164–183. [Google Scholar] [CrossRef]
- Diaconu-Gherasim, L.R.; Mardari, C.R.; Măirean, C. The relation between time perspectives and well-being: A meta-analysis on research. Curr. Psychol. 2021. Available online: https://link.springer.com/article/10.1007/s12144-021-01949-4 (accessed on 8 August 2022). [CrossRef]
- Webster, J.D. A New Measure of Time Perspective: Initial Psychometric Findings for the Balanced Time Perspective Scale (BTPS). Can. J. Behav. Sci. 2011, 43, 111–118. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, R.; Zhao, L.; Qiu, J.; Guo, C. Neural bases underlying the association between balanced time perspective and trait anxiety. Behav. Brain Res. 2019, 359, 206–214. [Google Scholar] [CrossRef]
- Griffin, E.; Wildbur, D. The role of balanced time perspective on student well-being and mental health: A mixed-methods study. Ment. Health Prev. 2020, 18, 200181. [Google Scholar] [CrossRef]
- McKay, M.T.; Cole, J.C. The relationship between balanced and negative time perspectives, and symptoms of anxiety and depression. Psychiatry Res. 2020, 293, 113383. [Google Scholar] [CrossRef]
- Pyszkowska, A.; Rönnlund, M. Psychological Flexibility and Self-Compassion as Predictors of Well-Being: Mediating Role of a Balanced Time Perspective. Front. Psychol. 2021, 12, 671746. [Google Scholar] [CrossRef] [PubMed]
- Orkibi, H.; Ronen, T. A Dual-Pathway Model. Linking Self-Control. Skills to Aggression in Adolescents: Happiness and Time Perspective as Mediators. J. Happiness Stud. 2019, 20, 729–742. [Google Scholar] [CrossRef]
- Stolarski, M.; Bitner, J.; Zimbardo, P.G. Time perspective, emotional intelligence and discounting of delayed awards. Time Soc. 2011, 20, 346–363. [Google Scholar] [CrossRef]
- Rönnlund, M.; Koudriavtseva, A.; Germundsjö, L.; Eriksson, T.; Åström, E.; Carelli, M.G. Mindfulness Promotes a More Balanced Time Perspective: Correlational and Intervention-Based Evidence. Mindfulness 2019, 10, 1579–1591. [Google Scholar] [CrossRef]
- Stolarski, M.; Cyniak-Cieciura, M. Balanced and less traumatized: Balanced time perspective mediates the relationship between temperament and severity of PTSD syndrome in motor vehicle accident survivor sample. Personal. Individ. Differ. 2016, 101, 456–461. [Google Scholar] [CrossRef]
- Styła, R.; Stolarski, M.; Szymanowska, A. Linking childhood adversities with schizophrenia: A mediating role of the balanced time perspective. Schizophr. Res. 2019, 209, 281–283. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Z.; Feng, T. Neural substrates underlying balanced time perspective: A combined voxel-based morphometry and resting-state functional connectivity study. Behav. Brain Res. 2017, 332, 237–242. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 2012, 18, 251–270. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef]
- Spreng, R.N.; Mar, R.A.; Kim, A.S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef]
- Buckner, R.L.; Carroll, D.C. Self-projection and the brain. Trends Cogn. Sci. 2007, 11, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Dosenbach, N.U.F.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.L.; Fox, K.C.; Christoff, K. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia 2014, 62, 321–330. [Google Scholar] [CrossRef]
- Zabelina, D.L.; Andrews-Hanna, J.R. Dynamic network interactions supporting internally-oriented cognition. Curr. Opin. Neurobiol. 2016, 40, 86–93. [Google Scholar] [CrossRef]
- Christoff, K.; Irving, Z.C.; Fox, K.C.R.; Spreng, R.N.; Andrews-Hanna, J.R. Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci. 2016, 17, 718–731. [Google Scholar] [CrossRef]
- Christoff, K.; Gordon, A.M.; Smallwood, J.; Smith, R.; Schooler, J.W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. USA 2009, 106, 8719–8724. [Google Scholar] [CrossRef]
- Chen, T.; LI, Z.; Cui, J.; Huang, J.; Irish, M.; Wang, Y.; Chan, R.C.K. The neural substrates of sex differences in balanced time perspective: A unique role for the precuneus. Brain Imaging Behav. 2022. Available online: https://link.springer.com/article/10.1007/s11682-022-00694-x (accessed on 8 August 2022). [CrossRef]
- Wang, Y.; Chen, X.; Cui, J.; Liu, L. Testing the Zimbardo Time Perspective Inventory in the Chinese context. Psych. J. 2015, 4, 166–175. [Google Scholar] [CrossRef]
- Stolarski, M.; Wiberg, B.; Osin, E. Assessing Temporal Harmony: The Issue of a Balanced Time Perspective. In Time Perspective Theory; Review, Research and Application: Essays in Honor of Philip G. Zimbardo; Stolarski, M., Fieulaine, N., van Beek, W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 57–71. [Google Scholar]
- Zhang, J.W.; Howell, R.T.; Stolarski, M. Comparing Three Methods to Measure a Balanced Time Perspective: The Relationship Between a Balanced Time Perspective and Subjective Well-Being. J. Happiness Stud. 2013, 14, 169–184. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.; Cui, J.; Chen, X.; Wang, Y. Developmental trajectory of time perspective: From children to older adults. PsyCh. J. 2016, 5, 245–255. [Google Scholar] [CrossRef]
- Yan, C.-G.; Zang, Y.-F. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tepfer, L.J.; Taren, A.A.; Smith, D.V. Functional parcellation of the default mode network: A large-scale meta-analysis. Sci. Rep. 2020, 10, 16096. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, J.; Bernhardt, B.C.; Leech, R.; Bzdok, D.; Jefferies, E.; Margulies, D.S. The default mode network in cognition: A topographical perspective. Nat. Rev. Neurosci. 2021, 22, 503–513. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Irish, M. Harnessing Visual Imagery and Oculomotor Behaviour to Understand Prospection. Trends Cogn. Sci. 2021, 25, 272–283. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Pizzagalli, D.A. Frontocingulate Dysfunction in Depression: Toward Biomarkers of Treatment Response. Neuropsychopharmacology 2011, 36, 183–206. [Google Scholar] [CrossRef]
- Lv, D.; Ou, Y.; Chen, Y.; Yang, R.; Zhong, Z.; Jia, C.; Sun, L.; Wang, Y.; Zhang, G.; Sun, Z.; et al. Increased cerebellar–default-mode network connectivity at rest in obsessive–compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 1015–1024. [Google Scholar] [CrossRef]
- Guo, W.; Liu, F.; Liu, J.; Yu, M.; Zhang, Z.; Liu, G.; Xiao, C.; Zhao, J. Increased cerebellar-default-mode-networ.rk connectivity in drug-naive major depressive disorder at rest. Medicine 2015, 94, e560. [Google Scholar] [CrossRef]
- McKay, M.T.; Cole, J.C.; Andretta, J.R. Temporal profiles relate meaningfully to anxiety and depression in university undergraduates. Personal. Individ. Differ. 2016, 101, 106–109. [Google Scholar] [CrossRef]
- Imburgio, M.J.; Orr, J.M. Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia 2018, 117, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Witowska, J.; Zajenkowski, M. Cognitive consequences of timeframe bias. On the link between working memory, cognitive switching, and time perspective. Curr. Psychol. 2019, 40, 3532–3545. [Google Scholar] [CrossRef]
- Zajenkowski, M.; Stolarski, M.; Witowska, J.; Maciantowicz, O.; Łowicki, P. Fluid Intelligence as a Mediator of the Relationship between Executive Control. and Balanced Time Perspective. Front. Psychol. 2016, 7, 1844. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Shine, J.M.; Hodges, J.R.; Andrews-Hanna, J.R.; Irish, M. Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 3316–3321. [Google Scholar] [CrossRef]
- Irish, M.; Hodges, J.R.; Piguet, O. Episodic future thinking is impaired in the behavioural variant of frontotemporal dementia. Cortex 2013, 49, 2377–2388. [Google Scholar]
- Irish, M.; Addis, D.R.; Hodges, J.R.; Piguet, O. Considering the role of semantic memory in episodic future thinking: Evidence from semantic dementia. Brain 2012, 135, 2178–2191. [Google Scholar] [CrossRef] [Green Version]


| Representative Region of the Cluster | Co-Ordinates of Maximum Voxel | Cluster Size (Voxels) | Anatomical Region | T/Z Value |
|---|---|---|---|---|
| Positive association with DBTP | ||||
| Seed: right amPFC | ||||
| Left Cerebellum Exterior | −18; −81; −21 | 82 | Left Cerebellum Exterior (41%), Left occipital fusiform gyrus (38%), Left lingual gyrus (16%) | 4.55/4.30 |
| Negative association with DBTP | ||||
| Seed: left amPFC | ||||
| Bilateral precuneus | 9; −57; 15 | 140 | Right precuneus (41%), left precuneus (24%), Right Cuneus (10%) | 4.30/4.09 |
| Seed: right amPFC | ||||
| Right DLPFC | 48; 30; 21 | 73 | Right middle frontal gyrus (74%) | 4.92/4.61 |
| Right precuneus | 12; −57; 15 | 51 | Right precuneus (61%), Right Cuneus (12%) | 3.90/3.74 |
| Seed: left PCC | ||||
| Bilateral ACC | 15; 42; 18 | 77 | Right anterior cingulate gyrus (27%), Right superior frontal gyrus medial segment (25%), Left anterior cingulate gyrus (21%) | 4.25/4.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Huang, J.; Cui, J.-f.; Li, Z.; Wang, Y.; Irish, M.; Chan, R.C.K. Functional Coupling between the Fronto-Parietal Network and Default Mode Network Is Associated with Balanced Time Perspective. Brain Sci. 2022, 12, 1201. https://doi.org/10.3390/brainsci12091201
Chen T, Huang J, Cui J-f, Li Z, Wang Y, Irish M, Chan RCK. Functional Coupling between the Fronto-Parietal Network and Default Mode Network Is Associated with Balanced Time Perspective. Brain Sciences. 2022; 12(9):1201. https://doi.org/10.3390/brainsci12091201
Chicago/Turabian StyleChen, Tao, Jia Huang, Ji-fang Cui, Zhi Li, Ya Wang, Muireann Irish, and Raymond C. K. Chan. 2022. "Functional Coupling between the Fronto-Parietal Network and Default Mode Network Is Associated with Balanced Time Perspective" Brain Sciences 12, no. 9: 1201. https://doi.org/10.3390/brainsci12091201






